Spectrum of Germline Pathogenic Variants in BRCA1/2 Genes in the Apulian Southern Italy Population: Geographic Distribution and Evidence for Targeted Genetic Testing
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Set
2.2. Molecular BRCA1/2 Analysis
2.3. Statistical Analysis
3. Results
3.1. Data Set Description
3.2. Phenotype Description
3.3. Genotype Description
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mann, G.J.; Thorne, H.; Balleine, R.L.; Butow, P.N.; Clarke, C.L.; Edkins, E.; Evans, G.M.; Fereday, S.; Haan, E.; Gattas, M.; et al. Analysis of cancer risk and BRCA1 and BRCA2 mutation prevalence in the kConFab familial breast cancer resource. Breast Cancer Res. 2006, 8, R12. [Google Scholar] [CrossRef] [PubMed]
- AIOM-Airtum “I Numeri del Cancro 2019”. Available online: https://www.aiom.it/i-numeri-del-cancro-in-italia/ (accessed on 10 October 2019).
- Hartmann, L.C.; Lindor, N.M. The Role of Risk-Reducing Surgery in Hereditary Breast and Ovarian Cancer. N. Engl. J. Med. 2016, 374, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Reis-Filho, J.S.; Ellis, I.O. Basal-like breast cancer: A critical review. J. Clin. Oncol. 2008, 26, 2568–2581. [Google Scholar] [CrossRef] [PubMed]
- Atchley, D.P.; Albarracin, C.T.; Lopez, A.; Valero, V.; Amos, C.I.; Gonzalez-Angulo, A.M.; Hortobagyi, G.N.; Arun, B.K. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J. Clin. Oncol. 2008, 26, 4282–4288. [Google Scholar] [CrossRef]
- Fujiwara, M.; McGuire, V.A.; Felberg, A.; Sieh, W.; Whittemore, A.S.; Longacre, T.A. Prediction of BRCA1 germline mutation status in women with ovarian cancer using morphology-based criteria: Identification of a BRCA1 ovarian cancer phenotype. Am. J. Surg. Pathol. 2012, 36, 1170–1177. [Google Scholar] [CrossRef]
- Rust, K.; Spiliopoulou, P.; Tang, C.Y.; Bell, C.; Stirling, D.; Phang, T.; Davidson, R.; Mackean, M.; Nussey, F.; Glasspool, R.M.; et al. Routine germline BRCA1 and BRCA2 testing in patients with ovarian carcinoma: Analysis of the Scottish real-life experience. BJOG 2018, 125, 1451–1458. [Google Scholar] [CrossRef]
- Petrucelli, N.; Daly, M.B.; Pal, T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. In GeneReviews; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle WA, USA, 1998; Available online: https://www.ncbi.nlm.nih.gov/books/NBK1247/ (accessed on 20 May 2020).
- Kurian, A.W.; Sigal, B.M.; Plevritis, S.K. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J. Clin. Oncol. 2010, 28, 222–231. [Google Scholar] [CrossRef]
- Le-Petross, H.T.; Whitman, G.J.; Atchley, D.P.; Yuan, Y.; Gutierrez-Barrera, A.; Hortobagyi, G.N.; Litton, J.K.; Arun, B.K. Effectiveness of alternating mammography and magnetic resonance imaging for screening women with deleterious BRCA mutations at high risk of breast cancer. Cancer 2011, 117, 3900–3907. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef]
- NCCN Guidelines Version 1. Genetic/Familial High-Risk Assessment: Breast, Ovarian and Pancreatic. 2020. Available online: https://www.nccn.org/guidelines/ (accessed on 10 October 2019).
- Palma, M.D.; Domchek, S.M.; Stopfer, J.; Erlichman, J.; Siegfried, J.D.; Tigges-Cardwell, J.; Mason, B.A.; Rebbeck, T.R.; Nathanson, K.L. The relative contribution of point mutations and genomic rearrangements in BRCA1 and BRCA2 in high-risk breast cancer families. Cancer Res. 2008, 68, 7006–7014. [Google Scholar] [CrossRef]
- Janavičius, R. Founder BRCA1/2 mutations in the Europe: Implications for hereditary breast-ovarian cancer prevention and control. EPMA J. 2010, 1, 397–412. [Google Scholar] [CrossRef]
- D’Andrea, E.; Marzuillo, C.; De Vito, C.; Di Marco, M.; Pitini, E.; Vacchio, M.R.; Villari, P. Which BRCA genetic testing programs are ready for implementation in health care? A systematic review of economic evaluations. Genet. Med. Off. J. Am. Coll. Med. Genet. 2016, 18, 1171–1180. [Google Scholar] [CrossRef]
- Manchanda, R.; Gaba, F. Population Based Testing for Primary Prevention: A Systematic Review. Cancers 2018, 10, 424. [Google Scholar] [CrossRef]
- Enigma Consortium. Available online: https://enigmaconsortium.org/ (accessed on 15 March 2019).
- Plon, S.E.; Eccles, D.M.; Easton, D.; Foulkes, W.D.; Genuardi, M.; Greenblatt, M.S.; Hogervorst, F.B.; Hoogerbrugge, N.; Spurdle, A.B.; Tavtigian, S.V.; et al. Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum. Mutat. 2008, 29, 1282–1291. [Google Scholar] [CrossRef]
- Sequence Variant Nomenclature. Available online: http://www.hgvs.org/mutnomen/examplesDNA.html/ (accessed on 21 September 2019).
- Skidmore, Z.L.; Wagner, A.H.; Lesurf, R.; Campbell, K.M.; Kunisaki, J.; Griffith, O.L.; Griffith, M. GenVisR: Genomic Visualizations in R. Bioinformatics 2016, 32, 3012–3014. [Google Scholar] [CrossRef]
- Incorvaia, L.; Fanale, D.; Badalamenti, G.; Bono, M.; Calò, V.; Cancelliere, D.; Castiglia, M.; Fiorino, A.; Pivetti, A.; Barraco, N.; et al. Hereditary Breast and Ovarian Cancer in Families from Southern Italy (Sicily)-Prevalence and Geographic Distribution of Pathogenic Variants in BRCA1/2 Genes. Cancers 2020, 12, 1158. [Google Scholar] [CrossRef]
- Foglietta, J.; Ludovini, V.; Bianconi, F.; Pistola, L.; Reda, M.S.; Al-Refaie, A.; Tofanetti, F.R.; Mosconi, A.; Minenza, E.; Anastasi, P.; et al. Prevalence and Spectrum of BRCA Germline Variants in Central Italian High Risk or Familial Breast/Ovarian Cancer Patients: A Monocentric Study. Genes 2020, 11, 925. [Google Scholar] [CrossRef]
- Cini, G.; Mezzavilla, M.; Della Puppa, L.; Cupelli, E.; Fornasin, A.; D’Elia, A.V.; Dolcetti, R.; Damante, G.; Bertok, S.; Miolo, G.; et al. Tracking of the origin of recurrent mutations of the BRCA1 and BRCA2 genes in the North-East of Italy and improved mutation analysis strategy. BMC Med. Genet. 2016, 17, 11. [Google Scholar] [CrossRef][Green Version]
- Figlioli, G.; De Nicolo, A.; Catucci, I.; Manoukian, S.; Peissel, B.; Azzollini, J.; Beltrami, B.; Bonanni, B.; Calvello, M.; Bondavalli, D.; et al. Analysis of Italian BRCA1/2 Pathogenic Variants Identifies a Private Spectrum in the Population from the Bergamo Province in Northern Italy. Cancers 2021, 13, 532. [Google Scholar] [CrossRef]
- Santonocito, C.; Rizza, R.; Paris, I.; Marchis, L.; Paolillo, C.; Tiberi, G.; Scambia, G.; Capoluongo, E. Spectrum of Germline BRCA1 and BRCA2 Variants Identified in 2351 Ovarian and Breast Cancer Patients Referring to a Reference Cancer Hospital of Rome. Cancers 2020, 12, 1286. [Google Scholar] [CrossRef]
- Palomba, G.; Loi, A.; Uras, A.; Fancello, P.; Piras, G.; Gabbas, A.; Cossu, A.; Budroni, M.; Contu, A.; Tanda, F.; et al. A role of BRCA1 and BRCA2 germline mutations in breast cancer susceptibility within Sardinian population. BMC Cancer 2009, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R.; Friebel, T.M.; Friedman, E.; Hamann, U.; Huo, D.; Kwong, A.; Olah, E.; Olopade, O.I.; Solano, A.R.; Teo, S.H.; et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum. Mutat. 2018, 39, 593–620. [Google Scholar] [CrossRef] [PubMed]
- Laitman, Y.; Friebel, T.M.; Yannoukakos, D.; Fostira, F.; Konstantopoulou, I.; Figlioli, G.; Bonanni, B.; Manoukian, S.; Zuradelli, M.; Tondini, C.; et al. The spectrum of BRCA1 and BRCA2 pathogenic sequence variants in Middle Eastern, North African, and South European countries. Hum. Mutat. 2019, 40, e1–e23. [Google Scholar] [CrossRef]
- Hamel, N.; Feng, B.J.; Foretova, L.; Stoppa-Lyonnet, D.; Narod, S.A.; Imyanitov, E.; Sinilnikova, O.; Tihomirova, L.; Lubinski, J.; Gronwald, J.; et al. On the origin and diffusion of BRCA1 c.5266dupC (5382insC) in European populations. Eur. J. Hum. Genet. EJHG 2011, 19, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Gianolio, E. Gli Ebrei a Trani e in Puglia nel Medioevo; Landriscina Editrice: Trani, Italy, 2008. [Google Scholar]
- Palmero, E.I.; Carraro, D.M.; Alemar, B.; Moreira, M.; Ribeiro-Dos-Santos, Â.; Abe-Sandes, K.; Galvão, H.; Reis, R.M.; de Pádua Souza, C. The germline mutational landscape of BRCA1 and BRCA2 in Brazil. Sci. Rep. 2018, 8, 9188. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Kasumi, F.; Yoshimoto, M.; Nomizu, T.; Asaishi, K.; Abe, R.; Tsuchiya, A.; Sugano, M.; Takai, S.; Yoneda, M.; et al. High proportion of missense mutations of the BRCA1 and BRCA2 genes in Japanese breast cancer families. J. Hum. Genet. 1998, 43, 42–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santos, C.; Peixoto, A.; Rocha, P.; Pinto, P.; Bizarro, S.; Pinheiro, M.; Pinto, C.; Henrique, R.; Teixeira, M.R. Pathogenicity evaluation of BRCA1 and BRCA2 unclassified variants identified in Portuguese breast/ovarian cancer families. J. Mol. Diagn. JMD 2014, 16, 324–334. [Google Scholar] [CrossRef]
| Feature | n (%) |
|---|---|
SEX
| 1947 (96%) 79 (4%) |
HEALTH STATE
| 46 (2%) 1980 (98%) |
| AGE [mean (range)] | 55 yrs. (21–92) |
BRCA1/2 STATUS
| 499 (24.6%) 1396 (69.1%) 131 (6.5%) |
BRCA 1/2 carriers
| 342 (68.4%) 158 (316%) |
| Geographical Area of Origin | BRCA1/2 Status | Probands Tested n (%) |
|---|---|---|
| BARI | BRCA1carriers BRCA2 carriers BRCA1/2 non-carriers BRCA1/2 VUS TOTAL | 105 (13%) 59(7%) 595 (72%) 63 (8%) 822 |
| BAT (Barletta, Andria and Trani) | BRCA1carriers BRCA2 carriers BRCA1/2 non-carriers BRCA1/2 VUS TOTAL | 53 (29%) 25 (14%) 101 (55%) 4 (2%) 182 |
| BRINDISI | BRCA1carriers BRCA2 carriers BRCA1/2 non-carriers BRCA1/2 VUS TOTAL | 25 (19%) 14 (10%) 90 (67%) 5 (4%) 134 |
| FOGGIA | BRCA1carriers BRCA2 carriers BRCA1/2 non-carriers BRCA1/2 VUS TOTAL | 10 (40%) 4 (16%) 6 (24%) 5 (20%) 25 |
| LECCE | BRCA1carriers BRCA2 carriers BRCA1/2 non-carriers BRCA1/2 VUS TOTAL | 103 (17%) 40 (6%) 422 (70%) 43 (7%) 618 |
| TARANTO | BRCA1carriers BRCA2 carriers BRCA1/2 non-carriers BRCA1/2 VUS TOTAL | 46 (19%) 16 (6%) 172 (70%) 11 (5%) 245 |
| Cancer Type | Mutational Status | n (%) | p-Value |
|---|---|---|---|
| BC | C | 358 (67.4) | |
| NC | 1102 (82.3) | <0.0001 | |
| VUS | 107 (84.9) | ||
| Melanoma | C | 0 (0) | |
| NC | 5 (0.37) | 0.2 | |
| VUS | 1 (0.79) | ||
| OC | C | 144 (27.1) | |
| NC | 167 (12.4) | <0.0001 | |
| VUS | 17 (13.5) | ||
| Pancreatic cancer | C | 2 (0.4) | |
| NC | 5 (0.3) | 0.7 | |
| VUS | 0 (0) | ||
| Prostate cancer | C | 1 (0,2) | |
| NC | 9 (0.7) | 0.2 | |
| VUS | 0 (0) | ||
| Other site | C | 11 (2) | |
| NC | 21 (1.6) | 0.2 | |
| VUS | 0 (0) | ||
| Healthy | C | 15 (2.8) | |
| NC | 30 (2.2) | 0.3 | |
| VUS | 0 (0) |
| Breast Cancer Histology | Mutational Status | n (%) | p-Value |
|---|---|---|---|
| Ductal | C | 265 (84.4) | |
| NC | 774 (74.3) | 0.0009 | |
| VUS | 70 (75.3) | ||
| in situ | C | 20 (6.4) | |
| NC | 102 (9.8) | 0.1 | |
| VUS | 10 (10.7) | ||
| Lobular | C | 8 (2.5) | |
| NC | 80 (7.7) | 0.05 | |
| VUS | 5 (5.3) | ||
| Mixed histology | C | 6 (1.9) | |
| NC | 26 (2.5) | 0.6 | |
| VUS | 1 (1) | ||
| Other histologies | C | 15 (4.7) | |
| NC | 60 (5.8) | 0.5 | |
| VUS | 7 (7.5) |
| Ovarian Cancer Histology | Mutational Status | n (%) | p-Value |
|---|---|---|---|
| Endometrioid | C | 15 (11.3) | |
| NC | 23 (14.1) | 0.3 | |
| VUS | 4 (23.5) | ||
| High-grade serous | C | 109 (81.9) | |
| NC | 127 (77.9) | 0.08 | |
| VUS | 10 (58.8) | ||
| Other histologies | C | 9 (6.7) | |
| NC | 13 (7.9) | 0.05 | |
| VUS | 3 (17.6) |
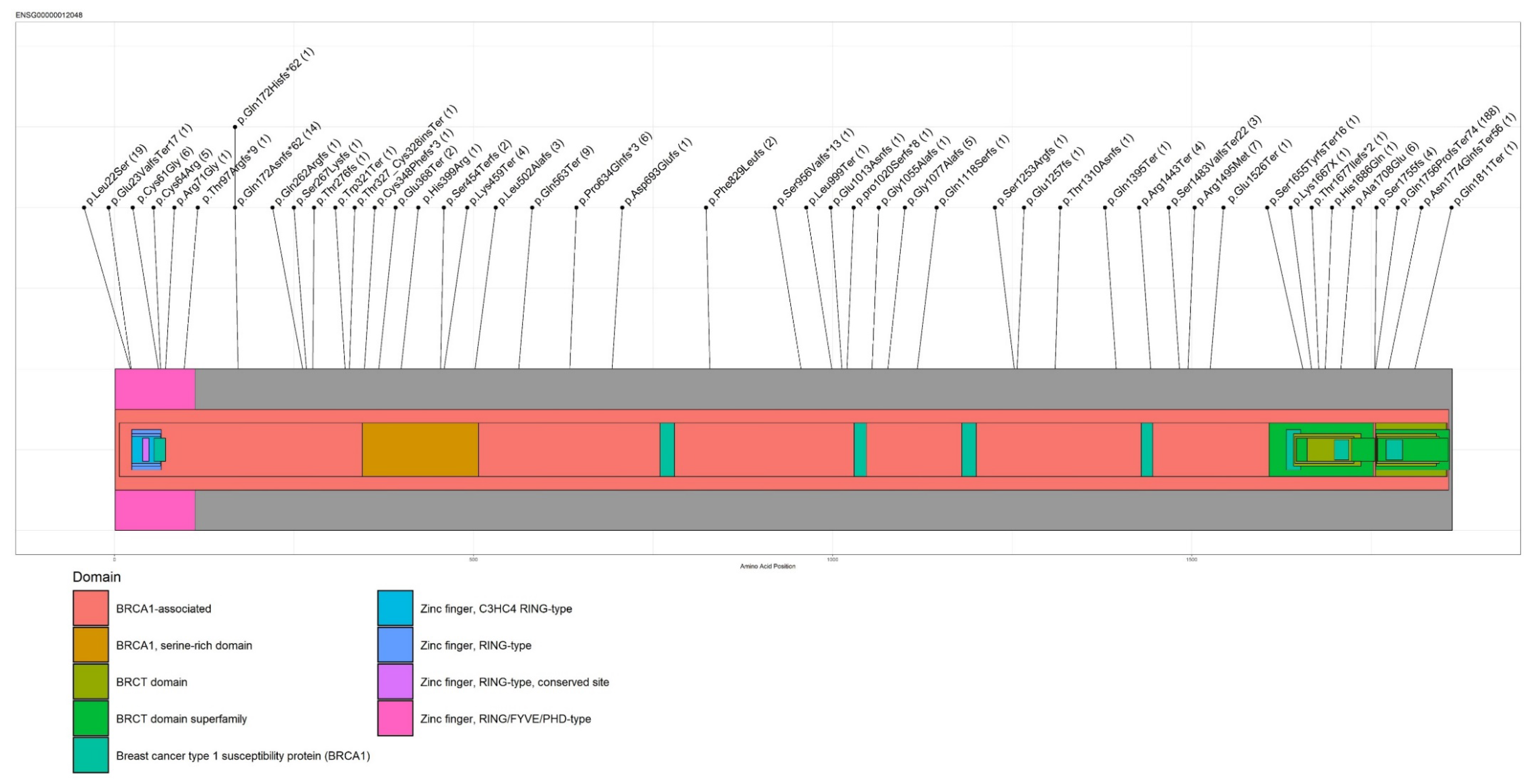
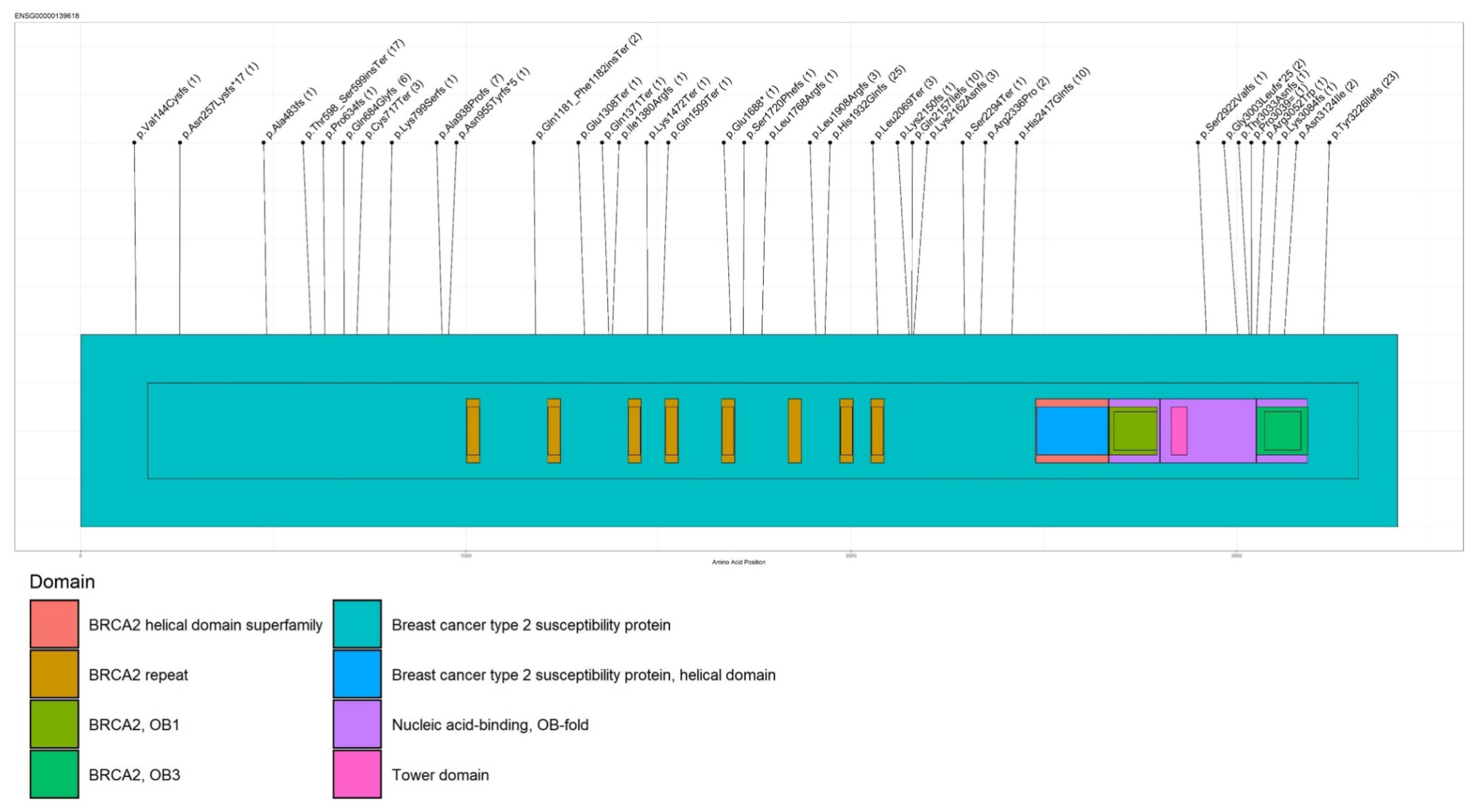
| Gene | HGVS Nomenclature | Proband Numbers |
|---|---|---|
| BRCA1 | c.5266dupC (p.Gln1756Profs) | 188 |
| BRCA1 | c.65T > C (p.Leu22Ser) | 19 |
| BRCA1 | c.514delC (p.Gln172fs) | 14 |
| BRCA1 | c.1687C > T (p.Gln563Ter) | 9 |
| BRCA1 | c.4484G > T (p.Arg1495Met) | 7 |
| BRCA2 | c.5796_5797delTA (p.His1932Glnfs) | 25 |
| BRCA2 | c.9676delT (p.Tyr3226Ilefs) | 23 |
| BRCA2 | c.1796_1800delCTTAT (p.Ser599Terfs) | 17 |
| BRCA2 | c.8755-1G > A | 12 |
| BRCA2 | c.6462_6463delTC (p.Gln2157Ilefs) | 10 |
| Recurrent PVs | European Allele Frequency | European Sample Size | Global Allele Frequency | Global Sample Size | Bari (%) | BAT (%) | Brindisi (%) | Foggia (%) | Lecce (%) | Taranto (%) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BRCA1:c.1687C > T | 0.00007 | 73,172 | 0.000041 | 121,058 | 6 (3) | 1 (1) | 0 (0) | 0 (0) | 2 (1) | 0 (0) | n.s. |
| BRCA1:c.5266dupC | 0.00026 | 73,354 | 0.000156 | 1,121,412 | 49 (29) | 46 (59) | 21 (53) | 4 (28) | 37 (25) | 31 (50) | <0.0001 |
| BRCA1:c.514delC | 0.00001 | 73,352 | 0.000008 | 121,410 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 14 (9) | 0 (0) | <0.0001 |
| BRCA1:c.4484G > T | 0.00001 | 73,324 | 0.000008 | 121,358 | 0 (0) | 0 (0) | 1 (2) | 1 (8) | 0 (0) | 5 (8) | <0.0001 |
| BRCA1:c.65T > C | / | / | / | / | 12 (7) | 0 (0) | 0 (0) | 0 (0) | 7 (4) | 0 (0) | 0.018 |
| BRCA2:c.5796_5797delTA | 0.00001 | 73,246 | 0.000008 | 121,048 | 14 (8) | 10 (12.9) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | <0.0001 |
| BRCA2:c.1796_1800delCTTAT | 0.000008 * | 132,644 * | 0.000004 * | 244,054 * | 12 (7) | 2 (2.5) | 1 (2.5) | 0 (0) | 2 (1.4) | 0 (0) | 0.025 |
| BRCA2: c.8755-1G > A | 0.000008 ** | 16,160 ** | 0.00047 ** | 17,014 ** | 0 (0) | 0 (0) | 2 (5.1) | 0 (0) | 10 (7) | 0 (0) | 0.0009 |
| BRCA2:c.6462_6463delTC | 0.00001 | 72,338 | 0.000008 | 118,538 | 6 (3.6) | 2 (2.6) | 0 (0) | 0 (0) | 1 (0.7) | 1 (1.6) | n.s. |
| BRCA2:C.9676delT | / | / | / | / | 1 (0.6) | 0 (0) | 1 (2) | 0 (0) | 17 (11.8) | 4 (6.4) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patruno, M.; De Summa, S.; Resta, N.; Caputo, M.; Costanzo, S.; Digennaro, M.; Pilato, B.; Bagnulo, R.; Pantaleo, A.; Simone, C.; et al. Spectrum of Germline Pathogenic Variants in BRCA1/2 Genes in the Apulian Southern Italy Population: Geographic Distribution and Evidence for Targeted Genetic Testing. Cancers 2021, 13, 4714. https://doi.org/10.3390/cancers13184714
Patruno M, De Summa S, Resta N, Caputo M, Costanzo S, Digennaro M, Pilato B, Bagnulo R, Pantaleo A, Simone C, et al. Spectrum of Germline Pathogenic Variants in BRCA1/2 Genes in the Apulian Southern Italy Population: Geographic Distribution and Evidence for Targeted Genetic Testing. Cancers. 2021; 13(18):4714. https://doi.org/10.3390/cancers13184714
Chicago/Turabian StylePatruno, Margherita, Simona De Summa, Nicoletta Resta, Mariapia Caputo, Silvia Costanzo, Maria Digennaro, Brunella Pilato, Rosanna Bagnulo, Antonino Pantaleo, Cristiano Simone, and et al. 2021. "Spectrum of Germline Pathogenic Variants in BRCA1/2 Genes in the Apulian Southern Italy Population: Geographic Distribution and Evidence for Targeted Genetic Testing" Cancers 13, no. 18: 4714. https://doi.org/10.3390/cancers13184714
APA StylePatruno, M., De Summa, S., Resta, N., Caputo, M., Costanzo, S., Digennaro, M., Pilato, B., Bagnulo, R., Pantaleo, A., Simone, C., Natalicchio, M. I., De Matteis, E., Tarantino, P., Tommasi, S., & Paradiso, A. (2021). Spectrum of Germline Pathogenic Variants in BRCA1/2 Genes in the Apulian Southern Italy Population: Geographic Distribution and Evidence for Targeted Genetic Testing. Cancers, 13(18), 4714. https://doi.org/10.3390/cancers13184714






