Capecitabine in Combination with Endocrine Therapy as Maintenance Therapy after Bevacizumab Plus Paclitaxel Induction Therapy for Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer: KBCSG-TR1214
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
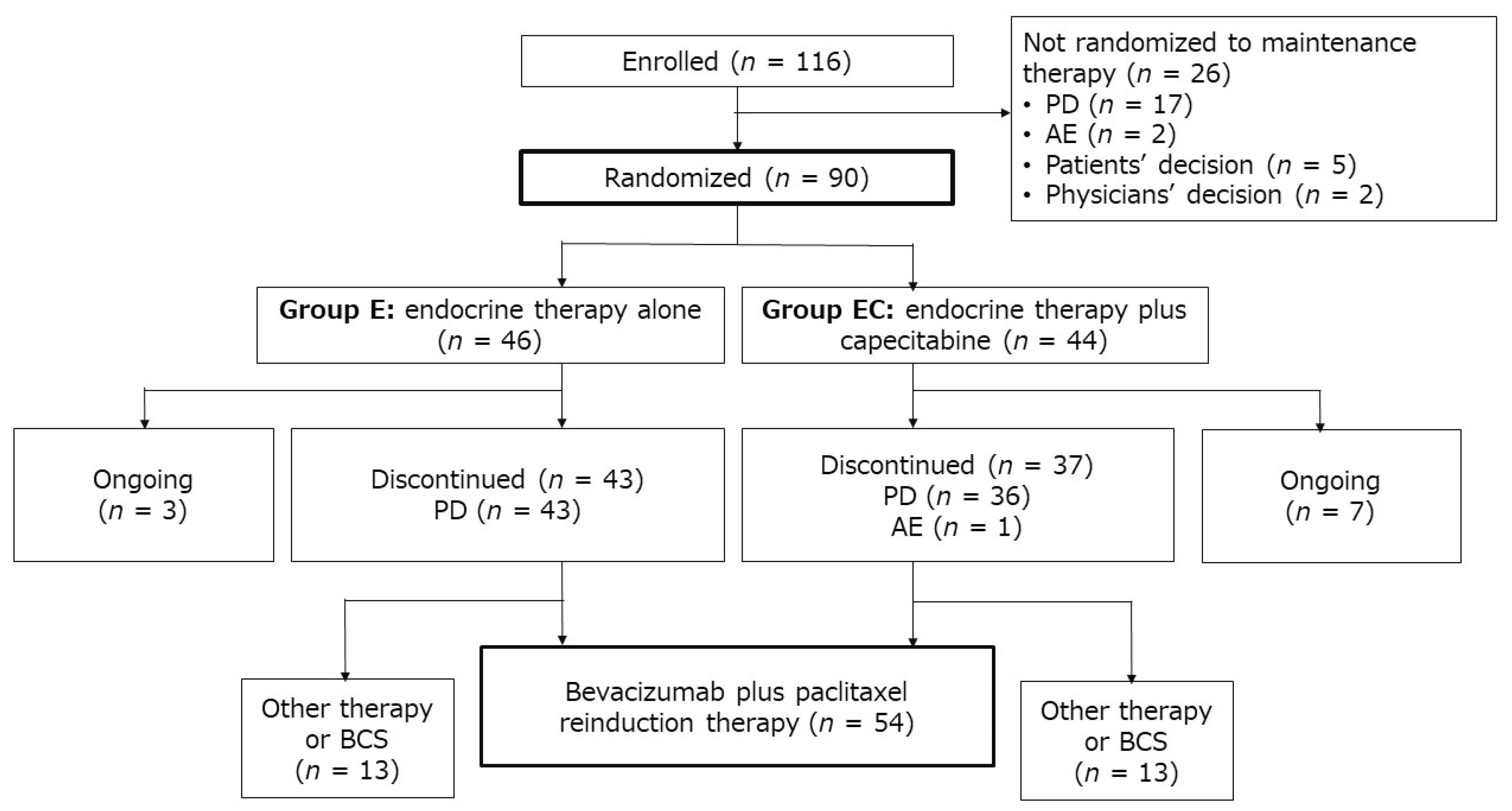
2.1. Patient Characteristics
2.2. Efficacy
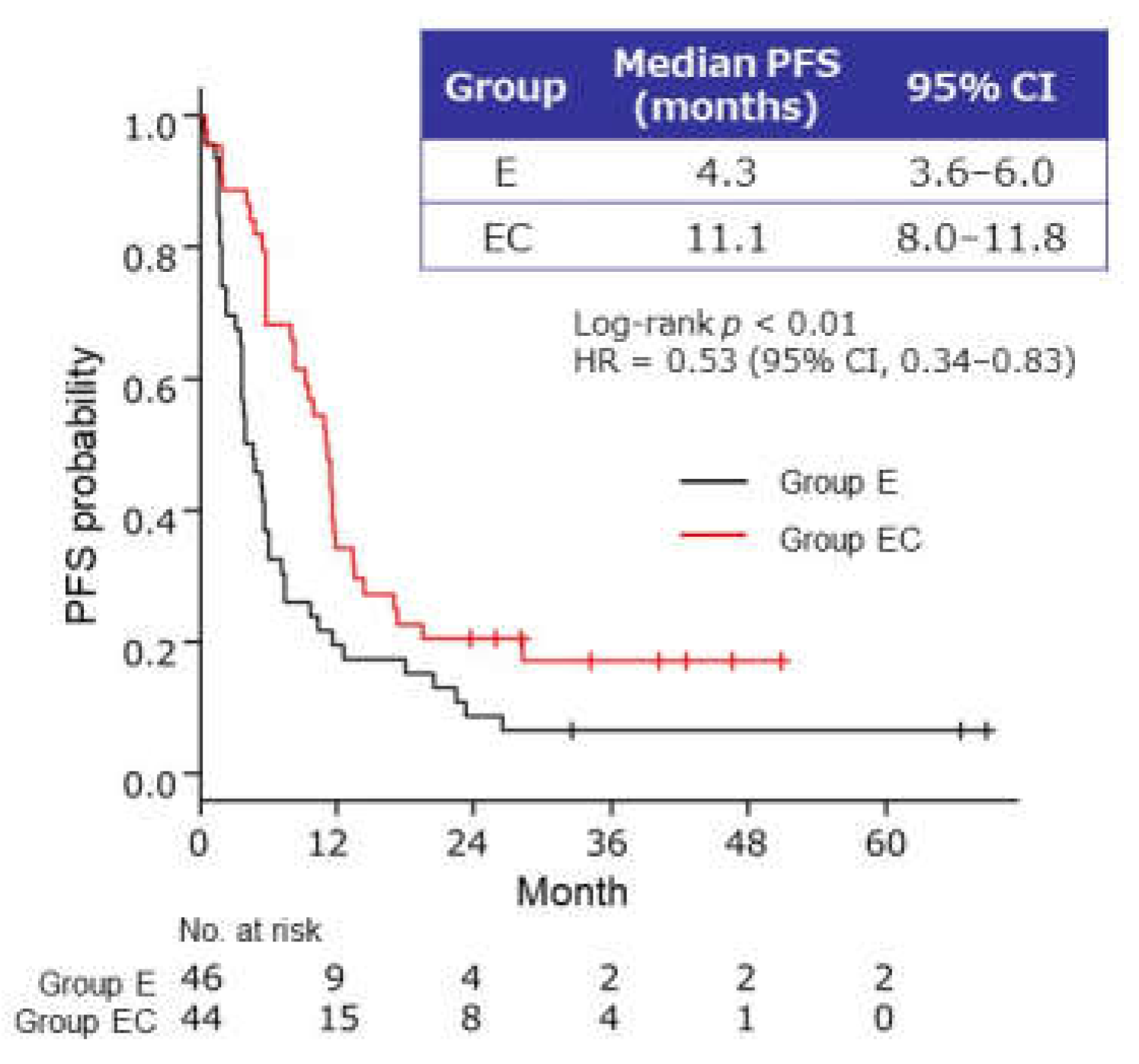
2.2.1. PFS of Maintenance Therapy
2.2.2. Efficacy of Reinduction Therapy
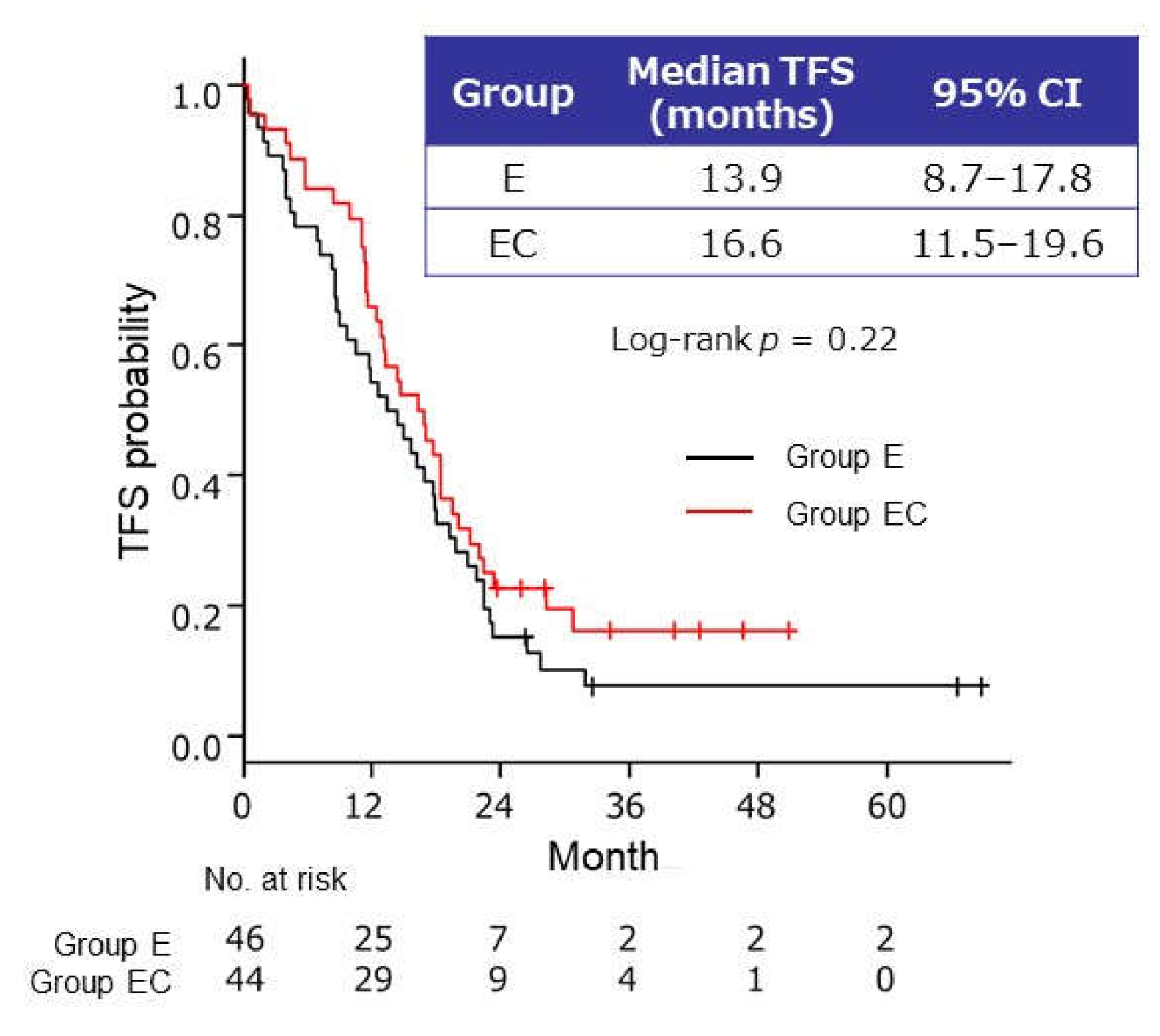
2.2.3. Time to Failure of Strategy
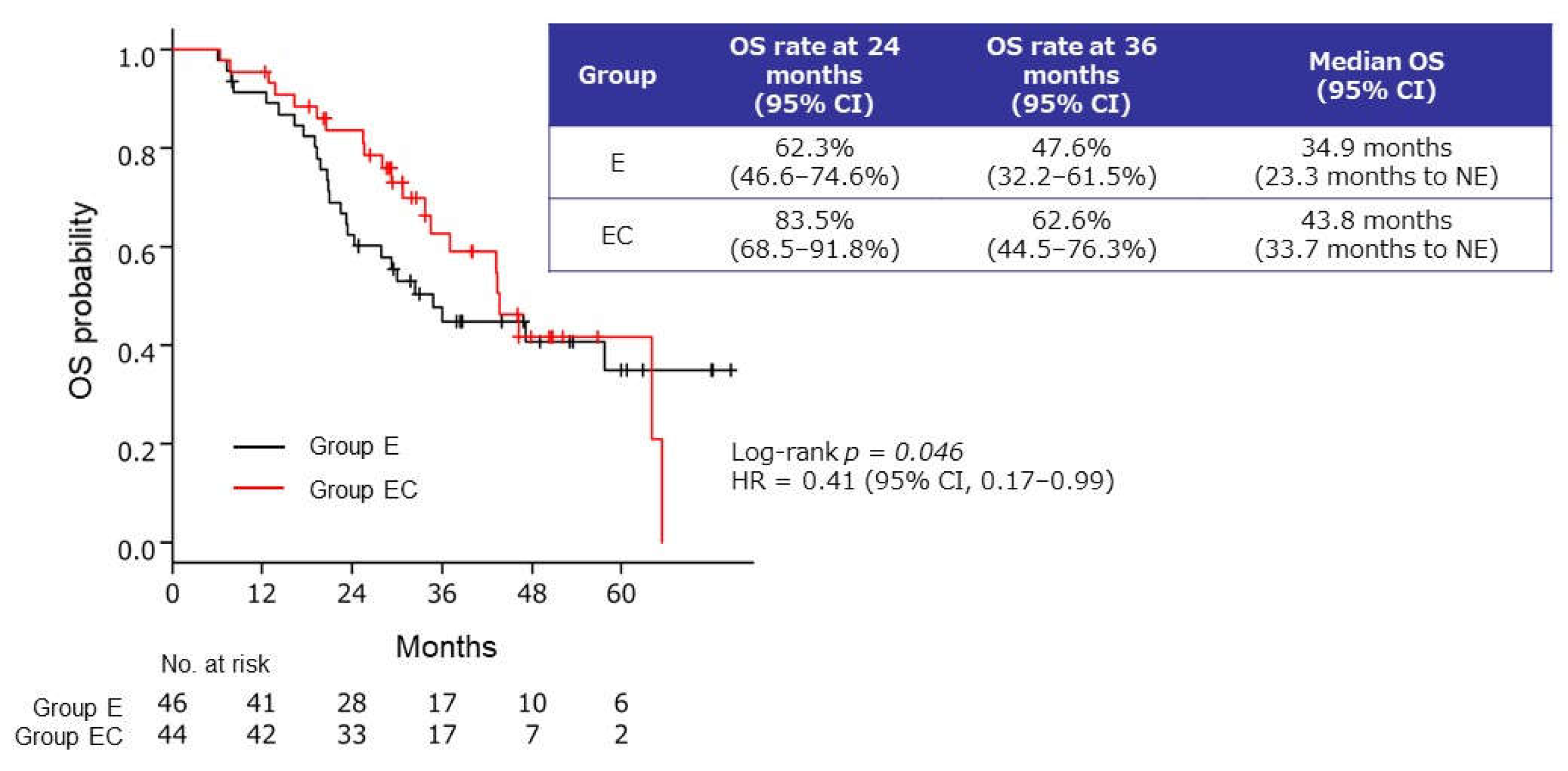
2.2.4. OS from the Start of Induction Therapy
2.3. Safety
3. Discussion
3.1. Maintenance Therapy: Clinical Usefulness of the Addition of Capecitabine to Endocrine Therapy
3.2. Efficacy of Bevacizumab–Paclitaxel Reinduction Therapy
3.3. Effects on OS from the Start of Induction Therapy
3.4. Implications for Therapeutic Strategy
3.5. Limitations and Future Research
4. Materials and Methods
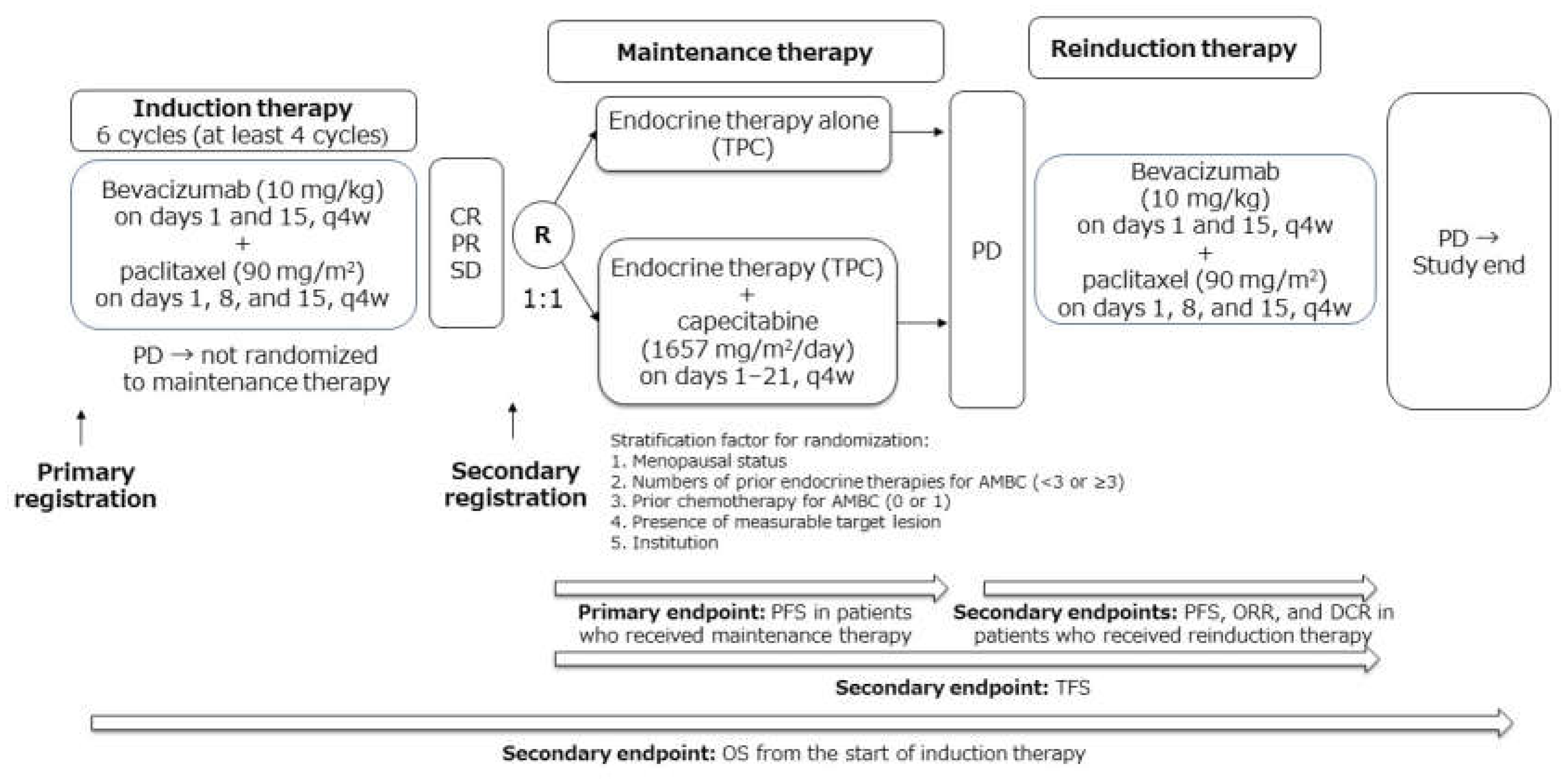
4.1. Study Design
4.2. Recruitment
4.3. Treatment
4.3.1. Induction Therapy
4.3.2. Randomization and Maintenance Therapy
4.3.3. Reinduction Therapy
4.4. Assessment of Efficacy and Safety
4.5. Endpoints
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hortobagyi, G.N. Treatment of breast cancer. N. Engl. J. Med. 1998, 339, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. PALOMA3 Study Group. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Miles, D.W.; Chan, A.; Dirix, L.Y.; Cortés, J.; Pivot, X.; Tomczak, P.; Delozier, T.; Sohn, J.H.; Provencher, L.; Puglisi, F.; et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2010, 28, 3239–3247. [Google Scholar] [CrossRef]
- Miles, D.W.; de Haas, S.L.; Dirix, L.Y.; Romieu, G.; Chan, A.; Pivot, X.; Tomczak, P.; Provencher, L.; Cortés, J.; Delmar, P.R.; et al. Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br. J. Cancer 2013, 108, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.; Cameron, D.; Bondarenko, I.; Manzyuk, L.; Alcedo, J.C.; Lopez, R.I.; Im, S.A.; Canon, J.L.; Shparyk, Y.; Yardley, D.A.; et al. Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): A double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur. J. Cancer 2017, 70, 146–155. [Google Scholar] [CrossRef]
- Miller, K.; Wang, M.; Gralow, J.; Dickler, M.; Cobleigh, M.; Perez, E.A.; Shenkier, T.; Cella, D.; Davidson, N.E. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007, 357, 2666–2676. [Google Scholar] [CrossRef] [Green Version]
- Masuda, N.; Takahashi, M.; Nakagami, K.; Okumura, Y.; Nakayama, T.; Sato, N.; Kanatani, K.; Tajima, K.; Kashiwaba, M. First-line bevacizumab plus paclitaxel in Japanese patients with HER2-negative metastatic breast cancer: Subgroup results from the randomized Phase III MERiDiAN trial. Jpn. J. Clin. Oncol. 2017, 47, 385–392. [Google Scholar] [CrossRef]
- Gennari, A.; Stockler, M.; Puntoni, M.; Sormani, M.; Nanni, O.; Amadori, D.; Wilcken, N.; D’Amico, M.; DeCensi, A.; Bruzzi, P. Duration of chemotherapy for metastatic breast cancer: A systematic review and meta-analysis of randomized clinical trials. J. Clin. Oncol. 2011, 29, 2144–2149. [Google Scholar] [CrossRef] [Green Version]
- Thomssen, C.; Lüftner, D.; Untch, M.; Haidinger, R.; Würstlein, R.; Harbeck, N.; Augustin, D.; Briest, S.; Ettl, J.; Fasching, P.A.; et al. International Consensus Conference for Advanced Breast Cancer, Lisbon 2019: ABC5 Consensus—Assessment by a German Group of Experts. Breast Care 2020, 15, 82–95. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef] [PubMed]
- Gligorov, J.; Doval, D.; Bines, J.; Alba, E.; Cortes, P.; Pierga, J.Y.; Gupta, V.; Costa, R.; Srock, S.; de Ducla, S.; et al. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1351–1360. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Ciruelos, E.; Pérez-García, J.M.; Gavilá, J.; Rodríguez, A.; de la Haba-Rodriguez, J. Maintenance Therapy in HER2-Negative Metastatic Breast Cancer: A New Approach for an Old Concept. Clin. Drug Investig. 2019, 39, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Yoshinami, T.; Yagi, T.; Okuno, J.; Kittaka, N.; Ishitobi, M.; Sugimoto, N.; Nakayama, T.; Tamaki, Y.; Imamura, F. Efficacy and safety of re-induction therapy with bevacizumab and paclitaxel for metastatic breast cancer. Breast Cancer 2017, 24, 147–151. [Google Scholar] [CrossRef]
- Rossi, S.; Schinzari, G.; Basso, M.; Strippoli, A.; Dadduzio, V.; D’Argento, E.; Cassano, A.; Barone, C. Maintenance hormonal and chemotherapy treatment in metastatic breast cancer: A systematic review. Future Oncol. 2016, 12, 1299–1307. [Google Scholar] [CrossRef]
- Trédan, O.; Follana, P.; Moullet, I.; Cropet, C.; Trager-Maury, S.; Dauba, J.; Lavau-Denes, S.; Diéras, V.; Béal-Ardisson, D.; Gouttebel, M.; et al. A phase III trial of exemestane plus bevacizumab maintenance therapy in patients with metastatic breast cancer after first-line taxane and bevacizumab: A GINECO group study. Ann. Oncol. 2016, 27, 1020–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claessens, A.K.M.; Bos, M.E.M.M.; Lopez-Yurda, M.; Bouma, J.M.; Rademaker-Lakhai, J.M.; Honkoop, A.H.; de Graaf, H.; van Druten, E.; van Warmerdam, L.J.C.; van der Sangen, M.J.C.; et al. Intermittent versus continuous first-line treatment for HER2-negative metastatic breast cancer: The Stop & Go study of the Dutch Breast Cancer Research Group (BOOG). Breast Cancer Res. Treat. 2018, 172, 413–423. [Google Scholar] [PubMed]
- Inoue, K.; Ninomiya, J.; Saito, T.; Kimizuka, K.; Kurosumi, M. Induction therapy with paclitaxel and bevacizumab followed by switch maintenance therapy with eribulin in Japanese patients with HER2-negative metastatic breast cancer: A multicenter, collaborative, open-label, phase II clinical study for the SBCCSG 35 investigators. BMC Cancer 2018, 18, 671. [Google Scholar]
- Toi, M.; Atiqur Rahman, M.; Bando, H.; Chow, L.W. Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol. 2005, 6, 158–166. [Google Scholar] [CrossRef]
- Giuliano, M.; Schettini, F.; Rognoni, C.; Milani, M.; Jerusalem, G.; Bachelot, T.; de Laurentiis, M.; Thomas, G.; de Placido, P.; Arpino, G.; et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol. 2019, 20, 1360–1369. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.H.; Kim, T.Y.; Kim, G.M.; Kang, S.Y.; Park, I.H.; Kim, J.H.; Lee, K.E.; Ahn, H.K.; Lee, M.H.; Kim, H.J.; et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019, 20, 1750–1759. [Google Scholar] [CrossRef]
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Turner, N.; Ciruelos, E.M.; Muñoz, M.; Bermejo, B.; Margeli, M.; Anton, A.; et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: A phase III randomised controlled trial-PEARL. Ann. Oncol. 2021, 32, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Chaudhury, A.; Solovieff, N.; Paré, L.; Martinez, D.; Chic, N.; Martínez-Sáez, O.; Brasó-Maristany, F.; Lteif, A.; Taran, T.; et al. Correlative Biomarker Analysis of Intrinsic Subtypes and Efficacy Across the MONALEESA Phase III Studies. J. Clin. Oncol. 2021, 39, 1458–1467. [Google Scholar] [CrossRef]
- Watanabe, T.; Katsumata, N.; Sasaki, Y.; Saeki, T.; Aogi, K.; Toi, M.; Holdener, E. A multicenter phase II trial of XelodaTM (Capecitabine) in patients with docetaxel-refractory advanced/metastatic breast cancer. Proc. Am. Soc. Clin. Oncol. 2001, 20, 61b (abstr 1991). [Google Scholar]
- Ishida, T.; Kiba, T.; Takeda, M.; Matsuyama, K.; Teramukai, S.; Ishiwata, R.; Masuda, N.; Takatsuka, Y.; Noguchi, S.; Ishioka, C.; et al. Phase II study of capecitabine and trastuzumab combination chemotherapy in patients with HER2 overexpressing metastatic breast cancers resistant to both anthracyclines and taxanes. Cancer Chemother. Pharmacol. 2009, 64, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute Common Terminology Criteria for Adverse Events, Version 4.0 (Japanese Clinical Oncology Group Edition). Available online: http://www.jcog.jp/doctor/tool/CTCAEv4J_20170310_miekeshi.pdf (accessed on 1 May 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 1 May 2021).

| Characteristic | Enrolled (n = 116) | Randomized (n = 90) | Group E (n = 46) | Group EC (n = 44) |
|---|---|---|---|---|
| Median Age, Years (Range) | 59.8 (31.5–81.0) | 59.8 (34.5–81.0) | 59.8 (41.5–75.8) | 59.9 (34.5–81.0) |
| Menopausal Status | ||||
| Premenopausal | 26 (22.4) | 20 (22.2) | 10 (21.7) | 10 (22.7) |
| Postmenopausal | 90 (77.6) | 70 (77.8) | 36 (78.3) | 34 (77.3) |
| ECOG PS | ||||
| 0 | 83 (71.6) | 64 (71.1) | 33 (71.7) | 31 (70.5) |
| 1 | 32 (27.6) | 25 (27.8) | 13 (28.3) | 12 (27.3) |
| 2 | 1 (0.9) | 1 (1.1) | 0 | 1 (02.3) |
| Measurable Lesion a | ||||
| Yes | 111 (95.7) | 85 (94.4) | 44 (95.7) | 41 (93.2) |
| No | 5 (4.3) | 5 (5.6) | 2 (4.3) | 3 (6.8) |
| Previous Endocrine Therapies with/without Targeted Therapy for AMBC | ||||
| 0 | 42 (36.2) | 35 (38.9) | 18 (39.1) | 17 (38.6) |
| 1 Regimen | 26 (22.4) | 13 (14.4) | 7 (15.2) | 6 (13.6) |
| 2 Regimens | 19 (16.4) | 16 (17.8) | 8 (17.4) | 8 (18.2) |
| ≥3 Regimens | 29 (25.0) | 26 (28.9) | 13 (28.3) | 13 (29.5) |
| Exemestane + mTOR Inhibitor | 8 (6.9) | 7 (7.8) | 4 (8.7) | 3 (6.8) |
| Previous Chemotherapy for AMBC | ||||
| 0 | 102 (87.9) | 81 (90.0) | 39 (84.8) | 42 (95.5) |
| 1 Regimen | 14 (12.1) | 9 (10.0) | 7 (15.2) | 2 (4.5) |
| Histological Grade | ||||
| 1 | 19 (16.4) | 15 (16.7) | 8 (17.4) | 7 (15.9) |
| 2 | 41 (35.3) | 32 (35.6) | 17 (37.0) | 15 (34.1) |
| 3 | 23 (19.8) | 15 (16.7) | 6 (13.0) | 9 (20.5) |
| Unknown | 33 (28.4) | 28 (31.1) | 15 (32.6) | 13 (29.5) |
| Hormone Receptor Status | ||||
| ER + PgR+ | 96 (82.8) | 75 (83.3) | 38 (82.6) | 37 (84.1) |
| ER + PgR− | 18 (15.5) | 13 (14.4) | 7 (15.2) | 6 (13.6) |
| ER + PgR Unknown | 2 (1.7) | 2 (2.2) | 1 (2.2) | 1 (2.3) |
| HER2 Status (IHC) | ||||
| 0 | 63 (54.3) | 48 (53.3) | 26 (56.5) | 22 (50.0) |
| 1+ | 40 (34.5) | 32 (35.6) | 16 (33.8) | 16 (36.4) |
| 2+ | 11 (9.5) | 8 (8.9) | 4 (8.7) | 4 (9.1) |
| Unknown | 2 (1.7) | 2 (2.2) | 0 | 2 (4.5) |
| Endocrine Agent(s) | Group E (n = 46) | Group EC (n = 44) |
|---|---|---|
| AI ± LHRH Agonist | 28 (60.9) | 27 (61.4) |
| SERM | 11 (23.9) | 8 (18.2) |
| SERD (Fulvestrant) | 6 (13.0) | 6 (13.6) |
| MPA | 1 (2.2) | 3 (6.8) |
| Response | Group E (n = 46) | Group EC (n = 44) |
|---|---|---|
| Complete Response | 0 | 1 (2.3) |
| Partial Response | 35 (76.1) | 33 (75.0) |
| Stable Disease | 11 (23.9) | 10 (22.7) |
| Progressive Disease | 0 | 0 |
| Not Evaluable | 0 | 0 |
| Adverse Event | Group E (n = 46) | Group EC (n = 44) | ||
|---|---|---|---|---|
| n | Grade ≥ 3 | n | Grade ≥ 3 | |
| Sensory Neuropathy | 31 (67.4) | 2 (4.3) | 34 (77.3) | 2 (4.5) |
| Alopecia | 30 (65.2) | 0 (0.0) | 30 (68.2) | 0 (0.0) |
| Hypertension | 16 (34.8) | 3 (6.5) | 21 (47.7) | 5 (11.4) |
| Hemorrhage | 9 (19.6) | 0 (0.0) | 11 (25.0) | 0 (0.0) |
| Proteinuria | 9 (19.6) | 0 (0.0) | 4 (9.1) | 1 (2.3) |
| Anemia | 8 (17.4) | 0 (0.0) | 9 (20.5) | 0 (0.0) |
| Malaise | 7 (15.2) | 0 (0.0) | 14 (31.8) | 0 (0.0) |
| Motor Neuropathy | 7 (15.2) | 0 (0.0) | 8 (18.2) | 0 (0.0) |
| Arthralgia | 7 (15.2) | 1 (2.2) | 7 (15.9) | 0 (0.0) |
| Anorexia | 6 (13.0) | 1 (2.2) | 14 (31.8) | 0 (0.0) |
| ALT Increased | 6 (13.0) | 2 (4.3) | 1 (2.3) | 0 (0.0) |
| Rash, Skin Changes (Dryness/Itchiness) | 5 (10.9) | 0 (0.0) | 12 (27.3) | 0 (0.0) |
| Limb Edema | 5 (10.9) | 0 (0.0) | 9 (20.5) | 0 (0.0) |
| Oral Mucositis | 5 (10.9) | 0 (0.0) | 8 (18.2) | 0 (0.0) |
| Fatigue | 4 (8.7) | 1 (2.2) | 17 (38.6) | 1 (2.3) |
| Nausea | 4 (8.7) | 0 (0.0) | 10 (22.7) | 0 (0.0) |
| AST Increased | 4 (8.7) | 2 (4.3) | 1 (2.3) | 0 (0.0) |
| Dysgeusia | 3 (6.5) | 0 (0.0) | 12 (27.3) | 0 (0.0) |
| Diarrhea | 3 (6.5) | 0 (0.0) | 7 (15.9) | 0 (0.0) |
| Muscle Pain | 2 (4.3) | 0 (0.0) | 6 (13.6) | 0 (0.0) |
| Palmar–Plantar Erythrodysesthesia Syndrome | 1 (2.2) | 0 (0) | 33 (75.0) | 6 (13.6) |
| Neutropenia | 1 (2.2) | 0 (0.0) | 10 (22.7) | 1 (2.3) |
| Leukopenia | 1 (2.2) | 0 (0.0) | 7 (15.9) | 0 (0.0) |
| Nail Disorders | 0 (0.0) | 0 (0.0) | 13 (29.5) | 0 (0.0) |
| CPK Increased | 0 (0.0) | 0 (0.0) | 1 (2.3) | 1 (2.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuda, N.; Yoshinami, T.; Ikeda, M.; Mizutani, M.; Yamaguchi, M.; Komoike, Y.; Takashima, T.; Yoshidome, K.; Tsurutani, J.; Iwamoto, M.; et al. Capecitabine in Combination with Endocrine Therapy as Maintenance Therapy after Bevacizumab Plus Paclitaxel Induction Therapy for Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer: KBCSG-TR1214. Cancers 2021, 13, 4399. https://doi.org/10.3390/cancers13174399
Masuda N, Yoshinami T, Ikeda M, Mizutani M, Yamaguchi M, Komoike Y, Takashima T, Yoshidome K, Tsurutani J, Iwamoto M, et al. Capecitabine in Combination with Endocrine Therapy as Maintenance Therapy after Bevacizumab Plus Paclitaxel Induction Therapy for Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer: KBCSG-TR1214. Cancers. 2021; 13(17):4399. https://doi.org/10.3390/cancers13174399
Chicago/Turabian StyleMasuda, Norikazu, Tetsuhiro Yoshinami, Masahiko Ikeda, Makiko Mizutani, Miki Yamaguchi, Yoshifumi Komoike, Tsutomu Takashima, Katsuhide Yoshidome, Junji Tsurutani, Mitsuhiko Iwamoto, and et al. 2021. "Capecitabine in Combination with Endocrine Therapy as Maintenance Therapy after Bevacizumab Plus Paclitaxel Induction Therapy for Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer: KBCSG-TR1214" Cancers 13, no. 17: 4399. https://doi.org/10.3390/cancers13174399
APA StyleMasuda, N., Yoshinami, T., Ikeda, M., Mizutani, M., Yamaguchi, M., Komoike, Y., Takashima, T., Yoshidome, K., Tsurutani, J., Iwamoto, M., Fujisawa, F., Yasojima, H., Yamamura, J., Morishima, H., Aki, F., Yamada, T., Morita, S., & Nakayama, T. (2021). Capecitabine in Combination with Endocrine Therapy as Maintenance Therapy after Bevacizumab Plus Paclitaxel Induction Therapy for Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer: KBCSG-TR1214. Cancers, 13(17), 4399. https://doi.org/10.3390/cancers13174399








