Performance of Narrow Band Imaging (NBI) and Photodynamic Diagnosis (PDD) Fluorescence Imaging Compared to White Light Cystoscopy (WLC) in Detecting Non-Muscle Invasive Bladder Cancer: A Systematic Review and Lesion-Level Diagnostic Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
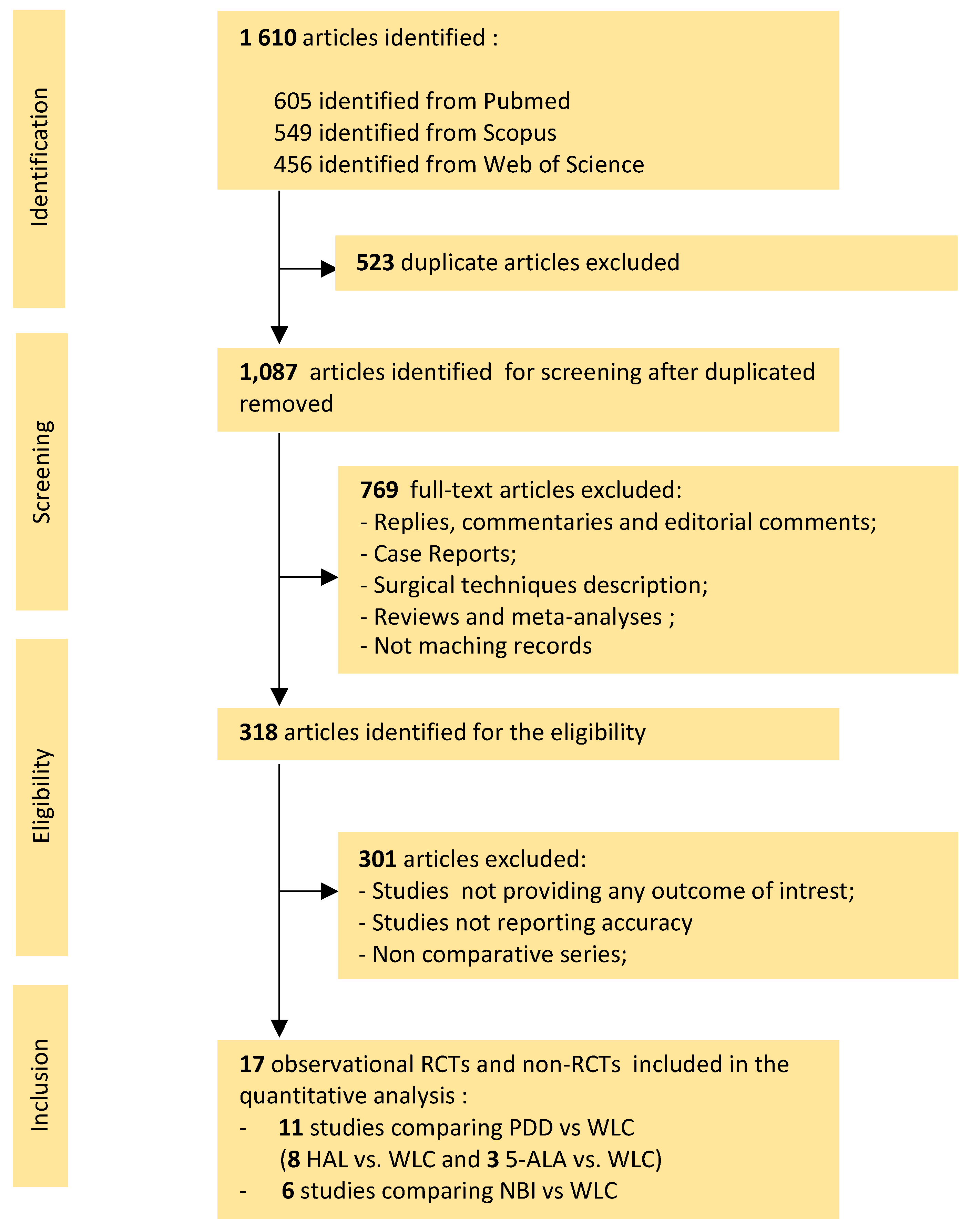
2.1. Search Strategy and Selection Criteria
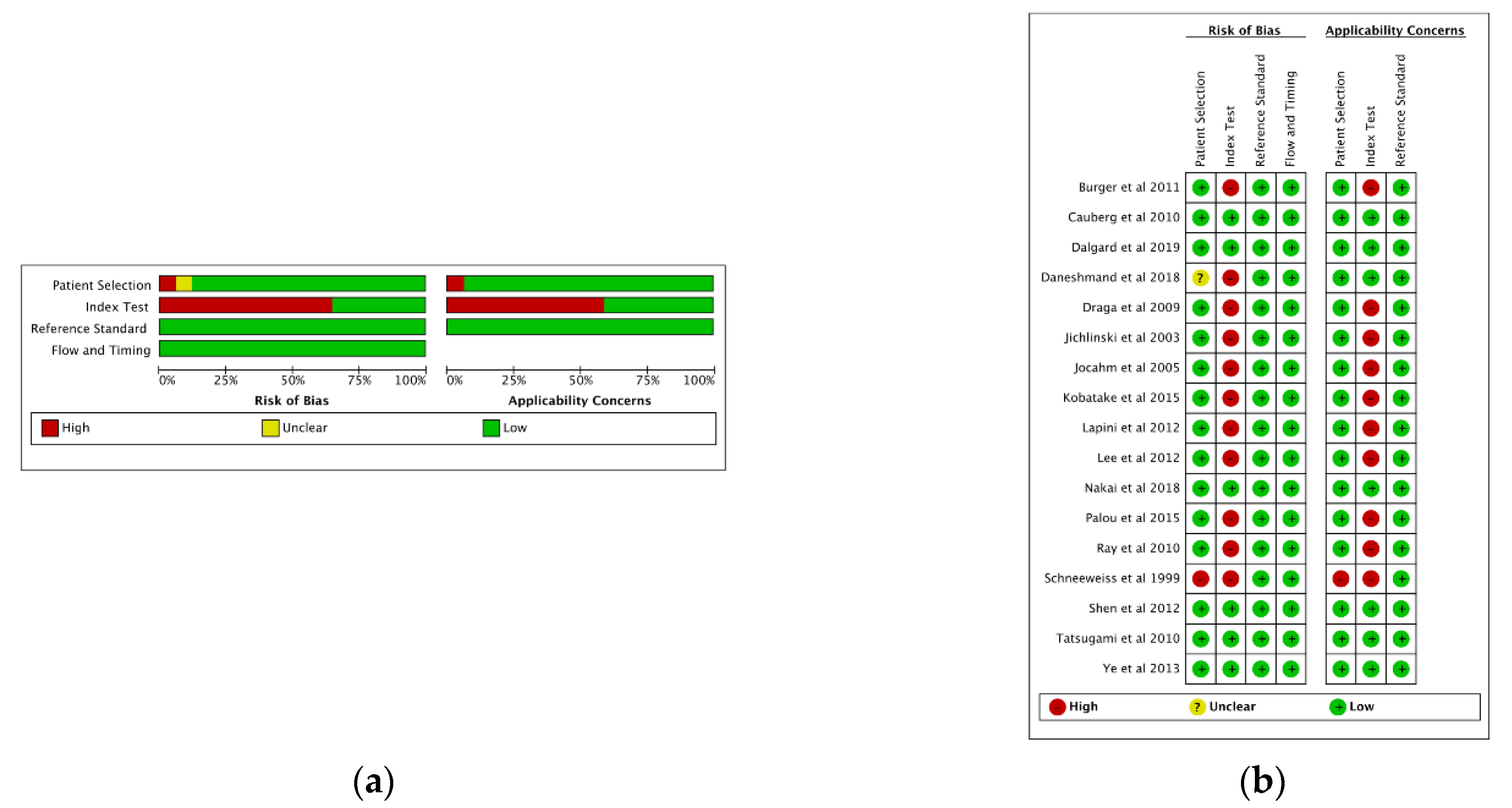
2.2. Selection Studies and Quality Assessment
2.3. Endpoints of Interests
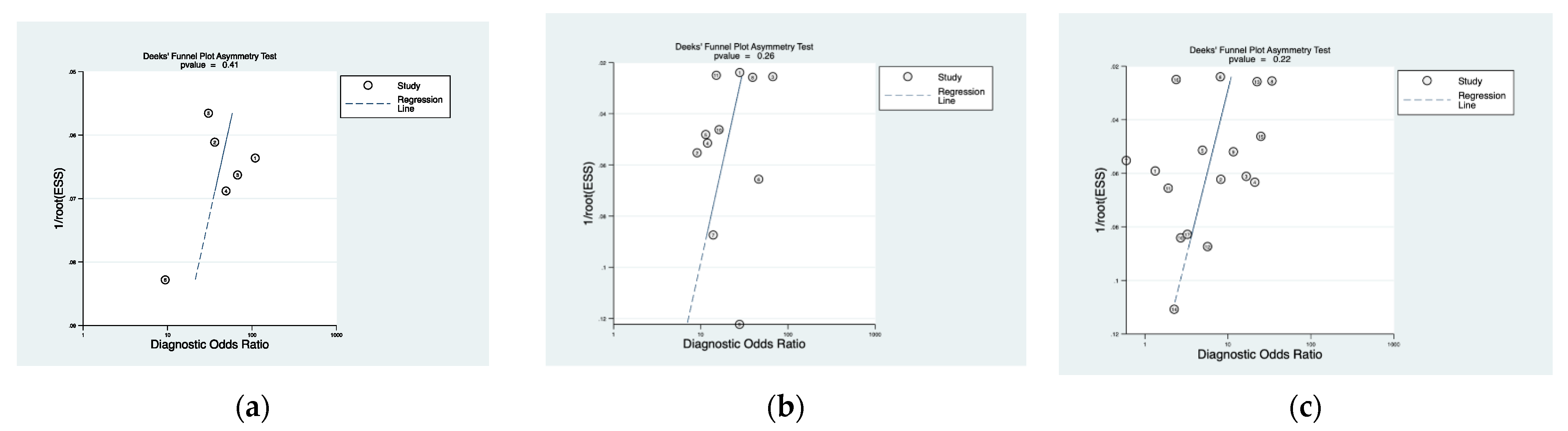
2.4. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Quality Assessment
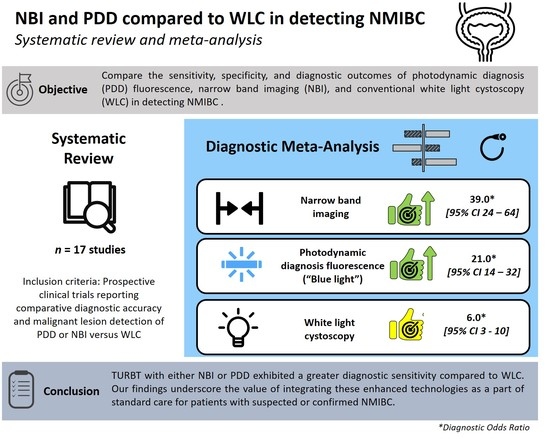
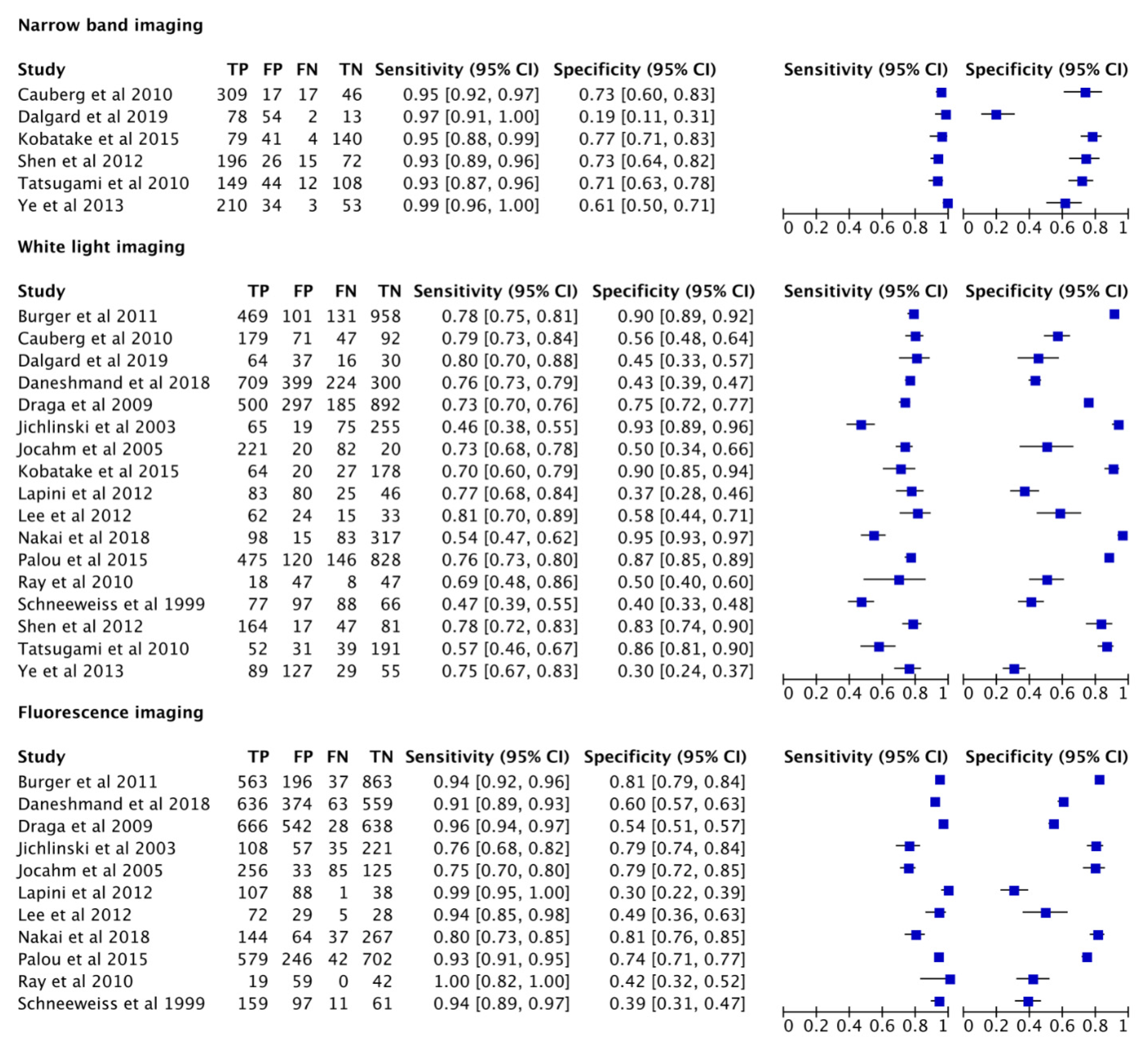
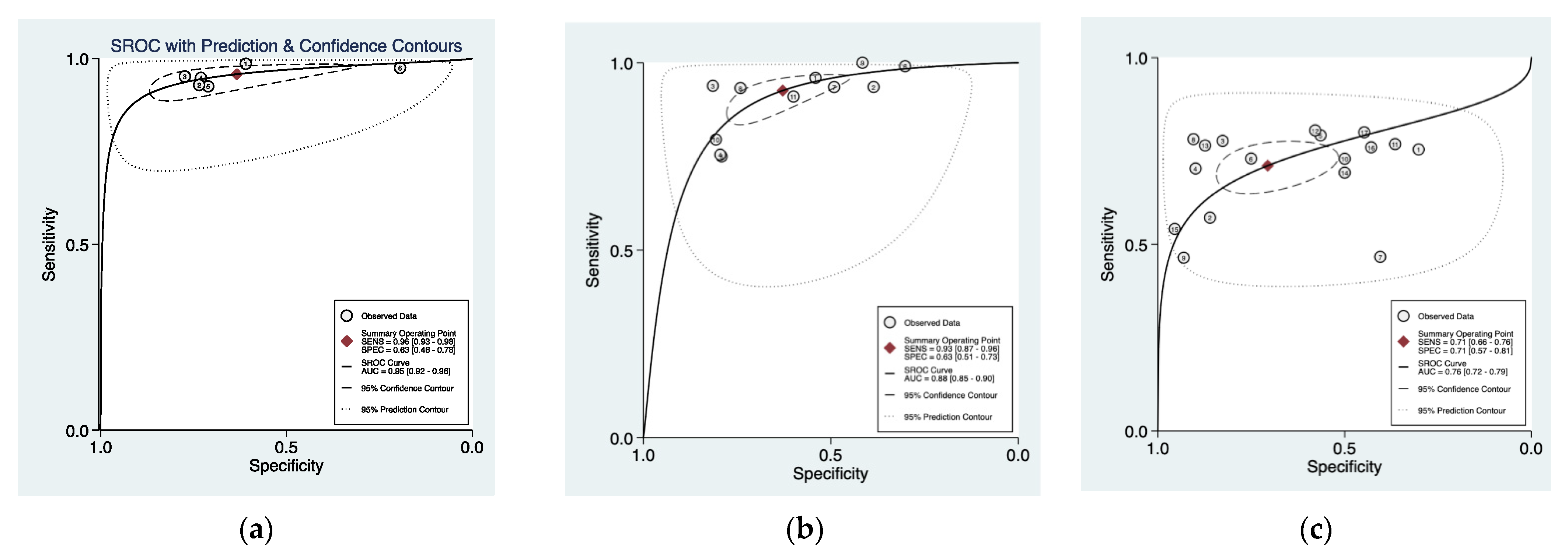
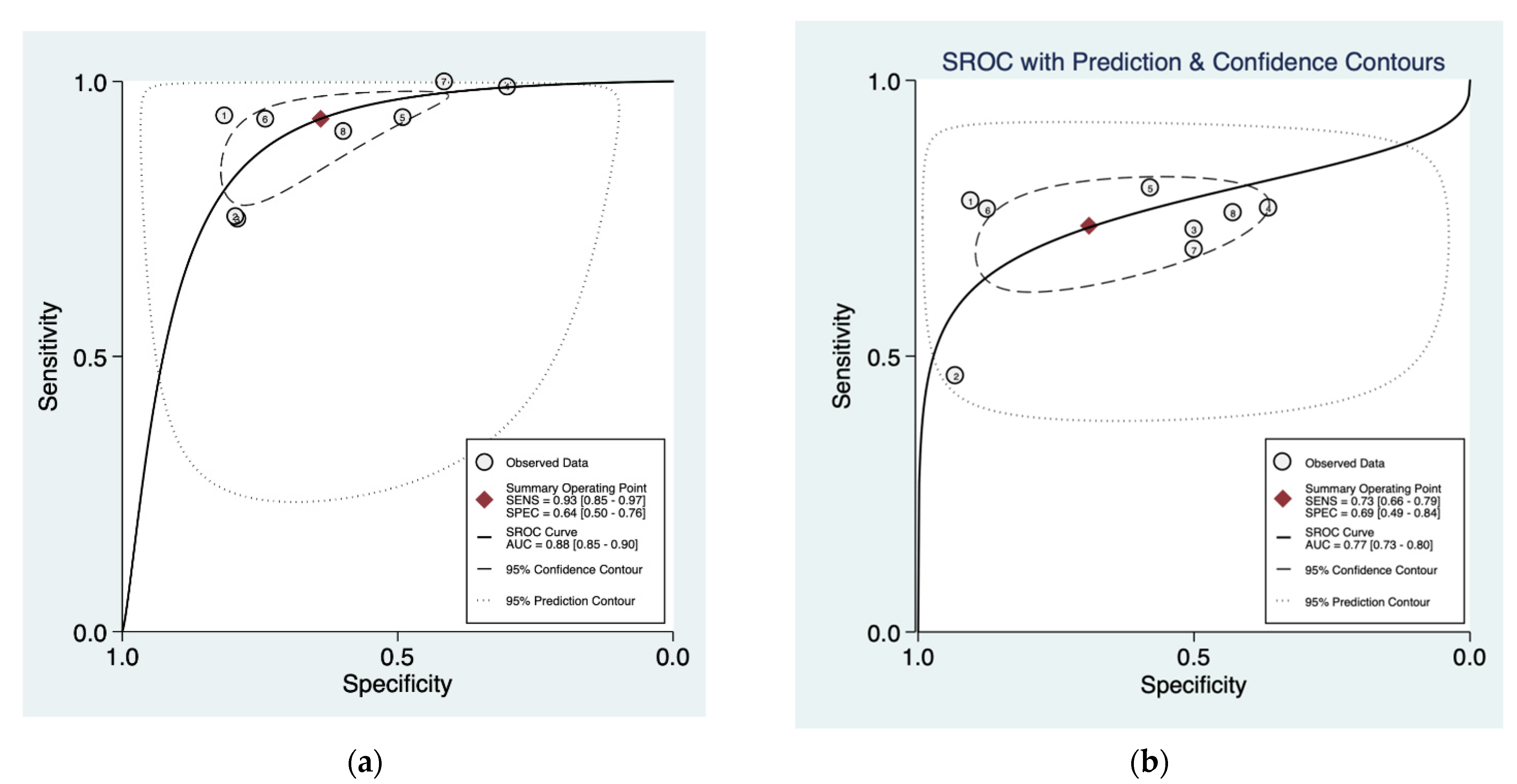
3.3. Cumulative Accuracy Meta-Analysis of Comparative Studies Reporting Diagnostic Accuracy
3.4. Subset Diagnostic Meta-Analysis of Studies Comparing HAL-PDD vs. WLC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Search Criteria and Translational Terms Used for the Literature Review
Appendix A.2. Translations
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Crundwell, M. Pathology and genetics of tumours of the urinary system and male genital organs. BJU Int. 2004, 94, 675. [Google Scholar] [CrossRef]
- Dhir, R. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. Yearb. Pathol. Lab. Med. 2007, 2007, 192–193. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R. EAU Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and CIS). Available online: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/ (accessed on 4 June 2021).
- Jichlinski, P.; Leisinger, H.-J. Fluorescence Cystoscopy in the Management of Bladder Cancer: A Help for the Urologist! Urol. Int. 2005, 74, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, W.F.; Bush, I.M.; Esquivel, E. Tetracycline ultraviolet fluorescence in bladder carcinoma. Cancer 1964, 17, 1528–1532. [Google Scholar] [CrossRef]
- Daneshmand, S.; Schuckman, A.K.; Bochner, B.H.; Cookson, M.S.; Downs, T.M.; Gomella, L.G.; Grossman, H.B.; Kamat, A.M.; Konety, B.R.; Lee, C.T.; et al. Hexaminolevulinate blue-light cystoscopy in non-muscle-invasive bladder cancer: Review of the clinical evidence and consensus statement on appropriate use in the USA. Nat. Rev. Urol. 2014, 11, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Lotan, Y.; Bivalacqua, T.J.; Downs, T.; Huang, W.; Jones, J.; Kamat, A.M.; Konety, B.; Malmström, P.U.; McKiernan, J.; O’Donnell, M.; et al. Blue light flexible cystoscopy with hexaminolevulinate in non-muscle-invasive bladder cancer: Review of the clinical evidence and consensus statement on optimal use in the USA—Update 2018. Nat. Rev. Urol. 2019, 16, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269.W264. [Google Scholar] [CrossRef] [Green Version]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Cauberg, E.C.; Kloen, S.; Visser, M.; de la Rosette, J.J.; Babjuk, M.; Soukup, V.; Pesl, M.; Duskova, J.; de Reijke, T.M. Narrow band imaging cystoscopy improves the detection of non-muscle-invasive bladder cancer. Urology 2010, 76, 658–663. [Google Scholar] [CrossRef]
- Kobatake, K.; Mita, K.; Ohara, S.; Kato, M. Advantage of transurethral resection with narrow band imaging for non-muscle invasive bladder cancer. Oncol. Lett. 2015, 10, 1097–1102. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.J.; Zhu, Y.P.; Ye, D.W.; Yao, X.D.; Zhang, S.L.; Dai, B.; Zhang, H.L.; Zhu, Y. Narrow-band imaging flexible cystoscopy in the detection of primary non-muscle invasive bladder cancer: A “second look” matters? Int. Urol. Nephrol. 2012, 44, 451–457. [Google Scholar] [CrossRef]
- Song, P.H.; Cho, S.; Ko, Y.H. Decision Based on Narrow Band Imaging Cystoscopy without a Referential Normal Standard Rather Increases Unnecessary Biopsy in Detection of Recurrent Bladder Urothelial Carcinoma Early after Intravesical Instillation. Cancer Res. Treat. 2016, 48, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Tatsugami, K.; Kuroiwa, K.; Kamoto, T.; Nishiyama, H.; Watanabe, J.; Ishikawa, S.; Shinohara, N.; Sazawa, A.; Fukushima, S.; Naito, S. Evaluation of narrow-band imaging as a complementary method for the detection of bladder cancer. J. Endourol. 2010, 24, 1807–1811. [Google Scholar] [CrossRef]
- Ye, Z.; Hu, J.; Song, X.; Li, F.; Zhao, X.; Chen, S.; Wang, X.; He, D.; Fan, J.; Ye, D.; et al. A comparison of NBI and WLI cystoscopy in detecting non-muscle-invasive bladder cancer: A prospective, randomized and multi-center study. Sci. Rep. 2015, 5, 10905. [Google Scholar] [CrossRef] [Green Version]
- Draga, R.O.; Grimbergen, M.C.; Kok, E.T.; Jonges, T.N.; Bosch, J.L. Predictors of false positives in 5-aminolevulinic acid-induced photodynamic diagnosis of bladder carcinoma: Identification of patient groups that may benefit most from highly specific optical diagnostics. Urology 2009, 74, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Inoue, K.; Tsuzuki, T.; Shimamoto, T.; Shuin, T.; Nagao, K.; Matsuyama, H.; Oyama, M.; Furuse, H.; Ozono, S.; et al. Oral 5-aminolevulinic acid-mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: A multicenter phase III study. Int. J. Urol. 2018, 25, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Schneeweiss, S.; Kriegmair, M.; Stepp, H. Is everything all right if nothing seems wrong? A simple method of assessing the diagnostic value of endoscopic procedures when a gold standard is absent. J. Urol. 1999, 161, 1116–1119. [Google Scholar] [CrossRef]
- Burgués, J.P.; Conde, G.; Oliva, J.; Abascal, J.M.; Iborra, I.; Puertas, M.; Ordoño, F. Hexaminolevulinate photodynamic diagnosis in non-muscle invasive bladder cancer: Experience of the BLUE group. Actas Urol. Esp. 2011, 35, 439–445. [Google Scholar] [CrossRef]
- Jichlinski, P.; Guillou, L.; Karlsen, S.J.; Malmstrom, P.U.; Jocham, D.; Brennhovd, B.; Johansson, E.; Gartner, T.; Lange, N.; van den Bergh, H.; et al. Hexyl aminolevulinate fluorescence cystoscopy: New diagnostic tool for photodiagnosis of superficial bladder cancer--a multicenter study. J. Urol. 2003, 170, 226–229. [Google Scholar] [CrossRef] [Green Version]
- Jocham, D.; Witjes, F.; Wagner, S.; Zeylemaker, B.; Van Moorselaar, J.; Grimm, M.O.; Muschter, R.; Popken, G.; König, F.; Knüchel, R.; et al. Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: A prospective, phase III multicenter study. J. Urol. 2005, 174, 862–866. [Google Scholar] [CrossRef] [Green Version]
- Lapini, A.; Minervini, A.; Masala, A.; Schips, L.; Pycha, A.; Cindolo, L.; Giannella, R.; Martini, T.; Vittori, G.; Zani, D.; et al. A comparison of hexaminolevulinate (Hexvix((R))) fluorescence cystoscopy and white-light cystoscopy for detection of bladder cancer: Results of the HeRo observational study. Surg. Endosc. 2012, 26, 3634–3641. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, S.Y.; Kim, W.J.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Choi, H.Y.; Jeong, B.C. Efficacy and safety of hexaminolevulinate fluorescence cystoscopy in the diagnosis of bladder cancer. Korean J. Urol. 2012, 53, 821–825. [Google Scholar] [CrossRef] [Green Version]
- Palou, J.; Hernandez, C.; Solsona, E.; Abascal, R.; Burgues, J.P.; Rioja, C.; Cabrera, J.A.; Gutierrez, C.; Rodriguez, O.; Iborra, I.; et al. Effectiveness of hexaminolevulinate fluorescence cystoscopy for the diagnosis of non-muscle-invasive bladder cancer in daily clinical practice: A Spanish multicentre observational study. BJU Int. 2015, 116, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Ray, E.R.; Chatterton, K.; Khan, M.S.; Chandra, A.; Thomas, K.; Dasgupta, P.; O’Brien, T.S. Hexylaminolaevulinate fluorescence cystoscopy in patients previously treated with intravesical bacille Calmette-Guerin. BJU Int. 2010, 105, 789–794. [Google Scholar] [CrossRef]
- Daneshmand, S.; Bazargani, S.T.; Bivalacqua, T.J.; Holzbeierlein, J.M.; Willard, B.; Taylor, J.M.; Liao, J.C.; Pohar, K.; Tierney, J.; Konety, B. Blue light cystoscopy for the diagnosis of bladder cancer: Results from the US prospective multicenter registry. Urol. Oncol. 2018, 36, 361.e1–361.e6. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Alfred Witjes, J.; Lebret, T.; Comperat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernandez, V.; Espinos, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Cho, K.S.; Kang, D.H.; Jung, H.D.; Kwon, J.K.; Oh, C.K.; Ham, W.S.; Choi, Y.D. A network meta-analysis of therapeutic outcomes after new image technology-assisted transurethral resection for non-muscle invasive bladder cancer: 5-aminolaevulinic acid fluorescence vs. hexylaminolevulinate fluorescence vs. narrow band imaging. BMC Cancer 2015, 15, 566. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Li, J.; Ma, S.; Ge, J.; Zhou, L.; Li, D.; Chen, Q. A meta-analysis of narrow band imaging for the diagnosis and therapeutic outcome of non-muscle invasive bladder cancer. PLoS ONE 2017, 12, e0170819. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Huang, H.; Zhao, Y.; Liu, H.; Sylvester, R.; Lin, T.; Huang, J. Diagnostic performance of image technique based transurethral resection for non-muscle invasive bladder cancer: Systematic review and diagnostic meta-analysis. BMJ Open 2019, 9, e028173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tschirdewahn, S.; Harke, N.N.; Hirner, L.; Stagge, E.; Hadaschik, B.; Andreas, E. Narrow-band imaging assisted cystoscopy in the follow-up of patients with transitional cell carcinoma of the bladder: A randomized study in comparison with white light cystoscopy. World J. Urol. 2020, 38, 1509–1515. [Google Scholar] [CrossRef]
- Lai, L.Y.; Tafuri, S.M.; Ginier, E.C.; Herrel, L.A.; Dahm, P.; Maisch, P.; Lane, G.I. Narrow band imaging versus white light cystoscopy alone for transurethral resection of non-muscle invasive bladder cancer. Cochrane Database Syst. Rev. 2021, 2021. [Google Scholar] [CrossRef]
- Cauberg, E.C.; de Bruin, D.M.; Faber, D.J.; van Leeuwen, T.G.; de la Rosette, J.J.; de Reijke, T.M. A new generation of optical diagnostics for bladder cancer: Technology, diagnostic accuracy, and future applications. Eur. Urol. 2009, 56, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Nata, F.B.; Tirelli, G.; Capriotti, V.; Marcuzzo, A.V.; Sacchet, E.; Suran-Brunelli, A.N.; de Manzini, N. NBI utility in oncologic surgery: An organ by organ review. Surg. Oncol. -Oxf. 2021, 36, 65–75. [Google Scholar] [CrossRef]
- Burger, M.; Grossman, H.B.; Droller, M.; Schmidbauer, J.; Hermann, G.; Dragoescu, O.; Ray, E.; Fradet, Y.; Karl, A.; Burgues, J.P.; et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: A meta-analysis of detection and recurrence based on raw data. Eur. Urol. 2013, 64, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Rink, M.; Babjuk, M.; Catto, J.W.; Jichlinski, P.; Shariat, S.F.; Stenzl, A.; Stepp, H.; Zaak, D.; Witjes, J.A. Hexyl aminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle-invasive bladder cancer: A critical review of the current literature. Eur. Urol. 2013, 64, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Matulewicz, R.S.; Ravvaz, K.; Weissert, J.A.; Porten, S.; Steinberg, G.D. Association of smoking status and recurrence of non-muscle invasive bladder cancer among patients managed with blue light cystoscopy. Urol. Oncol. 2021. [Google Scholar] [CrossRef]
- Daneshmand, S.; Patel, S.; Lotan, Y.; Pohar, K.; Trabulsi, E.; Woods, M.; Downs, T.; Huang, W.; Jones, J.; O’Donnell, M.; et al. Efficacy and Safety of Blue Light Flexible Cystoscopy with Hexaminolevulinate in the Surveillance of Bladder Cancer: A Phase III, Comparative, Multicenter Study. J. Urol. 2018, 199, 1158–1165. [Google Scholar] [CrossRef]
- Lotan, Y.; Chaplin, I.; Ahmadi, H.; Meng, X.; Roberts, S.; Ladi-Seyedian, S.; Bagrodia, A.; Margulis, V.; Woldu, S.; Daneshmand, S. Prospective evaluation of blue-light flexible cystoscopy with hexaminolevulinate in non-muscle-invasive bladder cancer. BJU Int. 2021, 127, 108–113. [Google Scholar] [CrossRef]
- Williams, S.B.; Gavaghan, M.B.; Fernandez, A.; Daneshmand, S.; Kamat, A.M. Macro and microeconomics of blue light cystoscopy with CYSVIEW® in non-muscle invasive bladder cancer. Urol. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B.; Daneshmand, S.; Patel, S.; Pohar, K.; Trabulsi, E.; Woods, M.; Downs, T.; Huang, W.; Taylor, J.; Jones, J.; et al. Patient-reported outcomes of blue-light flexible cystoscopy with hexaminolevulinate in the surveillance of bladder cancer: Results from a prospective multicentre study. BJU Int. 2019, 123, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sievert, K.D.; Amend, B.; Nagele, U.; Schilling, D.; Bedke, J.; Horstmann, M.; Hennenlotter, J.; Kruck, S.; Stenzl, A. Economic aspects of bladder cancer: What are the benefits and costs? World J. Urol. 2009, 27, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef]
- Hendricksen, K.; Aziz, A.; Bes, P.; Chun, F.K.; Dobruch, J.; Kluth, L.A.; Gontero, P.; Necchi, A.; Noon, A.P.; van Rhijn, B.W.G.; et al. Discrepancy Between European Association of Urology Guidelines and Daily Practice in the Management of Non-muscle-invasive Bladder Cancer: Results of a European Survey. Eur. Urol. Focus 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, M.; Nishimura, N.; Inoue, T.; Suzuki, S.; Fujii, T.; Owari, T.; Hori, S.; Nakai, Y.; Toritsuka, M.; Nakagawa, H.; et al. Fluorescent cystoscopy-assisted en bloc transurethral resection versus conventional transurethral resection in patients with non-muscle invasive bladder cancer: Study protocol of a prospective, open-label, randomized control trial (the FLEBER study). Trials 2021, 22, 136. [Google Scholar] [CrossRef]
| Study | Year | Institution | Type of Study | LOE | Type of Cystoscopy | Number of Samples |
|---|---|---|---|---|---|---|
| Ye et al. [16] | 2013 | Huazhong University of Science and Technology, Wuhan, China | RTC | 2 | NBI | 300 |
| WLC | 300 | |||||
| Tatsugami et al. [15] | 2010 | Kyushu University, Fukuoka, Japan | Prospective | 3 | NBI | 313 |
| WLC | 313 | |||||
| Song et al. [14] | 2014 | Department of Urology, Yeungnam University College of Medicine, Daeg, Korea | Prospective | 3 | NBI | 63 |
| WLC | 63 | |||||
| Shen et al. [13] | 2012 | Department of Urology, Fudan University Shanghai Cancer Center | Prospective | 3 | NBI | 309 |
| WLC | 309 | |||||
| Kobatake et al. [12] | 2015 | Department of Urology, Hiroshima City Asa Hospital, Hiroshima 731-0293, Japan | Prospective | 3 | NBI | 264 |
| WLC | 289 | |||||
| Cauberg et al. [11] | 2010 | Departments of Urology and Pathology, Medical Center, Amsterdam | Prospective | 3 | NBI | 389 |
| WLC | 389 |
| Study | Year | Institution | Type of Study | LOE | Type of Cystoscopy | Number of Samples |
|---|---|---|---|---|---|---|
| Draga et al. [17] | 2009 | Departments of Urology, Medical Physics, and Pathology, University Medical Center Utrecht, Utrecht, the Netherlands Medical Center Utrecht | Prospective | 3 | 5-ALA | 1874 |
| WLC | 1874 | |||||
| Schneeweiss et al. [19] | 1999 | Department of Epidemiology, Harvard University School of Public Health, Boston, Massachusetts | Prospective | 3 | 5-ALA | 328 |
| WLC | 328 | |||||
| Burgues et al. [20] | 2011 | Department of Urology, Caritas-St. Josef Medical Center, University of Regensburg, Regensburg, Germany | RCT | 2 | HAL | 1659 |
| WLC | 1659 | |||||
| Nakai et al. [18] | 2018 | Department of Urology, Nara Medical University, Japan | Prospective | 3 | 5-ALA | 61 |
| WLC | 61 | |||||
| Jichlinski et al. [21] | 2003 | Department of Urology and Institute of Pathology, CHUV University-Hospital | Prospective | 3 | HAL | 421 |
| WLC | 414 | |||||
| Jocahm et al. [22] | 2005 | Departments of Urology, University of Schleswig-Holstein, Campus Lübeck (DJ), Lübeck | RCT | 2 | HAL | 499 |
| WLC | 343 | |||||
| Lapini et al. [23] | 2012 | Department of Urology (PJ, H-JL) and Institute of Pathology (LG), CHUV University-Hospital | Prospective | 3 | HAL | 234 |
| WLC | 234 | |||||
| Lee et al. [24] | 2012 | Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul | Prospective | 3 | HAL | 110 |
| WLC | 134 | |||||
| Palou et al. [25] | 2015 | Fundacio Puigvert, Universitat Auto noma de Barcelona, Barcelona | Prospective | 3 | HAL | 1569 |
| WLC | 1569 | |||||
| Ray et al. [26] | 2010 | Urology Centre, Guy and St. Thomas’ NHS Foundation Trust, London, UK | Prospective | 3 | HAL | 120 |
| WLC | 120 | |||||
| Daneshmand et al. [27] | 2018 | US prospective multicenter registry | Prospective | 3 | HAL | 1632 |
| WLC | 1632 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, G.I.; Sholklapper, T.N.; Cocci, A.; Broggi, G.; Caltabiano, R.; Smith, A.B.; Lotan, Y.; Morgia, G.; Kamat, A.M.; Witjes, J.A.; et al. Performance of Narrow Band Imaging (NBI) and Photodynamic Diagnosis (PDD) Fluorescence Imaging Compared to White Light Cystoscopy (WLC) in Detecting Non-Muscle Invasive Bladder Cancer: A Systematic Review and Lesion-Level Diagnostic Meta-Analysis. Cancers 2021, 13, 4378. https://doi.org/10.3390/cancers13174378
Russo GI, Sholklapper TN, Cocci A, Broggi G, Caltabiano R, Smith AB, Lotan Y, Morgia G, Kamat AM, Witjes JA, et al. Performance of Narrow Band Imaging (NBI) and Photodynamic Diagnosis (PDD) Fluorescence Imaging Compared to White Light Cystoscopy (WLC) in Detecting Non-Muscle Invasive Bladder Cancer: A Systematic Review and Lesion-Level Diagnostic Meta-Analysis. Cancers. 2021; 13(17):4378. https://doi.org/10.3390/cancers13174378
Chicago/Turabian StyleRusso, Giorgio I., Tamir N. Sholklapper, Andrea Cocci, Giuseppe Broggi, Rosario Caltabiano, Angela B. Smith, Yair Lotan, Giuseppe Morgia, Ashish M. Kamat, J. Alfred Witjes, and et al. 2021. "Performance of Narrow Band Imaging (NBI) and Photodynamic Diagnosis (PDD) Fluorescence Imaging Compared to White Light Cystoscopy (WLC) in Detecting Non-Muscle Invasive Bladder Cancer: A Systematic Review and Lesion-Level Diagnostic Meta-Analysis" Cancers 13, no. 17: 4378. https://doi.org/10.3390/cancers13174378
APA StyleRusso, G. I., Sholklapper, T. N., Cocci, A., Broggi, G., Caltabiano, R., Smith, A. B., Lotan, Y., Morgia, G., Kamat, A. M., Witjes, J. A., Daneshmand, S., Desai, M. M., Gill, I. S., & Cacciamani, G. E. (2021). Performance of Narrow Band Imaging (NBI) and Photodynamic Diagnosis (PDD) Fluorescence Imaging Compared to White Light Cystoscopy (WLC) in Detecting Non-Muscle Invasive Bladder Cancer: A Systematic Review and Lesion-Level Diagnostic Meta-Analysis. Cancers, 13(17), 4378. https://doi.org/10.3390/cancers13174378