Facing Thyroid Nodules in Paediatric Patients Previously Treated with Radiotherapy for Non-Thyroidal Cancers: Are Adult Ultrasound Risk Stratification Systems Reliable?
Abstract
:Simple Summary
Abstract
1. Introduction
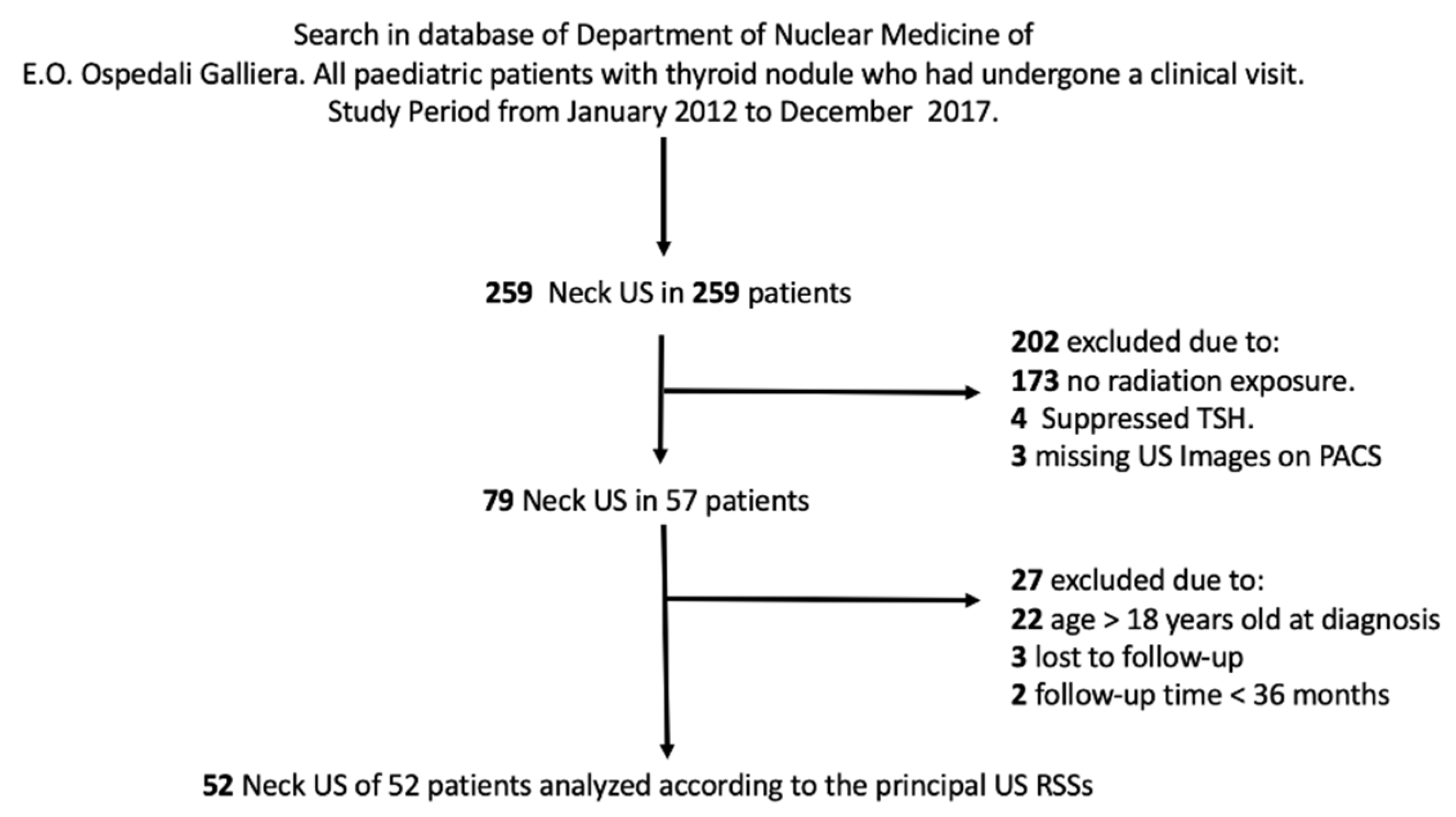
2. Materials and Methods
2.1. Neck Ultrasonography
2.2. Ultrasound Risk Stratification Systems
2.3. Imaging Review and Interpretation
2.4. Reference Standard
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cimbek, E.A.; Polat, R.; Sonmez, B.; Beyhun, N.E.; Dinc, H.; Saruhan, H.; Karaguzel, G. Clinical, sonographical, and pathological findings of pediatric thyroid nodules. Eur. J. Pediatr. 2021, 180, 2823–2829. [Google Scholar] [CrossRef]
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2015, 25, 716–759. [Google Scholar] [CrossRef] [Green Version]
- Richman, D.M.; Cherella, C.E.; Smith, J.R.; Modi, B.P.; Zendejas, B.; Frates, M.C.; Wassner, A.J. Clinical utility of sonographic features in indeterminate pediatric thyroid nodules. Eur. J. Endocrinol. 2021, 184, 657–665. [Google Scholar] [CrossRef]
- Elisei, R.; Romei, C.; Vorontsova, T.; Cosci, B.; Veremeychik, V.; Kuchinskaya, E.; Basolo, F.; Demidchik, E.P.; Miccoli, P.; Pinchera, A.; et al. RET/PTC rearrangements in thyroid nodules: Studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J. Clin. Endocrinol. Metab. 2001, 86, 3211–3216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shulan, J.M.; Vydro, L.; Schneider, A.B.; Mihailescu, D.V. Role of biomarkers in predicting the occurrence of thyroid neoplasms in radiation-exposed children. Endocr. Relat. Cancer 2018, 25, 481–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarzab, B.; Handkiewicz-Junak, D. Differentiated thyroid cancer in children and adults: Same or distinct disease? Hormones 2007, 6, 200–209. [Google Scholar] [PubMed]
- Piccardo, A.; Foppiani, L.; Puntoni, M.; Hanau, G.; Calafiore, L.; Garaventa, A.; Arlandini, A.; Villavecchia, G.; Bianchi, P.; Cabria, M. Role of low-cost thyroid follow-up in children treated with radiotherapy for primary tumors at high risk of developing a second thyroid tumor. Q. J. Nucl. Med. Mol. Imaging 2012, 56, 459–467. [Google Scholar] [PubMed]
- Iglesias, M.L.; Schmidt, A.; Ghuzlan, A.A.; Lacroix, L.; Vathaire, F.; Chevillard, S.; Schlumberger, M. Radiation exposure and thyroid cancer: A review. Arch. Endocrinol. Metab. 2017, 61, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistry, R.; Hillyar, C.; Nibber, A.; Sooriyamoorthy, T.; Kumar, N. Ultrasound Classification of Thyroid Nodules: A Systematic Review. Cureus 2020, 12, e7239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, E.G.; Tessler, F.N.; Hoang, J.K.; Langer, J.E.; Beland, M.D.; Berland, L.L.; Cronan, J.J.; Desser, T.S.; Frates, M.C.; Hamper, U.M.; et al. Thyroid Ultrasound Reporting Lexicon: White Paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. J. Am. Coll. Radiol. 2015, 12, 1272–1279. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, S.; Jing, M.; Sheng, R.; Cui, D.; Lu, S.; Zhang, X.; Jing, S.; Zhang, X.; Shan, T.; Shan, H.; et al. The analysis of differential diagnosis of benign and malignant thyroid nodules based on ultrasound reports. Gland. Surg. 2020, 9, 653–660. [Google Scholar] [CrossRef]
- Sakorafas, G.H.; Mastoraki, A.; Lappas, C.; Safioleas, M. Small (<10 mm) thyroid nodules; how aggressively should they be managed? Onkologie 2010, 33, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.; Trimboli, P.; Buffet, C. The New Era of TIRADSs to Stratify the Risk of Malignancy of Thyroid Nodules: Strengths, Weaknesses and Pitfalls. Cancers 2021, 13, 4316. [Google Scholar] [CrossRef]
- Castellana, M.; Castellana, C.; Treglia, G.; Giorgino, F.; Giovanella, L.; Russ, G.; Trimboli, P. Performance of Five Ultrasound Risk Stratification Systems in Selecting Thyroid Nodules for FNA. J. Clin. Endocrinol. Metab. 2020, 105, dgz170. [Google Scholar] [CrossRef] [PubMed]
- Fadda, G.; Basolo, F.; Bondi, A.; Bussolati, G.; Crescenzi, A.; Nappi, O.; Nardi, F.; Papotti, M.; Taddei, G.; Palombini, L.; et al. Cytological classification of thyroid nodules. Proposal of the SIAPEC-IAP Italian Consensus Working Group. Pathologica 2010, 102, 405–408. [Google Scholar] [PubMed]
- Nardi, F.; Basolo, F.; Crescenzi, A.; Fadda, G.; Frasoldati, A.; Orlandi, F.; Palombini, L.; Papini, E.; Zini, M.; Pontecorvi, A.; et al. Italian consensus for the classification and reporting of thyroid cytology. J. Endocrinol. Investig. 2014, 37, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, R.; Shankar, R.; Kort, K.; Khurana, K. Ultrasound-guided fine-needle aspiration in the management of thyroid nodules in children and adolescents. Thyroid 2009, 19, 703–705. [Google Scholar] [CrossRef]
- Buryk, M.A.; Simons, J.P.; Picarsic, J.; Monaco, S.E.; Ozolek, J.A.; Joyce, J.; Gurtunca, N.; Nikiforov, Y.E.; Feldman Witchel, S. Can malignant thyroid nodules be distinguished from benign thyroid nodules in children and adolescents by clinical characteristics? A review of 89 pediatric patients with thyroid nodules. Thyroid 2015, 25, 392–400. [Google Scholar] [CrossRef]
- Creo, A.; Alahdab, F.; Al Nofal, A.; Thomas, K.; Kolbe, A.; Pittock, S.T. Ultrasonography and the American Thyroid Association Ultrasound-Based Risk Stratification Tool: Utility in Pediatric and Adolescent Thyroid Nodules. Horm. Res. Paediatr. 2018, 90, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rios, C.; Daneman, A.; Bajno, L.; van der Kaay, D.C.M.; Moineddin, R.; Wasserman, J.D. Utility of adult-based ultrasound malignancy risk stratifications in pediatric thyroid nodules. Pediatr. Radiol. 2018, 48, 74–84. [Google Scholar] [CrossRef]
- Gupta, A.; Ly, S.; Castroneves, L.A.; Frates, M.C.; Benson, C.B.; Feldman, H.A.; Wassner, A.J.; Smith, J.R.; Marqusee, E.; Alexander, E.K.; et al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J. Clin. Endocrinol. Metab. 2013, 98, 3238–3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedziela, M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr. Relat. Cancer 2006, 13, 427–453. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.H.; Yoon, H.M.; Hwang, J.; Lee, J.S.; Jung, A.Y.; Cho, Y.A.; Baek, J.H. Diagnostic performance of adult-based ATA and ACR-TIRADS ultrasound risk stratification systems in pediatric thyroid nodules: A systematic review and meta-analysis. Eur. Radiol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Polat, Y.D.; Ozturk, V.S.; Ersoz, N.; Anik, A.; Karaman, C.Z. Is Thyroid Imaging Reporting and Data System Useful as an Adult Ultrasonographic Malignancy Risk Stratification Method in Pediatric Thyroid Nodules? J. Med. Ultrasound 2019, 27, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Castellana, M.; Piccardo, A.; Romanelli, F.; Grani, G.; Giovanella, L.; Durante, C. The ultrasound risk stratification systems for thyroid nodule have been evaluated against papillary carcinoma. A meta-analysis. Rev. Endocr. Metab. Disord. 2021, 22, 453–460. [Google Scholar] [CrossRef]
- Clement, S.C.; Lebbink, C.A.; Klein Hesselink, M.S.; Teepen, J.C.; Links, T.P.; Ronckers, C.M.; van Santen, H.M. Presentation and outcome of subsequent thyroid cancer among childhood cancer survivors compared to sporadic thyroid cancer: A matched national study. Eur. J. Endocrinol. 2020, 183, 169–180. [Google Scholar] [CrossRef]
- Uner, C.; Aydin, S.; Ucan, B. Thyroid Image Reporting and Data System Categorization: Effectiveness in Pediatric Thyroid Nodule Assessment. Ultrasound Q. 2020, 36, 15–19. [Google Scholar] [CrossRef]
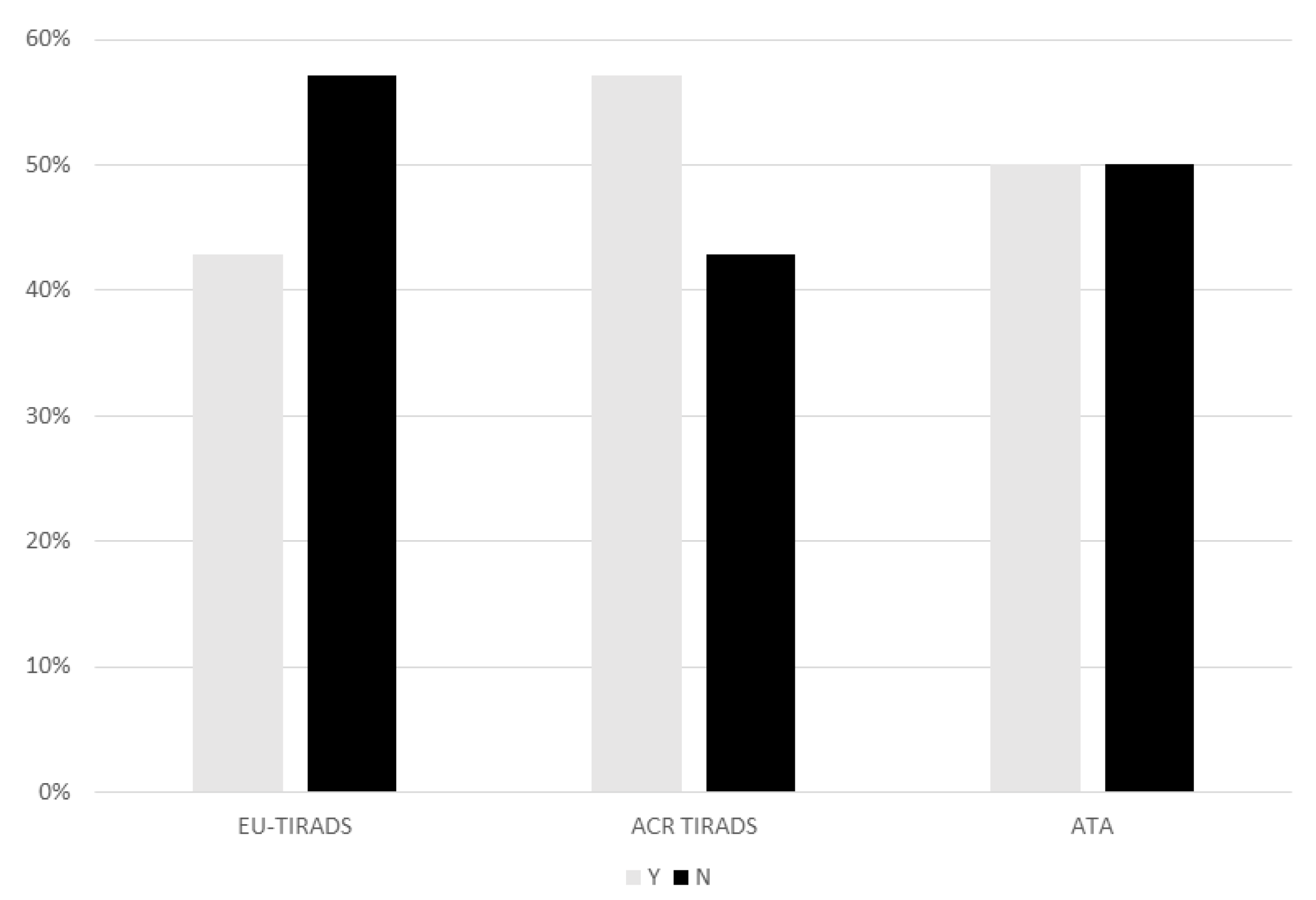
| Variable | Subjects Included (n = 52) |
|---|---|
| Sex | |
| Female, n. (%) | 32 (61.5) |
| Male, n. (%) | 20 (38.5) |
| Age on nodule diagnosis, median (IQR), years | 17 (15–18) |
| <15 years, n. (%) | 11 (21.1) |
| ≥15 years, n. (%) | 41 (78.9) |
| Age on irradiation, median (IQR), year | 5 (3–7) |
| <5 years, n. (%) | 24 (46.1) |
| ≥5 years, n. (%) | 28 (53.9) |
| Time from RT to thyroid nodule diagnosis median (IQR), year | 11 (8–14) |
| <10 years, n. (%) | 16 (30.8) |
| ≥10 years, n. (%) | 36 (69.2) |
| Nodule dimensions, median (IQR), mm | 13 (11–22) |
| <10 mm, n. (%) | 7 (13.4) |
| 10–15 mm, n. (%) | 26 (50.0) |
| 16–20 mm, n. (%) | 5 (9.6) |
| >20 mm, n. (%) | 16 (30.7) |
| Thyroid cytology * | |
| Tir 2, n. (%) | 36 (69.2) |
| Tir 3, n. (%) | 2 (3.8) |
| Tir 3b, n.(%) | 2 (3.8) |
| Tir 4, n. (%) | 3 (5.7) |
| Tir 5, n. (%) | 9 (17.3) |
| Thyroidectomy | |
| Yes, n. (%) | 19 (36.5) |
| No, n. (%) | 33 (63.5) |
| Pathology | |
| Papillary thyroid carcinoma, n. (%) | 14 (73.) |
| Follicular thyroid carcinoma, n. (%) | 0 (0) |
| Follicular hyperplasia | 4 (21.0) |
| Follicular adenoma | 1 (5.3) |
| Age on DTC diagnosis, median (IQR), years | 15 (14–18) |
| <15 years, no. (%) | 4 (28.5) |
| ≥15 years, no. (%) | 10 (71.5) |
| Time from RT to DTC diagnosis, median (IQR), year | 11 (10–12) |
| <10 years, no. (%) | 3 (21.4) |
| ≥10 years, no. (%) | 10 (78.6) |
| Clinico-pathological classification ** | |
| T1, no. (%) | 12 (85.7) |
| T2, no. (%) | 2 (14.3) |
| N0, no. (%) | 5 (35.7) |
| N1a, no. (%) | 7 (50.0) |
| N1b, no. (%) | 2 (14.3) |
| M0, no. (%) | 14 (100) |
| US-RSSs | ACR-TIRADS | EU-TIRADS | ATA | p-VALUE (ACR vs. EU TIRADS) | p-VALUE (ACR TIRADS vs. ATA) | p-VALUE (EU TIRADS vs. ATA) |
| Sensitivity | 71% | 71% | 64% | 0.66 | 0.4 | 0.68 |
| Specificity | 97% | 95% | 95% | 0.56 | 0.56 | 1 |
| NPV | 91% | 90% | 88% | 0.69 | 0.47 | 0.75 |
| PPV | 91% | 83% | 82% | 0.53 | 0.47 | 0.92 |
| Accuracy | 91% | 88% | 87% | 0.87 | 0.83 | 0.72 |
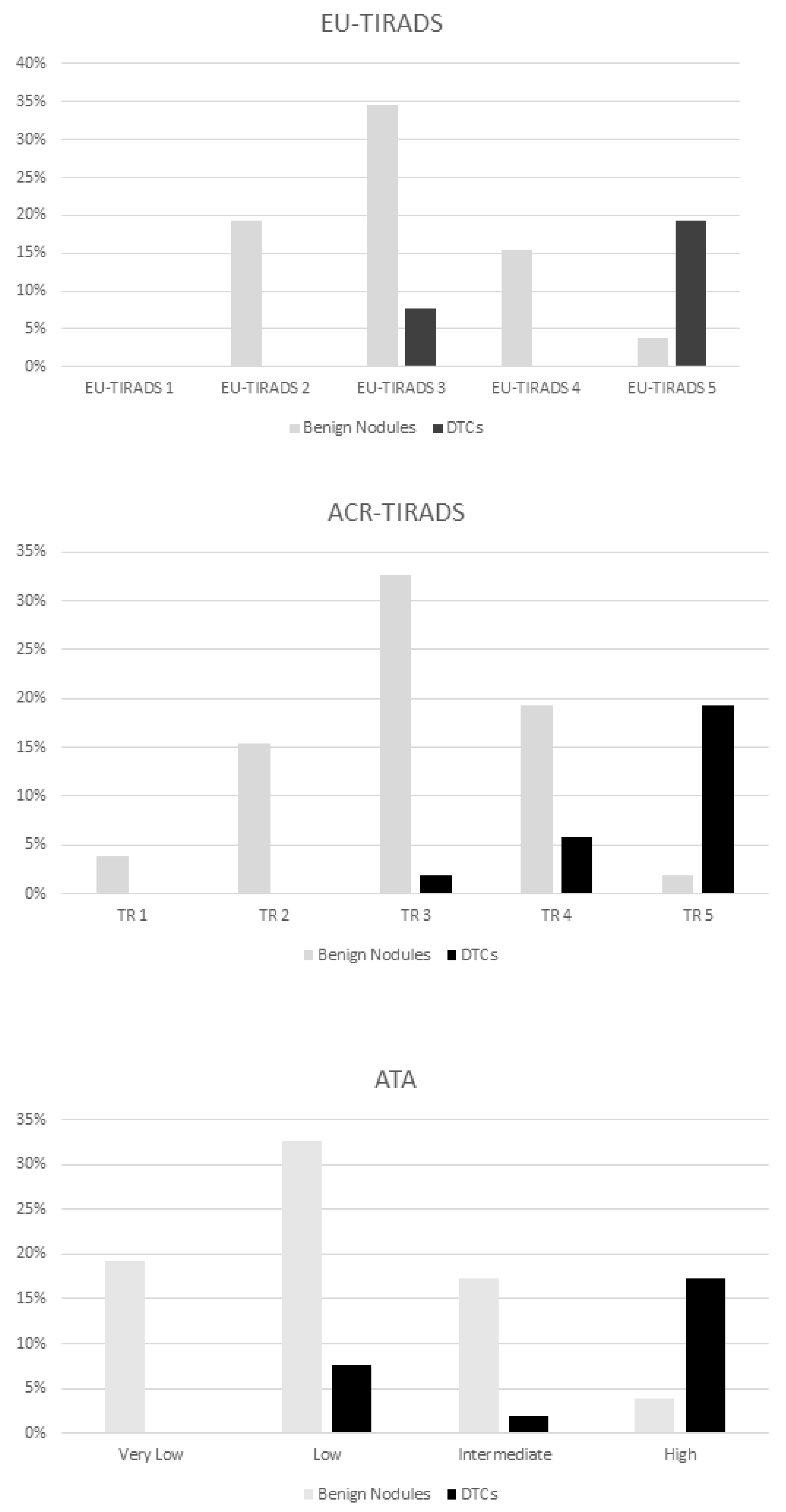
| Category | Prevalence of Malignancy |
|---|---|
| EU-TIRADS | |
| EU-TIRADS 2 | 0% |
| EU-TIRADS 3 | 4/14 (29%) |
| EU-TIRADS 4 | 0% |
| EU-TIRADS 4 | 10/14 (71%) |
| ACR TIRADS | |
| TR 1 | 0% |
| TR 2 | 0% |
| TR 3 | 1/14 (7%) |
| TR 4 | 3/14 (22%) |
| TR 5 | 10/14 (71%) |
| ATA | |
| Benign | 0% |
| Very low | 0% |
| Low | 4/14 (29%) |
| Intermediate | 1/14 (7%) |
| High | 9/14 (64%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccardo, A.; Fiz, F.; Bottoni, G.; De Luca, C.; Massollo, M.; Catrambone, U.; Foppiani, L.; Muraca, M.; Garaventa, A.; Trimboli, P. Facing Thyroid Nodules in Paediatric Patients Previously Treated with Radiotherapy for Non-Thyroidal Cancers: Are Adult Ultrasound Risk Stratification Systems Reliable? Cancers 2021, 13, 4692. https://doi.org/10.3390/cancers13184692
Piccardo A, Fiz F, Bottoni G, De Luca C, Massollo M, Catrambone U, Foppiani L, Muraca M, Garaventa A, Trimboli P. Facing Thyroid Nodules in Paediatric Patients Previously Treated with Radiotherapy for Non-Thyroidal Cancers: Are Adult Ultrasound Risk Stratification Systems Reliable? Cancers. 2021; 13(18):4692. https://doi.org/10.3390/cancers13184692
Chicago/Turabian StylePiccardo, Arnoldo, Francesco Fiz, Gianluca Bottoni, Camilla De Luca, Michela Massollo, Ugo Catrambone, Luca Foppiani, Monica Muraca, Alberto Garaventa, and Pierpaolo Trimboli. 2021. "Facing Thyroid Nodules in Paediatric Patients Previously Treated with Radiotherapy for Non-Thyroidal Cancers: Are Adult Ultrasound Risk Stratification Systems Reliable?" Cancers 13, no. 18: 4692. https://doi.org/10.3390/cancers13184692
APA StylePiccardo, A., Fiz, F., Bottoni, G., De Luca, C., Massollo, M., Catrambone, U., Foppiani, L., Muraca, M., Garaventa, A., & Trimboli, P. (2021). Facing Thyroid Nodules in Paediatric Patients Previously Treated with Radiotherapy for Non-Thyroidal Cancers: Are Adult Ultrasound Risk Stratification Systems Reliable? Cancers, 13(18), 4692. https://doi.org/10.3390/cancers13184692







