Lung-Resident Mesenchymal Stem Cell Fates within Lung Cancer
Abstract
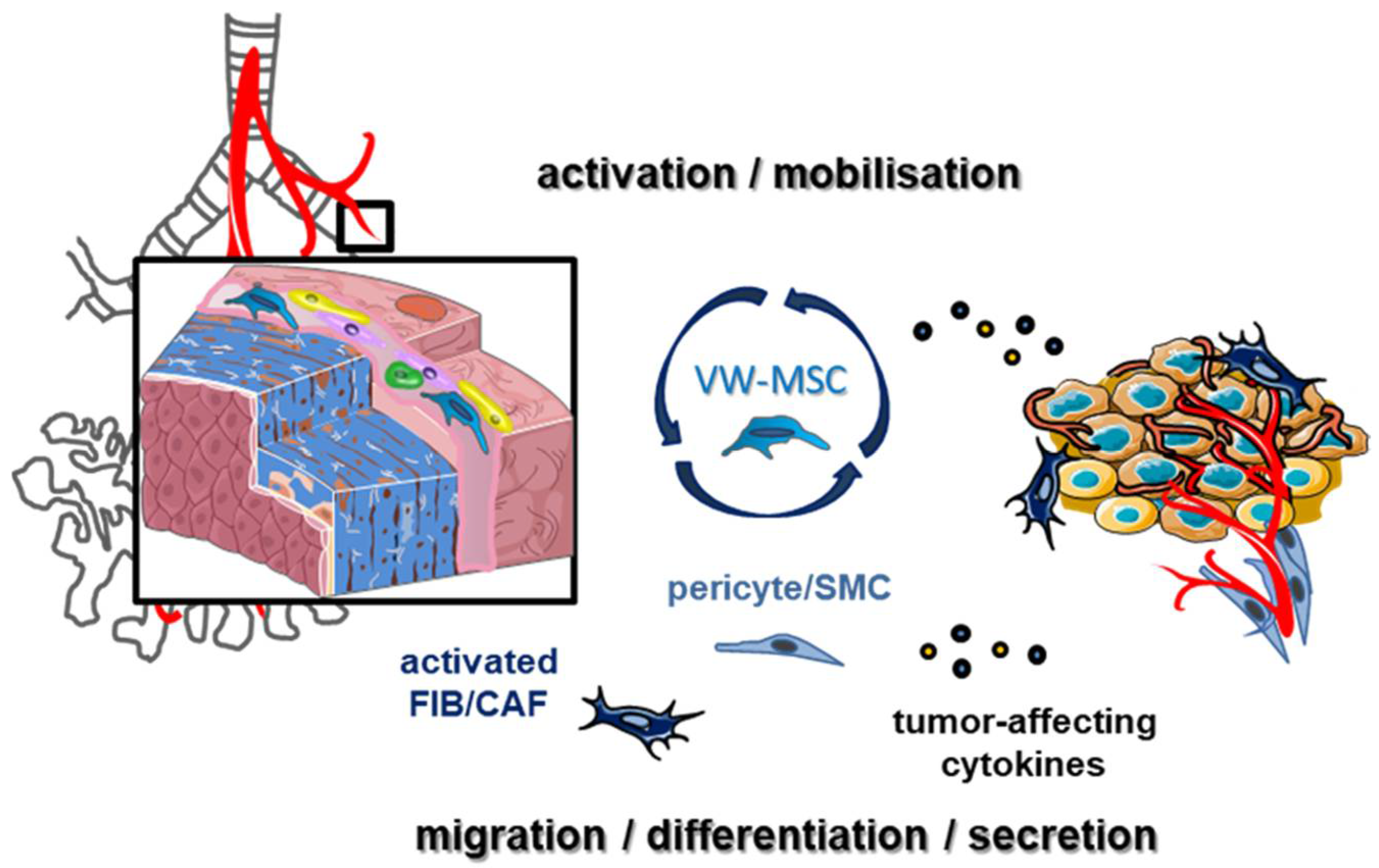
Simple Summary
Abstract
1. Introduction
2. The Tumor-Promoting Action of Lung Cancer-Associated MSCs
3. LR-MSCs as Source of Activated Fibroblasts
4. LR-MSCs as Mural Cell Precursors
5. The Challenges Regarding LR-MSCs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Osmani, L.; Askin, F.; Gabrielson, E.; Li, Q.K. Current who guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (nsclc): Moving from targeted therapy to immunotherapy. Semin. Cancer Biol. 2018, 52, 103–109. [Google Scholar] [CrossRef]
- Bissell, M.J. Thinking in three dimensions: Discovering reciprocal signaling between the extracellular matrix and nucleus and the wisdom of microenvironment and tissue architecture. Mol. Biol. Cell 2016, 27, 3205–3209. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V.; El Rayes, T.; Narula, N.; McGraw, T.E.; Altorki, N.K.; Barcellos-Hoff, M.H. The microenvironment of lung cancer and therapeutic implications. Adv. Exp. Med. Biol. 2016, 890, 75–110. [Google Scholar] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef]
- Wood, S.L.; Pernemalm, M.; Crosbie, P.A.; Whetton, A.D. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat. Rev. 2014, 40, 558–566. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited—The role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef]
- Chen, J.W.; Dhahbi, J. Lung adenocarcinoma and lung squamous cell carcinoma cancer classification, biomarker identification, and gene expression analysis using overlapping feature selection methods. Sci. Rep. 2021, 11, 13323. [Google Scholar] [CrossRef]
- Wu, F.; Fan, J.; He, Y.; Xiong, A.; Yu, J.; Li, Y.; Zhang, Y.; Zhao, W.; Zhou, F.; Li, W.; et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. 2021, 12, 2540. [Google Scholar] [CrossRef]
- Xi, K.X.; Wen, Y.S.; Zhu, C.M.; Yu, X.Y.; Qin, R.Q.; Zhang, X.W.; Lin, Y.B.; Rong, T.H.; Wang, W.D.; Chen, Y.Q.; et al. Tumor-stroma ratio (tsr) in non-small cell lung cancer (nsclc) patients after lung resection is a prognostic factor for survival. J. Thorac. Dis. 2017, 9, 4017–4026. [Google Scholar] [CrossRef]
- Harrell, C.R.; Sadikot, R.; Pascual, J.; Fellabaum, C.; Jankovic, M.G.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal stem cell-based therapy of inflammatory lung diseases: Current understanding and future perspectives. Stem Cells Int. 2019, 2019, 4236973. [Google Scholar] [CrossRef]
- Antoniou, K.M.; Karagiannis, K.; Tsitoura, E.; Bibaki, E.; Lasithiotaki, I.; Proklou, A.; Spandidos, D.A.; Tzanakis, N. Clinical applications of mesenchymal stem cells in chronic lung diseases. Biomed. Rep. 2018, 8, 314–318. [Google Scholar] [CrossRef]
- Klein, D. Lung multipotent stem cells of mesenchymal nature: Cellular basis, clinical relevance, and implications for stem cell therapy. Antioxid. Redox Signal. 2021, 35, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.F.; Rocco, P.R.M. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev. Respir. Med. 2020, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wick, K.D.; Leligdowicz, A.; Zhuo, H.; Ware, L.B.; Matthay, M.A. Mesenchymal stromal cells reduce evidence of lung injury in patients with ards. JCI Insight 2021, 6, e148983. [Google Scholar] [CrossRef]
- Behnke, J.; Kremer, S.; Shahzad, T.; Chao, C.M.; Bottcher-Friebertshauser, E.; Morty, R.E.; Bellusci, S.; Ehrhardt, H. Msc based therapies-new perspectives for the injured lung. J. Clin. Med. 2020, 9, 682. [Google Scholar] [CrossRef]
- Durand, N.; Mallea, J.; Zubair, A.C. Insights into the use of mesenchymal stem cells in covid-19 mediated acute respiratory failure. NPJ Regen. Med. 2020, 5, 17. [Google Scholar] [CrossRef]
- Rolandsson Enes, S.; Andersson Sjoland, A.; Skog, I.; Hansson, L.; Larsson, H.; Le Blanc, K.; Eriksson, L.; Bjermer, L.; Scheding, S.; Westergren-Thorsson, G. Msc from fetal and adult lungs possess lung-specific properties compared to bone marrow-derived msc. Sci. Rep. 2016, 6, 29160. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, C.; Sun, Z.; Wu, Y.; You, W.; Mao, Z.; Wang, W. Mesenchymal stem cells engineered by nonviral vectors: A powerful tool in cancer gene therapy. Pharmaceutics 2021, 13, 913. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Kusamori, K.; Tsukimori, C.; Shimizu, Y.; Hayashi, M.; Kiyama, I.; Katsumi, H.; Sakane, T.; Yamamoto, A.; Nishikawa, M. Anticancer drug-loaded mesenchymal stem cells for targeted cancer therapy. J. Control. Release 2021, 329, 1090–1101. [Google Scholar] [CrossRef]
- Javan, M.R.; Khosrojerdi, A.; Moazzeni, S.M. New insights into implementation of mesenchymal stem cells in cancer therapy: Prospects for anti-angiogenesis treatment. Front. Oncol. 2019, 9, 840. [Google Scholar] [CrossRef]
- Petrella, F.; Cocce, V.; Masia, C.; Milani, M.; Sale, E.O.; Alessandri, G.; Parati, E.; Sisto, F.; Pentimalli, F.; Brini, A.T.; et al. Paclitaxel-releasing mesenchymal stromal cells inhibit in vitro proliferation of human mesothelioma cells. Biomed. Pharmacother. 2017, 87, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Sadhukha, T.; Panyam, J.; Prabha, S. Nano-engineered mesenchymal stem cells increase therapeutic efficacy of anticancer drug through true active tumor targeting. Mol. Cancer Ther. 2018, 17, 1196–1206. [Google Scholar] [CrossRef]
- Mohr, A.; Zwacka, R. The future of mesenchymal stem cell-based therapeutic approaches for cancer-from cells to ghosts. Cancer Lett. 2018, 414, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Dericks, L.; Galetta, D. The therapeutic potential of mesenchymal stem cells in lung cancer: Benefits, risks and challenges. Cell. Oncol. 2019, 42, 727–738. [Google Scholar] [CrossRef]
- Li, L.; Pan, J.; Cai, X.; Gong, E.; Xu, C.; Zheng, H.; Cao, Z.; Yin, Z. Human umbilical cord mesenchymal stem cells suppress lung cancer via tlr4/nf-κb signalling pathway. Biotechnol. Biotechnol. Equip. 2020, 34, 24–29. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, H.; Sun, J.; Liang, G.; Zhao, Q.; Zhang, X.; Liang, S.; Guo, R.; Zhong, L. Abstract 6011: Mesenchymal stem cells promote pd-l1 expression in lung cancer cells. AACR 2020, 80, 6011. [Google Scholar]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Klein, D. Vascular wall-resident multipotent stem cells of mesenchymal nature within the process of vascular remodeling: Cellular basis, clinical relevance, and implications for stem cell therapy. Stem Cells Int. 2016, 2016, 1905846. [Google Scholar] [CrossRef]
- Steens, J.; Klar, L.; Hansel, C.; Slama, A.; Hager, T.; Jendrossek, V.; Aigner, C.; Klein, D. The vascular nature of lung-resident mesenchymal stem cells. Stem Cells Transl. Med. 2021, 10, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Du, L.; Lin, L.; Wang, Y. Tumour-associated mesenchymal stem/stromal cells: Emerging therapeutic targets. Nat. Rev. Drug Discov. 2017, 16, 35–52. [Google Scholar] [CrossRef]
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE 2009, 4, e4992. [Google Scholar] [CrossRef]
- Sai, B.; Dai, Y.; Fan, S.; Wang, F.; Wang, L.; Li, Z.; Tang, J.; Wang, L.; Zhang, X.; Zheng, L.; et al. Cancer-educated mesenchymal stem cells promote the survival of cancer cells at primary and distant metastatic sites via the expansion of bone marrow-derived-pmn-mdscs. Cell Death Dis. 2019, 10, 941. [Google Scholar] [CrossRef]
- Ren, W.; Hou, J.; Yang, C.; Wang, H.; Wu, S.; Wu, Y.; Zhao, X.; Lu, C. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage m2 polarization via mir-21-5p delivery. J. Exp. Clin. Cancer Res. 2019, 38, 62. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic bmsc-derived exosomal mirnas promote metastasis of lung cancer cells via stat3-induced emt. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef]
- Gu, J.J.; Hoj, J.; Rouse, C.; Pendergast, A.M. Mesenchymal stem cells promote metastasis through activation of an abl-mmp9 signaling axis in lung cancer cells. PLoS ONE 2020, 15, e0241423. [Google Scholar] [CrossRef]
- Chan, T.S.; Shaked, Y.; Tsai, K.K. Targeting the interplay between cancer fibroblasts, mesenchymal stem cells, and cancer stem cells in desmoplastic cancers. Front. Oncol. 2019, 9, 688. [Google Scholar] [CrossRef]
- Timaner, M.; Letko-Khait, N.; Kotsofruk, R.; Benguigui, M.; Beyar-Katz, O.; Rachman-Tzemah, C.; Raviv, Z.; Bronshtein, T.; Machluf, M.; Shaked, Y. Therapy-educated mesenchymal stem cells enrich for tumor-initiating cells. Cancer Res. 2018, 78, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Attar-Schneider, O.; Drucker, L.; Gottfried, M. The effect of mesenchymal stem cells’ secretome on lung cancer progression is contingent on their origin: Primary or metastatic niche. Lab. Investig. J. Tech. Methods Pathol. 2018, 98, 1549–1561. [Google Scholar] [CrossRef]
- Attar-Schneider, O.; Drucker, L.; Gottfried, M. Mesenchymal stem cells’ microvesicles from primary and metastatic nsclc niches differentially modulate lung cancer cells. J. Thorac. Oncol. 2018, 13, S5. [Google Scholar] [CrossRef]
- Attar-Schneider, O.; Dabbah, M.; Drucker, L.; Gottfried, M. Niche origin of mesenchymal stem cells derived microvesicles determines opposing effects on nsclc: Primary versus metastatic. Cell. Signal. 2020, 65, 109456. [Google Scholar] [CrossRef] [PubMed]
- Fregni, G.; Quinodoz, M.; Moller, E.; Vuille, J.; Galland, S.; Fusco, C.; Martin, P.; Letovanec, I.; Provero, P.; Rivolta, C.; et al. Reciprocal modulation of mesenchymal stem cells and tumor cells promotes lung cancer metastasis. EBioMedicine 2018, 29, 128–145. [Google Scholar] [CrossRef]
- Saforo, D.; Omer, L.; Smolenkov, A.; Barve, A.; Casson, L.; Boyd, N.; Clark, G.; Siskind, L.; Beverly, L. Primary lung cancer samples cultured under microenvironment-mimetic conditions enrich for mesenchymal stem-like cells that promote metastasis. Sci. Rep. 2019, 9, 4177. [Google Scholar] [CrossRef]
- Park, E.; Park, S.Y.; Sun, P.L.; Jin, Y.; Kim, J.E.; Jheon, S.; Kim, K.; Lee, C.T.; Kim, H.; Chung, J.H. Prognostic significance of stem cell-related marker expression and its correlation with histologic subtypes in lung adenocarcinoma. Oncotarget 2016, 7, 42502–42512. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Xu, S.; Zhang, J.; Zou, J.; Yang, C.; Zhang, Y.; Gong, C.; Kai, Y.; Li, Y. Hoxc8 promotes proliferation and migration through transcriptional up-regulation of tgfbeta1 in non-small cell lung cancer. Oncogenesis 2018, 7, 1. [Google Scholar] [CrossRef]
- Monterisi, S.; Lo Riso, P.; Russo, K.; Bertalot, G.; Vecchi, M.; Testa, G.; Di Fiore, P.P.; Bianchi, F. Hoxb7 overexpression in lung cancer is a hallmark of acquired stem-like phenotype. Oncogene 2018, 37, 3575–3588. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, X.; Song, X.; Tang, L.; Cao, Y.; Liu, X.; Wang, X.; Li, Y.; Yu, M.; Wan, H.; et al. Evidence for an oncogenic role of hoxc6 in human non-small cell lung cancer. PeerJ 2019, 7, e6629. [Google Scholar] [CrossRef]
- Yan, C.; Chang, J.; Song, X.; Qi, Y.; Ji, Z.; Liu, T.; Yu, W.; Wei, F.; Yang, L.; Ren, X. Lung cancer-associated mesenchymal stem cells promote tumor metastasis and tumorigenesis by induction of epithelial-mesenchymal transition and stem-like reprogram. Aging 2021, 13, 9780–9800. [Google Scholar] [CrossRef]
- Dama, E.; Colangelo, T.; Fina, E.; Cremonesi, M.; Kallikourdis, M.; Veronesi, G.; Bianchi, F. Biomarkers and lung cancer early detection: State of the art. Cancers 2021, 13, 3919. [Google Scholar] [CrossRef]
- Rahimi Jamnani, F. The state of the art in the development of a panel of biomarkers for the early detection of lung cancer. J. Thorac. Dis. 2018, 10, 625–627. [Google Scholar] [CrossRef]
- Ahn, S.Y. The role of mscs in the tumor microenvironment and tumor progression. Anticancer Res. 2020, 40, 3039–3047. [Google Scholar] [CrossRef]
- Domen, A.; Quatannens, D.; Zanivan, S.; Deben, C.; Van Audenaerde, J.; Smits, E.; Wouters, A.; Lardon, F.; Roeyen, G.; Verhoeven, Y.; et al. Cancer-associated fibroblasts as a common orchestrator of therapy resistance in lung and pancreatic cancer. Cancers 2021, 13, 987. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Han, C.; Liu, T.; Yin, R. Biomarkers for cancer-associated fibroblasts. Biomark. Res. 2020, 8, 64. [Google Scholar] [CrossRef]
- Lambrechts, D.; Wauters, E.; Boeckx, B.; Aibar, S.; Nittner, D.; Burton, O.; Bassez, A.; Decaluwe, H.; Pircher, A.; Van den Eynde, K.; et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018, 24, 1277–1289. [Google Scholar] [CrossRef]
- Kobayashi, H.; Enomoto, A.; Woods, S.L.; Burt, A.D.; Takahashi, M.; Worthley, D.L. Cancer-associated fibroblasts in gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 282–295. [Google Scholar] [CrossRef]
- Chen, C.; Hou, J.; Yu, S.; Li, W.; Wang, X.; Sun, H.; Qin, T.; Claret, F.X.; Guo, H.; Liu, Z. Role of cancer-associated fibroblasts in the resistance to antitumor therapy, and their potential therapeutic mechanisms in non-small cell lung cancer. Oncol. Lett. 2021, 21, 413. [Google Scholar] [CrossRef]
- Ishibashi, M.; Neri, S.; Hashimoto, H.; Miyashita, T.; Yoshida, T.; Nakamura, Y.; Udagawa, H.; Kirita, K.; Matsumoto, S.; Umemura, S.; et al. Cd200-positive cancer associated fibroblasts augment the sensitivity of epidermal growth factor receptor mutation-positive lung adenocarcinomas to egfr tyrosine kinase inhibitors. Sci. Rep. 2017, 7, 46662. [Google Scholar] [CrossRef]
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. Cd10(+)gpr77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 2018, 172, 841–856.e16. [Google Scholar] [CrossRef] [PubMed]
- Kawase, A.; Ishii, G.; Nagai, K.; Ito, T.; Nagano, T.; Murata, Y.; Hishida, T.; Nishimura, M.; Yoshida, J.; Suzuki, K.; et al. Podoplanin expression by cancer associated fibroblasts predicts poor prognosis of lung adenocarcinoma. Int. J. Cancer 2008, 123, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zeltz, C.; Pintilie, M.; Li, Q.; Sakashita, S.; Wang, T.; Cabanero, M.; Martins-Filho, S.N.; Wang, D.Y.; Pasko, E.; et al. Characterization of distinct populations of carcinoma-associated fibroblasts from non-small cell lung carcinoma reveals a role for st8sia2 in cancer cell invasion. Neoplasia 2019, 21, 482–493. [Google Scholar] [CrossRef]
- Alcaraz, J.; Carrasco, J.L.; Millares, L.; Luis, I.C.; Fernandez-Porras, F.J.; Martinez-Romero, A.; Diaz-Valdivia, N.; De Cos, J.S.; Rami-Porta, R.; Seijo, L.; et al. Stromal markers of activated tumor associated fibroblasts predict poor survival and are associated with necrosis in non-small cell lung cancer. Lung Cancer 2019, 135, 151–160. [Google Scholar] [CrossRef]
- Schulze, A.B.; Schmidt, L.H.; Heitkotter, B.; Huss, S.; Mohr, M.; Marra, A.; Hillejan, L.; Gorlich, D.; Barth, P.J.; Rehkamper, J.; et al. Prognostic impact of cd34 and sma in cancer-associated fibroblasts in stage i-iii nsclc. Thorac. Cancer 2020, 11, 120–129. [Google Scholar] [CrossRef]
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.; Takashi, S.; Baik, G.H.; Shibata, W.; Diprete, B.; Betz, K.S.; et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011, 19, 257–272. [Google Scholar] [CrossRef]
- Shibata, W.; Ariyama, H.; Westphalen, C.B.; Worthley, D.L.; Muthupalani, S.; Asfaha, S.; Dubeykovskaya, Z.; Quante, M.; Fox, J.G.; Wang, T.C. Stromal cell-derived factor-1 overexpression induces gastric dysplasia through expansion of stromal myofibroblasts and epithelial progenitors. Gut 2013, 62, 192–200. [Google Scholar] [CrossRef]
- Gottschling, S.; Granzow, M.; Kuner, R.; Jauch, A.; Herpel, E.; Xu, E.C.; Muley, T.; Schnabel, P.A.; Herth, F.J.; Meister, M. Mesenchymal stem cells in non-small cell lung cancer—Different from others? Insights from comparative molecular and functional analyses. Lung Cancer 2013, 80, 19–29. [Google Scholar] [CrossRef]
- Wels, J.; Kaplan, R.N.; Rafii, S.; Lyden, D. Migratory neighbors and distant invaders: Tumor-associated niche cells. Genes Dev. 2008, 22, 559–574. [Google Scholar] [CrossRef]
- Hung, C.; Linn, G.; Chow, Y.H.; Kobayashi, A.; Mittelsteadt, K.; Altemeier, W.A.; Gharib, S.A.; Schnapp, L.M.; Duffield, J.S. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2013, 188, 820–830. [Google Scholar] [CrossRef]
- Klein, D.; Steens, J.; Wiesemann, A.; Schulz, F.; Kaschani, F.; Rock, K.; Yamaguchi, M.; Wirsdorfer, F.; Kaiser, M.; Fischer, J.W.; et al. Mesenchymal stem cell therapy protects lungs from radiation-induced endothelial cell loss by restoring superoxide dismutase 1 expression. Antioxid. Redox Signal. 2017, 26, 563–582. [Google Scholar] [CrossRef]
- Sinclair, K.A.; Yerkovich, S.T.; Chen, T.; McQualter, J.L.; Hopkins, P.M.; Wells, C.A.; Chambers, D.C. Mesenchymal stromal cells are readily recoverable from lung tissue, but not the alveolar space, in healthy humans. Stem Cells 2016, 34, 2548–2558. [Google Scholar] [CrossRef] [PubMed]
- Marriott, S.; Baskir, R.S.; Gaskill, C.; Menon, S.; Carrier, E.J.; Williams, J.; Talati, M.; Helm, K.; Alford, C.E.; Kropski, J.A.; et al. Abcg2pos lung mesenchymal stem cells are a novel pericyte subpopulation that contributes to fibrotic remodeling. Am. J. Physiol. Cell Physiol. 2014, 307, C684–C698. [Google Scholar] [CrossRef]
- Arena, S.; Salati, M.; Sorgentoni, G.; Barbisan, F.; Orciani, M. Characterization of tumor-derived mesenchymal stem cells potentially differentiating into cancer-associated fibroblasts in lung cancer. Clin. Transl. Oncol. 2018, 20, 1582–1591. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, Y.; Ren, X.; Xiang, H.; Xi, Y.; Ma, X.; Zhu, K.; Guo, Z.; Zhou, C.; Zhang, G.; et al. Epidural adipose tissue-derived mesenchymal stem cell activation induced by lung cancer cells promotes malignancy and emt of lung cancer. Stem Cell Res. Ther. 2019, 10, 168. [Google Scholar] [CrossRef]
- Kerbel, R.S. Tumor angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Klein, D. The tumor vascular endothelium as decision maker in cancer therapy. Front. Oncol. 2018, 8, 367. [Google Scholar] [CrossRef]
- Ergun, S.; Tilki, D.; Oliveira-Ferrer, L.; Schuch, G.; Kilic, N. Significance of vascular stabilization for tumor growth and metastasis. Cancer Lett. 2006, 238, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Nehls, V.; Denzer, K.; Drenckhahn, D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992, 270, 469–474. [Google Scholar] [CrossRef]
- Ergun, S.; Tilki, D.; Klein, D. Vascular wall as a reservoir for different types of stem and progenitor cells. Antioxid. Redox Signal. 2011, 15, 981–995. [Google Scholar] [CrossRef]
- Klein, D.; Hohn, H.P.; Kleff, V.; Tilki, D.; Ergun, S. Vascular wall-resident stem cells. Histol. Histopathol. 2010, 25, 681–689. [Google Scholar] [PubMed]
- Klein, D. Improved isolation of human vascular wall-resident mesenchymal stem cells. Methods Mol. Biol. 2020, 2155, 71–81. [Google Scholar]
- Passman, J.N.; Dong, X.R.; Wu, S.P.; Maguire, C.T.; Hogan, K.A.; Bautch, V.L.; Majesky, M.W. A sonic hedgehog signaling domain in the arterial adventitia supports resident sca1+ smooth muscle progenitor cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9349–9354. [Google Scholar] [CrossRef]
- Tang, J.; Wang, H.; Huang, X.; Li, F.; Zhu, H.; Li, Y.; He, L.; Zhang, H.; Pu, W.; Liu, K.; et al. Arterial sca1(+) vascular stem cells generate de novo smooth muscle for artery repair and regeneration. Cell Stem Cell 2020, 26, 81–96.e84. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.; Weisshardt, P.; Kleff, V.; Jastrow, H.; Jakob, H.G.; Ergun, S. Vascular wall-resident cd44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation. PLoS ONE 2011, 6, e20540. [Google Scholar] [CrossRef]
- Yi, D.; Xiang, W.; Zhang, Q.; Cen, Y.; Su, Q.; Zhang, F.; Lu, Y.; Zhao, H.; Fu, P. Human glioblastoma-derived mesenchymal stem cell to pericytes transition and angiogenic capacity in glioblastoma microenvironment. Cell. Physiol. Biochem. 2018, 46, 279–290. [Google Scholar] [CrossRef]
- Guerra, D.A.P.; Paiva, A.E.; Sena, I.F.G.; Azevedo, P.O.; Silva, W.N.; Mintz, A.; Birbrair, A. Targeting glioblastoma-derived pericytes improves chemotherapeutic outcome. Angiogenesis 2018, 21, 667–675. [Google Scholar] [CrossRef]
- Joensuu, K.; Uusitalo-Kylmala, L.; Hentunen, T.A.; Heino, T.J. Angiogenic potential of human mesenchymal stromal cell and circulating mononuclear cell cocultures is reflected in the expression profiles of proangiogenic factors leading to endothelial cell and pericyte differentiation. J. Tissue Eng. Regen. Med. 2018, 12, 775–783. [Google Scholar] [CrossRef]
- Menezes, K.; Rosa, B.G.; Freitas, C.; da Cruz, A.S.; de Siqueira Santos, R.; Nascimento, M.A.; Alves, D.V.L.; Bonamino, M.; Rossi, M.I.; Borojevic, R.; et al. Human mesenchymal stromal/stem cells recruit resident pericytes and induce blood vessels maturation to repair experimental spinal cord injury in rats. Sci. Rep. 2020, 10, 19604. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.E.; Malta, T.M.; Kashima Haddad, S.; Covas, D.T. Mesenchymal stem cells and pericytes: To what extent are they related? Stem Cells Dev. 2016, 25, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.P.; Rowley, J.E.; Redpath, A.N.; Tilman, J.D.; Fellous, T.G.; Johnson, J.R. Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol. Ther. 2015, 151, 107–120. [Google Scholar] [CrossRef]
- Klein, D.; Meissner, N.; Kleff, V.; Jastrow, H.; Yamaguchi, M.; Ergun, S.; Jendrossek, V. Nestin(+) tissue-resident multipotent stem cells contribute to tumor progression by differentiating into pericytes and smooth muscle cells resulting in blood vessel remodeling. Front. Oncol. 2014, 4, 169. [Google Scholar] [CrossRef]
- Wang, H.H.; Cui, Y.L.; Zaorsky, N.G.; Lan, J.; Deng, L.; Zeng, X.L.; Wu, Z.Q.; Tao, Z.; Guo, W.H.; Wang, Q.X.; et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett. 2016, 375, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.R.; Komuta, Y.; Iwata, C.; Oka, M.; Shirai, Y.T.; Morishita, Y.; Ouchi, Y.; Kataoka, K.; Miyazono, K. Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor-beta receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer Sci. 2009, 100, 173–180. [Google Scholar] [CrossRef]
- Ruan, J.; Luo, M.; Wang, C.; Fan, L.; Yang, S.N.; Cardenas, M.; Geng, H.; Leonard, J.P.; Melnick, A.; Cerchietti, L.; et al. Imatinib disrupts lymphoma angiogenesis by targeting vascular pericytes. Blood 2013, 121, 5192–5202. [Google Scholar] [CrossRef]
- Paez-Ribes, M.; Allen, E.; Hudock, J.; Takeda, T.; Okuyama, H.; Vinals, F.; Inoue, M.; Bergers, G.; Hanahan, D.; Casanovas, O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009, 15, 220–231. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Krampera, M.; Galipeau, J.; Shi, Y.; Tarte, K.; Sensebe, L. Immunological characterization of multipotent mesenchymal stromal cells—The international society for cellular therapy (isct) working proposal. Cytotherapy 2013, 15, 1054–1061. [Google Scholar] [CrossRef]
- Liu, X.; Rowan, S.C.; Liang, J.; Yao, C.; Huang, G.; Deng, N.; Xie, T.; Wu, D.; Wang, Y.; Burman, A.; et al. Categorization of lung mesenchymal cells in development and fibrosis. iScience 2021, 24, 102551. [Google Scholar] [CrossRef]
- McCulley, D.; Wienhold, M.; Sun, X. The pulmonary mesenchyme directs lung development. Curr. Opin. Genet. Dev. 2015, 32, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Matsumoto, Y.; Takanami, K.; Ota, H.; Nishimiya, K.; Sugisawa, J.; Tsuchiya, S.; Amamizu, H.; Uzuka, H.; Suda, A.; et al. Coronary adventitial and perivascular adipose tissue inflammation in patients with vasospastic angina. J. Am. Coll. Cardiol. 2018, 71, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Thim, T.; Kristensen, I.B. Atherosclerotic plaque, adventitia, perivascular fat, and carotid imaging. JACC Cardiovasc. Imaging 2009, 2, 183–186. [Google Scholar] [CrossRef][Green Version]
- Majesky, M.W. Adventitia and perivascular cells. Arter. Thromb. Vasc. Biol. 2015, 35, e31–e35. [Google Scholar] [CrossRef]
- Chow, K.; Fessel, J.P.; Kaoriihida, S.; Schmidt, E.P.; Gaskill, C.; Alvarez, D.; Graham, B.; Harrison, D.G.; Wagner, D.H., Jr.; Nozik-Grayck, E.; et al. Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulm. Circ. 2013, 3, 31–49. [Google Scholar] [CrossRef]
- Kramann, R.; Schneider, R.K.; DiRocco, D.P.; Machado, F.; Fleig, S.; Bondzie, P.A.; Henderson, J.M.; Ebert, B.L.; Humphreys, B.D. Perivascular gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 2015, 16, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Denu, R.A.; Nemcek, S.; Bloom, D.D.; Goodrich, A.D.; Kim, J.; Mosher, D.F.; Hematti, P. Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable. Acta Haematol. 2016, 136, 85–97. [Google Scholar] [CrossRef]
- Steens, J.; Unger, K.; Klar, L.; Neureiter, A.; Wieber, K.; Hess, J.; Jakob, H.G.; Klump, H.; Klein, D. Direct conversion of human fibroblasts into therapeutically active vascular wall-typical mesenchymal stem cells. Cell. Mol. Life Sci. 2019, 77, 3401–3422. [Google Scholar] [CrossRef] [PubMed]
- Zepp, J.A.; Zacharias, W.J.; Frank, D.B.; Cavanaugh, C.A.; Zhou, S.; Morley, M.P.; Morrisey, E.E. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 2017, 170, 1134–1148.e10. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.L.M. The Alveolar Stem Cell Niche of the Mammalian Lung; Springer: Singapore, 2020; pp. 7–12. [Google Scholar]
- Lee, J.H.; Tammela, T.; Hofree, M.; Choi, J.; Marjanovic, N.D.; Han, S.; Canner, D.; Wu, K.; Paschini, M.; Bhang, D.H.; et al. Anatomically and functionally distinct lung mesenchymal populations marked by lgr5 and lgr6. Cell 2017, 170, 1149–1163.e12. [Google Scholar] [CrossRef]
- Molnar, K.; Meszaros, A.; Fazakas, C.; Kozma, M.; Gyori, F.; Reisz, Z.; Tiszlavicz, L.; Farkas, A.E.; Nyul-Toth, A.; Hasko, J.; et al. Pericyte-secreted igf2 promotes breast cancer brain metastasis formation. Mol. Oncol. 2020, 14, 2040–2057. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Flores, L.; Gutierrez, R.; Madrid, J.F.; Varela, H.; Valladares, F.; Acosta, E.; Martin-Vasallo, P.; Diaz-Flores, L., Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol. Histopathol. 2009, 24, 909–969. [Google Scholar] [PubMed]
- Yuan, K.; Agarwal, S.; Chakraborty, A.; Condon, D.F.; Patel, H.; Zhang, S.; Huang, F.; Mello, S.A.; Kirk, O.I.; Vasquez, R.; et al. Lung pericytes in pulmonary vascular physiology and pathophysiology. Compr. Physiol. 2021, 11, 2227–2247. [Google Scholar]
- Hung, C.F.; Wilson, C.L.; Schnapp, L.M. Pericytes in the lung. Adv. Exp. Med. Biol. 2019, 1122, 41–58. [Google Scholar]
- Sims, D.E. The pericyte—A review. Tissue Cell 1986, 18, 153–174. [Google Scholar] [CrossRef]
- Guimaraes-Camboa, N.; Cattaneo, P.; Sun, Y.; Moore-Morris, T.; Gu, Y.; Dalton, N.D.; Rockenstein, E.; Masliah, E.; Peterson, K.L.; Stallcup, W.B.; et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 2017, 20, 345–359.e5. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sentek, H.; Klein, D. Lung-Resident Mesenchymal Stem Cell Fates within Lung Cancer. Cancers 2021, 13, 4637. https://doi.org/10.3390/cancers13184637
Sentek H, Klein D. Lung-Resident Mesenchymal Stem Cell Fates within Lung Cancer. Cancers. 2021; 13(18):4637. https://doi.org/10.3390/cancers13184637
Chicago/Turabian StyleSentek, Hanna, and Diana Klein. 2021. "Lung-Resident Mesenchymal Stem Cell Fates within Lung Cancer" Cancers 13, no. 18: 4637. https://doi.org/10.3390/cancers13184637
APA StyleSentek, H., & Klein, D. (2021). Lung-Resident Mesenchymal Stem Cell Fates within Lung Cancer. Cancers, 13(18), 4637. https://doi.org/10.3390/cancers13184637





