Socio-Territorial Inequities in the French National Breast Cancer Screening Programme—A Cross-Sectional Multilevel Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Sample
2.2. Variables
- Age at invitation
- Travel time to the nearest accredited radiology centre
- French version of the European Deprivation Index (EDI)
- Opportunistic screening
- Care offer
- Département socioeconomic level
2.3. Statistics
2.3.1. Centring and Standardization
2.3.2. Model Building
2.3.3. Additional Measures
3. Results
3.1. Population
3.2. Results
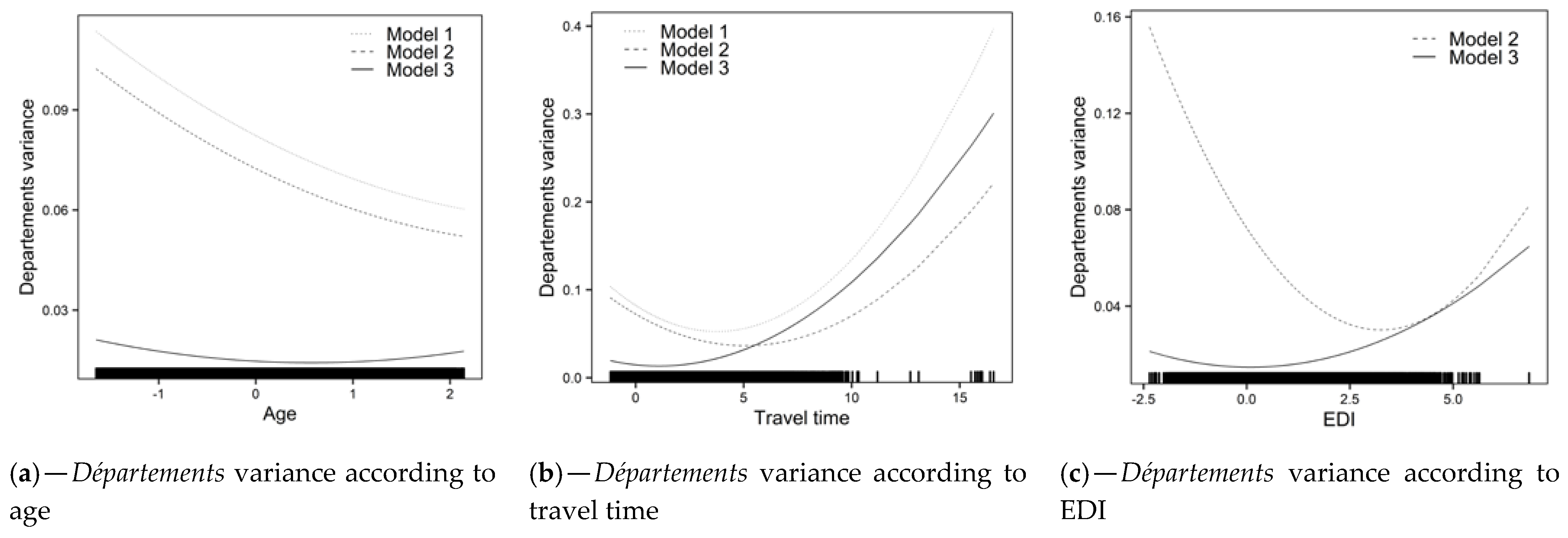
- Model 0: There was heterogeneity in FNBCSP participation (i.e., random intercepts variance) around the fixed intercept (OR = 1.32 [1.22–1.45]) at both IRIS (σ2 = 0.055; VPC = 1.6%) and département levels (σ2 = 0.082; VPC = 2.4%). Shrunken residuals used to estimate these variances are illustrated in Figure 2a,b (Model 0).
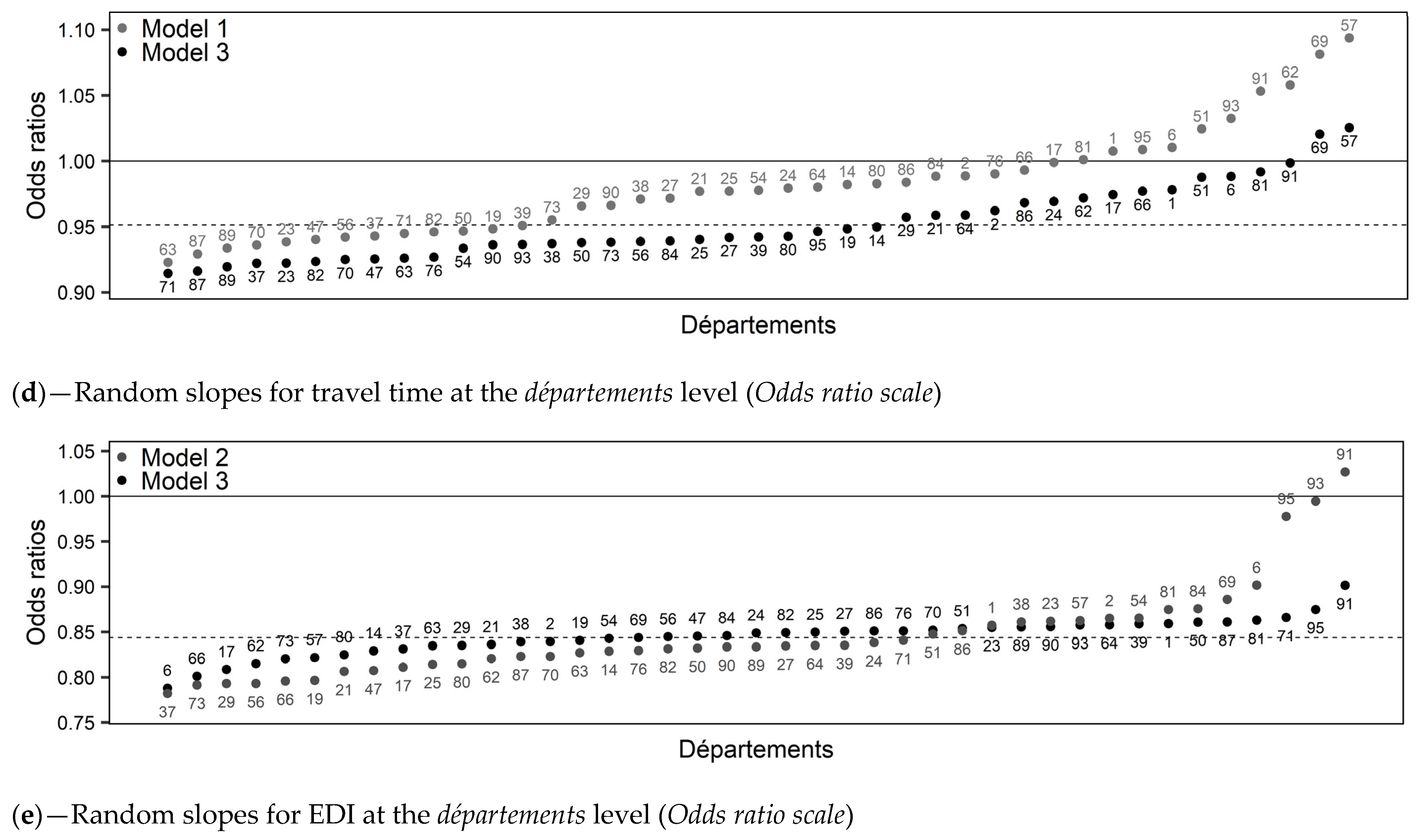
- Model 1: Overall, FNBCSP participation increased with age (OR = 1.05 [1.03–1.07]) and decreased with travel time (OR = 0.98 [0.96–0.99]). As shown by the random slopes, and illustrated in Figure 2c,d (Model 1), strength of these effects varied across départements, in such a way that the relation was insignificant or reversed in some cases. Random effects correlations showed that départements with higher intercepts tended to have a stronger effect for travel time and a weaker effect for age. It led to higher heterogeneity between départements for younger women and those closest to and furthest from the NARC (Figure 3a,b (Model 1)). There was an interaction between age and travel time (OR = 0.99 [0.98–1.00]), illustrated in Figure 4a.
- Model 2: Overall, an increase in EDI was associated with lower probability of FNBCS participation (OR = 0.84 [0.82–0.86]). Accounting for EDI reduced travel time effect heterogeneity (Figure 3b (Model 2)). As shown by the random slope and illustrated in Figure 2e, strength of the association between EDI and FNBCSP participation varied across départements, but few had a weak relationship. Random effects correlations showed that départements with higher random intercepts tended to have a stronger effect of EDI. It led to more heterogeneity in FNBCSP participation among the wealthiest women, and, to a lesser extent, the most deprived (Figure 3c (Model 2)). Accounting for EDI also reduced random intercepts variances at IRIS and département levels by 34% (Figure 2a (Model 2) and 12.2%.
- Model 3: FNBCSP participation was lower as départements’ opportunistic screening rates (OR = 0.84 [0.79–0.87]) and départements’ deprivation (OR = 0.91 [0.88–0.96]) increased. There were cross-level interactions between opportunistic screening rates and both age (OR = 1.02 [1.01–1.04]) and EDI (OR = 1.04 [1.03–1.06]). As illustrated in Figure 4b,c, FNBCSP participation in départements with high opportunistic screening rates was lower as age and deprivation decreased. There was a cross-level interaction between départements’ deprivation and EDI (OR = 1.02 [1.00–1.03]), with lower participation as deprivation decreased (Figure 4d). These effects reduced the remaining variance across départements by 79.2% (Figure 2b (Model 3)). They also strongly reduced heterogeneities between départements in the strength of the effects of age, travel time and EDI (Figure 2c–e (Model 3)). In addition, random effects correlations were reduced to statistical insignificance. Unexplained remaining variances between départements were thus independent of the lower-level variables. (Figure 3a–c (Model 3)). GDP-PPP and the number of radiology centres per 100,000 eligible women were not associated with FNBCSP participation.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
Consent for Publication
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Defossez, G.; Le Guyader-Peyrou, S.; Uhry, Z.; Grosclaude, P.; Colonna, M.; Dantony, E.; Delafosse, P.; Molinié, F.; Woronoff, A.-S.; Bouvier, A.-M. National Estimates of Cancer Incidence and Mortality in Metropolitan France between 1990 and 2018; Santé Publique France: Saint-Maurice, France, 2019; p. 372. [Google Scholar]
- Broeders, M.; Moss, S.; Nyström, L.; Njor, S.; Jonsson, H.; Paap, E.; Massat, N.; Duffy, S.; Lynge, E.; Paci, E.; et al. The Impact of Mammographic Screening on Breast Cancer Mortality in Europe: A Review of Observational Studies. J. Med. Screen 2012, 19 (Suppl. 1), 14–25. [Google Scholar] [CrossRef]
- Perry, N.; Broeders, M.; de Wolf, C.; Törnberg, S.; Holland, R.; von Karsa, L. European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis. Fourth Edition-Summary Document. Ann. Oncol. 2008, 19, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Taux de Participation au Programme de Dépistage Organisé du Cancer du Sein 2018–2019 et Évolution Depuis 2005. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers/cancer-du-sein/articles/taux-de-participation-au-programme-de-depistage-organise-du-cancer-du-sein-2018-2019-et-evolution-depuis-2005 (accessed on 12 April 2021).
- Dépistage du Cancer du Sein. Available online: https://www.has-sante.fr/jcms/r_1501534/fr/depistage-du-cancer-du-sein (accessed on 12 April 2021).
- Le Plan Cancer 2009–2013. Available online: https://www.e-cancer.fr/Institut-national-du-cancer/Strategie-de-lutte-contre-les-cancers-en-France/Les-Plans-cancer/Le-Plan-cancer-2009–2013 (accessed on 23 August 2021).
- Devaux, M. Income-Related Inequalities and Inequities in Health Care Services Utilisation in 18 Selected OECD Countries. Eur. J. Health Econ. 2015, 16, 21–33. [Google Scholar] [CrossRef]
- Carrieri, V.; Wuebker, A. Assessing Inequalities in Preventive Care Use in Europe. Health Policy 2013, 113, 247–257. [Google Scholar] [CrossRef]
- Jusot, F.; Goldzahl, L. Les déterminants du recours régulier au dépistage du cancer du sein en France. Rev. Fr. D’economie 2016, 31, 109–152. [Google Scholar]
- Sicsic, J.; Franc, C. Obstacles to the Uptake of Breast, Cervical, and Colorectal Cancer Screenings: What Remains to Be Achieved by French National Programmes? BMC Health Serv. Res. 2014, 14, 465. [Google Scholar] [CrossRef]
- Menvielle, G.; Richard, J.-B.; Ringa, V.; Dray-Spira, R.; Beck, F. To What Extent Is Women’s Economic Situation Associated with Cancer Screening Uptake When Nationwide Screening Exists? A Study of Breast and Cervical Cancer Screening in France in 2010. Cancer Causes Control 2014, 25, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Bussière, C.; Le Vaillant, M.; Pelletier-Fleury, N. The Use of Cervical, Breast and Colorectal Cancer Screening among People with Disability Living in Institution in France, April 2013: Clémence Bussière. Eur. J. Public Health 2013, 23, ckt126.083. [Google Scholar] [CrossRef][Green Version]
- Duport, N. Characteristics of Women Using Organized or Opportunistic Breast Cancer Screening in France. Analysis of the 2006 French Health, Health Care and Insurance Survey. Rev. Epidemiol. Sante Publique 2012, 60, 421–430. [Google Scholar] [CrossRef]
- Guillaume, E.; Launay, L.; Dejardin, O.; Bouvier, V.; Guittet, L.; Déan, P.; Notari, A.; De Mil, R.; Launoy, G. Could Mobile Mammography Reduce Social and Geographic Inequalities in Breast Cancer Screening Participation? Prev. Med. 2017, 100, 84–88. [Google Scholar] [CrossRef]
- Ouédraogo, S.; Dabakuyo-Yonli, T.S.; Roussot, A.; Pornet, C.; Sarlin, N.; Lunaud, P.; Desmidt, P.; Quantin, C.; Chauvin, F.; Dancourt, V.; et al. European Transnational Ecological Deprivation Index and Participation in Population-Based Breast Cancer Screening Programmes in France. Prev. Med. 2014, 63, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Pornet, C.; Dejardin, O.; Morlais, F.; Bouvier, V.; Launoy, G. Socioeconomic and Healthcare Supply Statistical Determinants of Compliance to Mammography Screening Programs: A Multilevel Analysis in Calvados, France. Cancer Epidemiol. 2010, 34, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Deborde, T.; Chatignoux, E.; Quintin, C.; Beltzer, N.; Hamers, F.F.; Rogel, A. Breast Cancer Screening Programme Participation and Socioeconomic Deprivation in France. Prev. Med. 2018, 115, 53–60. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Bull. World Health Organ. 2007, 85, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Pornet, C.; Delpierre, C.; Dejardin, O.; Grosclaude, P.; Launay, L.; Guittet, L.; Lang, T.; Launoy, G. Construction of an Adaptable European Transnational Ecological Deprivation Index: The French Version. J. Epidemiol. Community Health 2012, 66, 982–989. [Google Scholar] [CrossRef]
- Deborde, T. Participation Au Dépistage Organisé Du Cancer Du Sein et Défavorisation Socioéconomique En France. 2018. Available online: http://www.sudoc.abes.fr/cbs/xslt//DB=2.1/SET=2/TTL=1/CLK?IKT=1016&TRM=Participation+au+de%CC%81pistage+organise%CC%81+du+cancer+du+sein+et+de%CC%81favorisation+socioe%CC%81conomique+en+France (accessed on 29 August 2021).
- Statistiques OCDE. Available online: https://stats.oecd.org/?lang=fr (accessed on 23 August 2021).
- Merlo, J.; Chaix, B.; Yang, M.; Lynch, J.; Råstam, L. A Brief Conceptual Tutorial of Multilevel Analysis in Social Epidemiology: Linking the Statistical Concept of Clustering to the Idea of Contextual Phenomenon. J. Epidemiol. Community Health 2005, 59, 443–449. [Google Scholar] [CrossRef]
- Goldstein, H.; Browne, W.; Rasbash, J. Partitioning Variation in Multilevel Models. Underst. Stat. 2002, 1, 223–231. [Google Scholar] [CrossRef]
- Steele, F. LEMMA: 5.3 Allowing for Different Slopes across Groups: Random Slope Models: C 5-3. LEMMA VLE, University of Bristol, Centre for Multilevel Modelling. Available online: https://www.cmm.bris.ac.uk/lemma/mod/lesson/view.php?id=280&pageid=345 (accessed on 23 August 2021).
- Merlo, J.; Yang, M.; Chaix, B.; Lynch, J.; Rastam, L. A Brief Conceptual Tutorial on Multilevel Analysis in Social Epidemiology: Investigating Contextual Phenomena in Different Groups of People. J. Epidemiol. Community Health 2005, 59, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Rollet, Q.; Tron, L.; De Mil, R.; Launoy, G.; Guillaume, É. Contextual Factors Associated with Cancer Screening Uptake: A Systematic Review of Observational Studies. Prev. Med. 2021, 150, 106692. [Google Scholar] [CrossRef]
- Rey, G.; Jougla, E.; Fouillet, A.; Hémon, D. Ecological Association between a Deprivation Index and Mortality in France over the Period 1997–2001: Variations with Spatial Scale, Degree of Urbanicity, Age, Gender and Cause of Death. BMC Public Health 2009, 9, 33. [Google Scholar] [CrossRef]
- Ouédraogo, S.; Dabakuyo-Yonli, T.S.; Roussot, A.; Dialla, P.O.; Pornet, C.; Poillot, M.-L.; Soler-Michel, P.; Sarlin, N.; Lunaud, P.; Desmidt, P.; et al. Dépistage du cancer du sein dans treize départements français. Bull. Cancer 2015, 102, 126–138. [Google Scholar] [CrossRef]
- SPF Baromètre Santé 2005. Available online: https://www.santepubliquefrance.fr/docs/barometre-sante-2005 (accessed on 29 January 2021).
- Kalecinski, J.; Régnier-Denois, V.; Ouédraogo, S.; Dabakuyo-Yonli, T.S.; Dumas, A.; Arveux, P.; Chauvin, F. Dépistage organisé ou individuel du cancer du sein ? Attitudes et représentations des femmes. Sante Publique 2015, 27, 213–220. [Google Scholar] [CrossRef]
- Palència, L.; Espelt, A.; Rodríguez-Sanz, M.; Puigpinós, R.; Pons-Vigués, M.; Pasarín, M.I.; Spadea, T.; Kunst, A.E.; Borrell, C. Socio-Economic Inequalities in Breast and Cervical Cancer Screening Practices in Europe: Influence of the Type of Screening Program. Int. J. Epidemiol. 2010, 39, 757–765. [Google Scholar] [CrossRef]
- Walsh, B.; Silles, M.; O’Neill, C. The Importance of Socio-Economic Variables in Cancer Screening Participation: A Comparison between Population-Based and Opportunistic Screening in the EU-15. Health Policy 2011, 101, 269–276. [Google Scholar] [CrossRef]
- Dalton, S.O.; Düring, M.; Ross, L.; Carlsen, K.; Mortensen, P.B.; Lynch, J.; Johansen, C. The Relation between Socioeconomic and Demographic Factors and Tumour Stage in Women Diagnosed with Breast Cancer in Denmark, 1983–1999. Br. J. Cancer 2006, 95, 653–659. [Google Scholar] [CrossRef][Green Version]
- Bryere, J.; Dejardin, O.; Launay, L.; Colonna, M.; Grosclaude, P.; Launoy, G. Socioeconomic Status and Site-Specific Cancer Incidence, a Bayesian Approach in a French Cancer Registries Network Study. Eur. J. Cancer Prev. 2018, 27, 391–398. [Google Scholar] [CrossRef] [PubMed]
- La Participation au Dépistage du Cancer du Sein Des Femmes de 50 à 74 ans en France. Available online: https://www.has-sante.fr/jcms/c_1194998/fr/la-participation-au-depistage-du-cancer-du-sein-des-femmes-de-50-a-74-ans-en-france (accessed on 23 August 2021).
- SPF Dépistage Individuel du Cancer du Sein des Femmes de 50 à 74 ans en France en 2009. Numéro Thématique. Dépistage Organisé du Cancer du sein. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers/cancer-du-sein/depistage-individuel-du-cancer-du-sein-des-femmes-de-50-a-74-ans-en-france-en-2009.-numero-thematique.-depistage-organise-du-cancer-du-sein (accessed on 23 August 2021).
- Theall, K.P.; Scribner, R.; Broyles, S.; Yu, Q.; Chotalia, J.; Simonsen, N.; Schonlau, M.; Carlin, B.P. Impact of Small Group Size on Neighbourhood Influences in Multilevel Models. J. Epidemiol. Community Health 2011, 65, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Pickett, K.E.; Pearl, M. Multilevel Analyses of Neighbourhood Socioeconomic Context and Health Outcomes: A Critical Review. J. Epidemiol. Community Health 2001, 55, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Ponti, A.; Anttila, A.; Ronco, G.; Senore, C.; Basu, P.; Segnan, N.; Tomatis, M.; Primic Žakelj, M.; Dillner, J.; Fernan, M.; et al. Cancer Screening in the European Union. Report on the Implementation of the Council Recommendation on Cancer Screening (Second Report). Available online: https://screening.iarc.fr/EUreport.php (accessed on 23 August 2021).
- Deandrea, S.; Molina-Barceló, A.; Uluturk, A.; Moreno, J.; Neamtiu, L.; Peiró-Pérez, R.; Saz-Parkinson, Z.; Lopez-Alcalde, J.; Lerda, D.; Salas, D. Presence, Characteristics and Equity of Access to Breast Cancer Screening Programmes in 27 European Countries in 2010 and 2014. Results from an International Survey. Prev. Med. 2016, 91, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Thomson, K.; Bambra, C.; Todd, A. The Breast Cancer Paradox: A Systematic Review of the Association between Area-Level Deprivation and Breast Cancer Screening Uptake in Europe. Cancer Epidemiol. 2019, 60, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Mihor, A.; Tomsic, S.; Zagar, T.; Lokar, K.; Zadnik, V. Socioeconomic Inequalities in Cancer Incidence in Europe: A Comprehensive Review of Population-Based Epidemiological Studies. Radiol. Oncol. 2020, 54, 1–13. [Google Scholar] [CrossRef]
- Tron, L.; Belot, A.; Fauvernier, M.; Remontet, L.; Bossard, N.; Launay, L.; Bryere, J.; Monnereau, A.; Dejardin, O.; Launoy, G. Socioeconomic Environment and Disparities in Cancer Survival for 19 Solid Tumor Sites: An Analysis of the French Network of Cancer Registries (FRANCIM) Data. Int. J. Cancer 2019, 144, 1262–1274. [Google Scholar] [CrossRef]
- Lundqvist, A.; Andersson, E.; Ahlberg, I.; Nilbert, M.; Gerdtham, U. Socioeconomic Inequalities in Breast Cancer Incidence and Mortality in Europe-a Systematic Review and Meta-Analysis. Eur J. Public Health 2016, 26, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, S.; Dabakuyo-Yonli, T.S.; Amiel, P.; Dancourt, V.; Dumas, A.; Arveux, P. Breast Cancer Screening Programmes: Challenging the Coexistence with Opportunistic Mammography. Patient Educ. Couns. 2014, 97, 410–417. [Google Scholar] [CrossRef]
- Gøtzsche, P.C.; Jørgensen, K.J. Screening for Breast Cancer with Mammography. Cochrane Database Syst. Rev. 2013, 2013, CD001877. [Google Scholar] [CrossRef] [PubMed]
- Campergue, R.; Junod, B. No Mammo—Enquete Depistage; MAX MILO: Paris, France, 2011; ISBN 978-2-315-00293-1. [Google Scholar]
- Zielonke, N.; Kregting, L.M.; Heijnsdijk, E.A.M.; Veerus, P.; Heinävaara, S.; McKee, M.; de Kok, I.M.C.M.; de Koning, H.J.; van Ravesteyn, N.T. The Potential of Breast Cancer Screening in Europe. Int. J. Cancer 2021, 148, 406–418. [Google Scholar] [CrossRef]
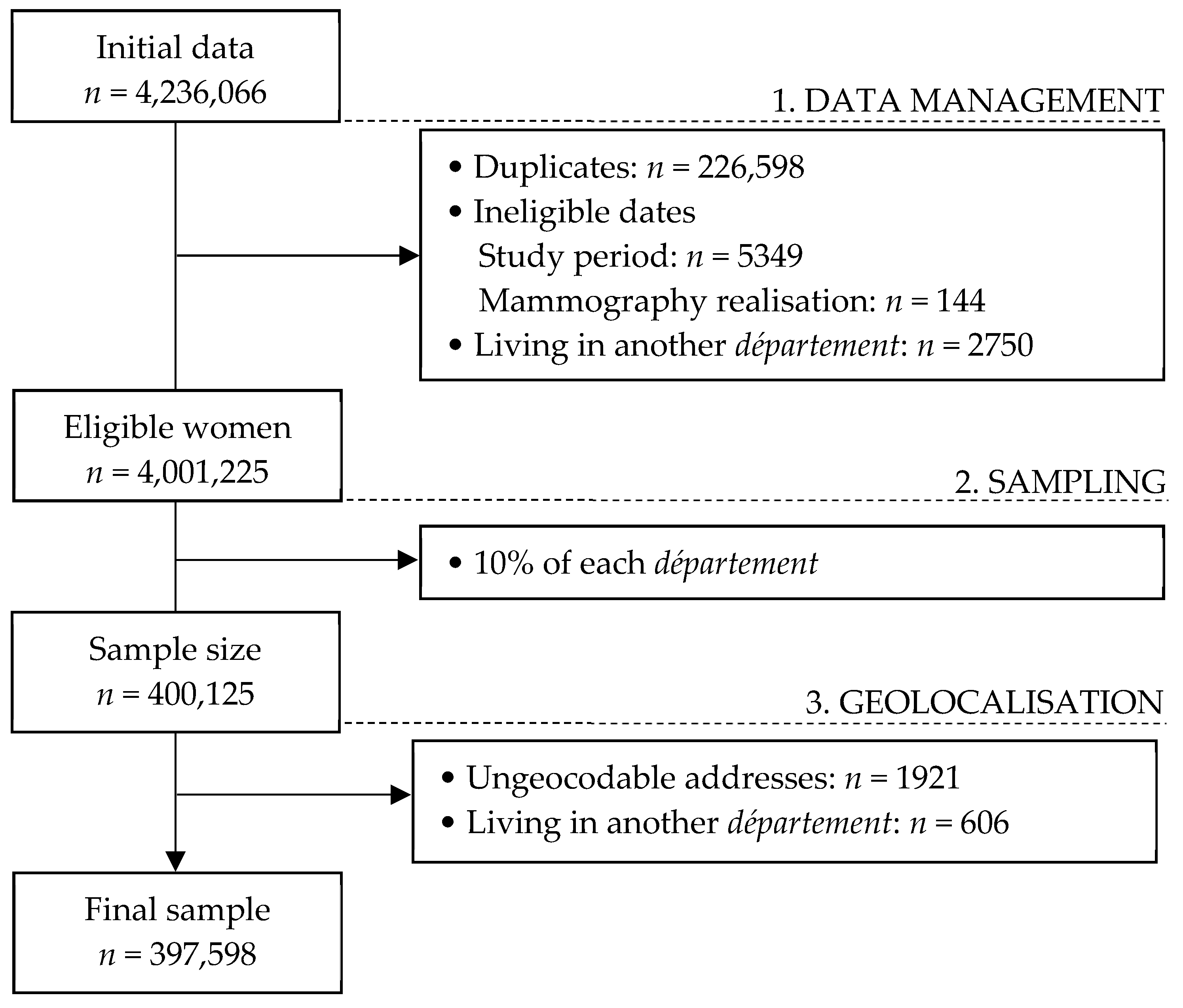


| Level 1—Individual Level | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Travel time (minutes) | 8.70 | 7.47 | 0.00 | 132.48 |
| Age (years) | 60.73 | 7.11 | 50 | 74 |
| FNBCSP a participation (%) | 55.06 | / | / | / |
| Level 2—IRIS b | ||||
| EDI c,d (dimensionless) | 0.97 | 5.12 | −11.08 | 35.96 |
| Level 3—Départements | ||||
| Opportunistic screening rates (%) | 8.91 | 6.06 | 2.30 | 28.00 |
| Mean of EDI c,d (dimensionless) | 0.97 | 2.29 | −1.67 | 9.63 |
| GDP (PPP) e per capita (US$) | 20,638 | 6134 | 17,310 | 23,360 |
| Number of accredited radiology centres/100,000 eligible women | 21.88 | 8.55 | 7.69 | 59.06 |
| Model 0: Empty Model | Model 1: Level 1 Variables | Model 2: Level 2 Variable | Model 3: Level 3 Variables | |
|---|---|---|---|---|
| Level 1—Individuals | ||||
| Intercept | 1.32 [1.22–1.45] | 1.35 [1.24–1.47] | 1.32 [1.22–1.43] | 1.32 [1.27–1.37] |
| Age | / | 1.05 [1.03–1.07] | 1.05 [1.03–1.07] | 1.05 [1.04–1.06] |
| Travel time | / | 0.98 [0.96–0.99] | 0.95 [0.93–0.96] | 0.94 [0.93–0.95] |
| Age × travel time | / | 0.99 [0.98–1.00] | 0.99 [0.98–1.00] | 0.99 [0.98–1.00] |
| Level 2—IRIS | ||||
| Fixed effects | ||||
| EDI | / | / | 0.84 [0.82–0.86] | 0.84 [0.83–0.85] |
| Random effects | ||||
| Random intercept (σ20I) | 0.055 [0.048–0.058] | 0.053 [0.048–0.058] | 0.035 [0.030–0.039] | 0.035 [0.031–0.039] |
| VCP | 1.60% | 1.55% | 1.03% | 1.05% |
| PCV (compared with empty model) | / | −3.64% | −36.36% | −36.36% |
| Level 3—Départements | ||||
| Fixed effects | ||||
| Individual screening rates | / | / | / | 0.84 [0.79–0.87] |
| Deprivation | / | / | / | 0.91 [0.88–0.96] |
| Cross-level interactions | ||||
| Individual screening rates × Age | / | / | / | 1.02 [1.01–1.04] |
| Individual screening rates × EDI | / | / | / | 1.04 [1.03–1.06] |
| Mean of EDI × EDI | / | / | / | 1.02 [1.00–1.03] |
| Random effects | ||||
| Random intercept (σ20D) | 0.082 [0.053–0.130] | 0.082 [0.048–0.123] | 0.072 [0.044–0.108] | 0.015 [0.007–0.021] |
| VPC | 2.39% | 2.39% | 2.12% | 0.45% |
| PCV (compared with empty model) | / | 0% | −12.20% | −81.71% |
| Age random slope (σ21D) | / | 2.3 × 10−3 [1.2 × 10−3–3.7 × 10−3] | 2.3 × 10−3 [1.2 × 10−3–3.6 × 10−3] | 1.4 × 10−3 [5.3 × 10−4–2.2 × 10−3] |
| Travel time random slope (σ22D) | / | 2.1 × 10−3 [1.0 × 10−3–3.4 × 10−3] | 1.4 × 10−3 [5.3 × 10−4–2.3 × 10−3] | 1.2 × 10−3 [4.0 × 10−4–2.2 × 10−3] |
| EDI random slope (σ23D) | / | / | 4.1 × 10−3 [1.8 × 10−3–6.7 × 10−3] | 1.1 × 10−3 [1.0 × 10−4–1.8 × 10−3] |
| Random effects correlation | ||||
| σ20D, σ21D | / | −0.55 [−0.77; −0.19] | −0.55 [−0.78; −0.23] | −0.18 [−0.57; 0.23] |
| σ20D, σ22D | / | −0.60 [−0.83; −0.31] | −0.71 [−0.94; −0.42] | −0.32 [−0.67; 0.13] |
| σ20D, σ23D | / | / | −0.76 [−0.91; −0.54] | −0.03 [−0.65; 0.59] |
| σ21D, σ22D | / | 0.49 [0.12; 0.82] | 0.68 [0.34; 0.95] | 0.55 [0.10; 0.93] |
| σ21D, σ23D | / | / | 0.43 [0.08; 0.75] | −0.04 [−0.72; 0.70] |
| σ22D, σ23D | / | / | 0.60 [0.17; 0.89] | −0.09 [−0.87; 0.52] |
| Deviance | 536,474 | 535,848 | 534,615 | 534,549 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rollet, Q.; Guillaume, É.; Launay, L.; Launoy, G. Socio-Territorial Inequities in the French National Breast Cancer Screening Programme—A Cross-Sectional Multilevel Study. Cancers 2021, 13, 4374. https://doi.org/10.3390/cancers13174374
Rollet Q, Guillaume É, Launay L, Launoy G. Socio-Territorial Inequities in the French National Breast Cancer Screening Programme—A Cross-Sectional Multilevel Study. Cancers. 2021; 13(17):4374. https://doi.org/10.3390/cancers13174374
Chicago/Turabian StyleRollet, Quentin, Élodie Guillaume, Ludivine Launay, and Guy Launoy. 2021. "Socio-Territorial Inequities in the French National Breast Cancer Screening Programme—A Cross-Sectional Multilevel Study" Cancers 13, no. 17: 4374. https://doi.org/10.3390/cancers13174374
APA StyleRollet, Q., Guillaume, É., Launay, L., & Launoy, G. (2021). Socio-Territorial Inequities in the French National Breast Cancer Screening Programme—A Cross-Sectional Multilevel Study. Cancers, 13(17), 4374. https://doi.org/10.3390/cancers13174374






