Minimal Residual Disease in Multiple Myeloma: Something Old, Something New
Abstract
Simple Summary
Abstract
1. Introduction
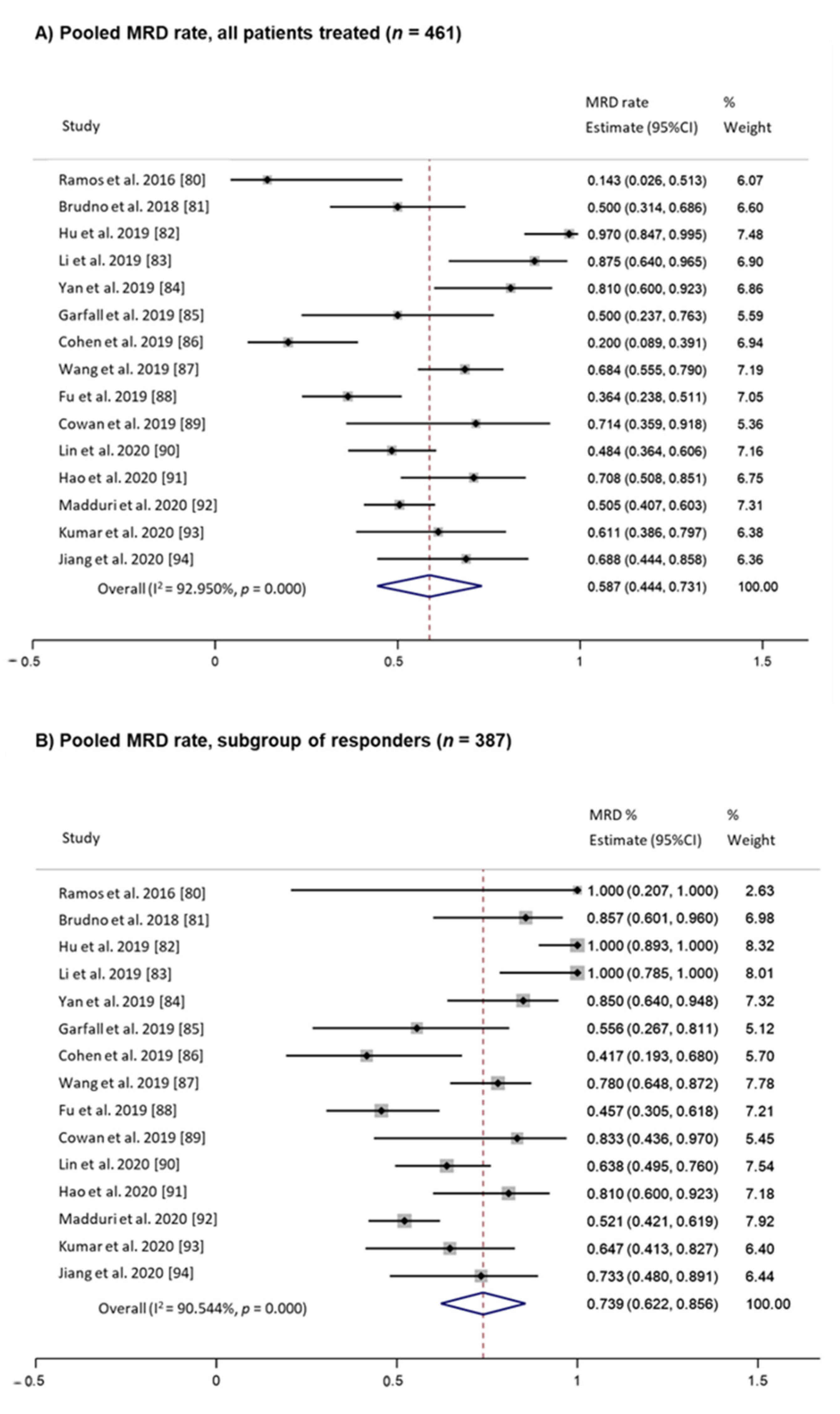
2. Something Old: MRD and MM Past
3. Something New: MRD and MM Present and Future
4. Something Blue (Reliable): Methods for BM MRD Assessment
5. Something Borrowed: Functional Imaging and Liquid Biopsy
6. A Silver Sixpence in Her Shoe: MRD Cost-Effectiveness
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thorsteinsdottir, S.; Dickman, P.W.; Landgren, O.; Blimark, C.; Hultcrantz, M.; Turesson, I.; Björkholm, M.; Kristinsson, S.Y. Dramatically improved survival in multiple myeloma patients in the recent decade: Results from a Swedish population-based study. Haematologica 2018, 103, e412–e415. [Google Scholar] [CrossRef]
- Chapman, P.B.; Einhorn, L.H.; Meyers, M.L.; Saxman, S.; Destro, A.N.; Panageas, K.S.; Begg, C.B.; Agarwala, S.S.; Schuchter, L.M.; Ernstoff, M.S.; et al. Phase III Multicenter Randomized Trial of the Dartmouth Regimen Versus Dacarbazine in Patients With Metastatic Melanoma. J. Clin. Oncol. 1999, 17, 2745. [Google Scholar] [CrossRef] [PubMed]
- Zabell, J. Docetaxel Plus Prednisone or Mitoxantrone Plus Prednisone for Advanced Prostate Cancer. In 50 Studies Every Urologist Should Know; Oxford University Press: Oxford, UK, 2021; pp. 83–88. [Google Scholar] [CrossRef]
- Anderson, K.C.; Auclair, D.; Kelloff, G.J.; Sigman, C.C.; Avet-Loiseau, H.; Farrell, A.T.; Gormley, N.J.; Kumar, S.K.; Landgren, O.; Munshi, N.C.; et al. The Role of Minimal Residual Disease Testing in Myeloma Treatment Selection and Drug Development: Current Value and Future Applications. Clin. Cancer Res. 2017, 23, 3980–3993. [Google Scholar] [CrossRef]
- Luskin, M.R.; Murakami, M.A.; Manalis, S.R.; Weinstock, D.M. Targeting Minimal Residual Disease: A Path to Cure? Nat. Rev. Cancer 2018, 18, 255–263. [Google Scholar] [CrossRef]
- Stevens, S.M. Something old, something new…. Blood J. Am. Soc. Hematol. 2020, 135, 1307–1308. [Google Scholar] [CrossRef]
- George, J.N. Management of Immune Thrombocytopenia—Something Old, Something New. N. Engl. J. Med. 2010, 363, 1959–1961. [Google Scholar] [CrossRef]
- Sekeres, M.A. Treatment of MDS: Something old, something new, something borrowed. Hematology 2009, 2009, 656–663. [Google Scholar] [CrossRef][Green Version]
- Wolmark, N.; Rockette, H.; Fisher, B.; Wickerham, D.L.; Redmond, C.; Fisher, E.R.; Jones, J.; Mamounas, E.P.; Ore, L.; Petrelli, N.J. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: Results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J. Clin. Oncol. 1993, 11, 1879–1887. [Google Scholar] [CrossRef]
- Frei, I.E.; Karon, M.; Levin, R.H.; Freireich, E.J.; Taylor, R.J.; Hananian, J.; Selawry, O.; Holland, J.F.; Hoogstraten, B.; Wolman, I.J.; et al. The Effectiveness of Combinations of Antileukemic Agents in Inducing and Maintaining Remission in Children with Acute Leukemia. Blood 1965, 26, 642–656. [Google Scholar] [CrossRef]
- Pieters, R.; de Groot-Kruseman, H.; Van der Velden, V.; Fiocco, M.; Berg, H.V.D.; de Bont, E.; Egeler, R.M.; Hoogerbrugge, P.; Kaspers, G.; Van der Schoot, E.; et al. Successful Therapy Reduction and Intensification for Childhood Acute Lymphoblastic Leukemia Based on Minimal Residual Disease Monitoring: Study ALL10 From the Dutch Childhood Oncology Group. J. Clin. Oncol. 2016, 34, 2591–2601. [Google Scholar] [CrossRef]
- Soverini, S.; Bavaro, L.; De Benedittis, C.; Martelli, M.; Iurlo, A.; Orofino, N.; Sica, S.; Sorà, F.; Lunghi, F.; Ciceri, F.; et al. Prospective assessment of NGS-detectable mutations in CML patients with nonoptimal response: The NEXT-in-CML study. Blood 2020, 135, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.; Ross, D. Moving treatment-free remission into mainstream clinical practice in CML. Blood 2016, 128, 17–23. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Kapoor, P.; Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Buadi, F.; Dingli, D.; Russell, S.J.; Hayman, S.R.; Witzig, T.E.; Lust, J.A.; et al. Importance of Achieving Stringent Complete Response After Autologous Stem-Cell Transplantation in Multiple Myeloma. J. Clin. Oncol. 2013, 31, 4529–4535. [Google Scholar] [CrossRef] [PubMed]
- Child, J.A.; Morgan, G.; Davies, F.; Owen, R.G.; Bell, S.E.; Hawkins, K.; Brown, J.; Drayson, M.; Selby, P.J. High-Dose Chemotherapy with Hematopoietic Stem-Cell Rescue for Multiple Myeloma. N. Engl. J. Med. 2003, 348, 1875–1883. [Google Scholar] [CrossRef]
- Landgren, O.; Devlin, S.; Boulad, M.; Mailankody, S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: A meta-analysis. Bone Marrow Transplant. 2016, 51, 1565–1568. [Google Scholar] [CrossRef]
- Munshi, N.C.; Avet-Loiseau, H.; Rawstron, A.; Owen, R.G.; Child, J.A.; Thakurta, A.; Sherrington, P.; Samur, M.K.; Georgieva, A.; Anderson, K.C.; et al. Association of Minimal Residual Disease with Superior Survival Outcomes in Patients with Multiple Myeloma. JAMA Oncol. 2017, 3, 28–35. [Google Scholar] [CrossRef]
- Paiva, B.; Vidriales, M.-B.; Cerveró, J.; Mateo, G.; Pérez, J.J.; Montalbán, M.A.; Sureda, A.; Montejano, L.; Gutiérrez, N.C.; de Coca, A.G.; et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood 2008, 112, 4017–4023. [Google Scholar] [CrossRef]
- Davies, F.E. Is molecular remission the goal of multiple myeloma therapy? Hematology 2017, 2017, 205–211. [Google Scholar] [CrossRef]
- Gay, F.; Cerrato, C.; Petrucci, M.T.; Zambello, R.; Gamberi, B.; Ballanti, S.; Omedè, P.; Palmieri, S.; Troia, R.; Spada, S.; et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: Results from the FORTE trial. J. Clin. Oncol. 2019, 37, 8002. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Kumar, S.K.; Lupparelli, G.; Usmani, S.; Waage, A.; Larocca, A.; van der Holt, B.; Musto, P.; Offidani, M.; et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: A meta-analysis of individual patient data. Lancet Oncol. 2014, 15, 333–342. [Google Scholar] [CrossRef]
- Takamatsu, H.; Yoroidaka, T.; Yamashita, T.; Murata, R.; Yoshihara, K.; Yoshihara, S.; Ueda, M.; Nakao, S.; Matsue, K. Minimal residual disease assessment using EuroFlow in patients with relapsed/refractory multiple myeloma who received carfilzomib+lenalidomide+dexamethasone (KRD) therapy. Clin. Lymphoma Myeloma Leuk. 2019, 19, e184. [Google Scholar] [CrossRef]
- Munshi, N.C.; Avet-Loiseau, H.; Anderson, K.C.; Neri, P.; Paiva, B.; Samur, M.; Dimopoulos, M.; Kulakova, M.; Lam, A.; Hashim, M.; et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv. 2020, 4, 5988–5999. [Google Scholar] [CrossRef]
- Rawstron, A.C.; Davies, F.E.; Dasgupta, R.; Ashcroft, A.J.; Patmore, R.; Drayson, M.T.; Owen, R.G.; Jack, A.S.; Child, J.A.; Morgan, G.J. Flow cytometric disease monitoring in multiple myeloma: The relationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood 2002, 100, 3095–3100. [Google Scholar] [CrossRef]
- Rawstron, A.; Child, J.A.; De Tute, R.M.; Davies, F.; Gregory, W.M.; Bell, S.E.; Szubert, A.J.; Navarro-Coy, N.; Drayson, M.; Feyler, S.; et al. Minimal Residual Disease Assessed by Multiparameter Flow Cytometry in Multiple Myeloma: Impact on Outcome in the Medical Research Council Myeloma IX Study. J. Clin. Oncol. 2013, 31, 2540–2547. [Google Scholar] [CrossRef]
- Martín-Mateos, M.-L.; Oriol, A.; Martinez-Lopez, J.; Teruel, A.-I.; De La Guía, A.L.; López, J.; Bengoechea, E.; Pérez, M.; Martínez, R.; Palomera, L.; et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: Do we still need alkylators? Blood 2014, 124, 1887–1893. [Google Scholar] [CrossRef]
- Silvennoinen, R.; Lundan, T.; Kairisto, V.; Pelliniemi, T.-T.; Putkonen, M.; Anttila, P.; Huotari, V.; Mantymaa, P.; Siitonen, S.; Uotila, L.; et al. Comparative analysis of minimal residual disease detection by multiparameter flow cytometry and enhanced ASO RQ-PCR in multiple myeloma. Blood Cancer J. 2014, 4, e250. [Google Scholar] [CrossRef]
- Korde, N.; Roschewski, M.; Zingone, A.; Kwok, M.; Manasanch, E.E.; Bhutani, M.; Tageja, N.; Kazandjian, D.; Mailankody, S.; Wu, P.; et al. Treatment with Carfilzomib-Lenalidomide-Dexamethasone With Lenalidomide Extension in Patients With Smoldering or Newly Diagnosed Multiple Myeloma. JAMA Oncol. 2015, 1, 746–754. [Google Scholar] [CrossRef]
- Paiva, B.D.L.; Chandia, M.; Puig, N.; Vidriales, M.-B.; Perez, J.J.; Corral, L.L.; Ocio, E.M.; Garcia-Sanz, R.; Gutierrez, N.; Jimenez-Ubieto, A.; et al. The prognostic value of multiparameter flow cytometry minimal residual disease assessment in relapsed multiple myeloma. Haematologica 2014, 100, e53–e55. [Google Scholar] [CrossRef][Green Version]
- Ludwig, H.; Greil, R.; Masszi, T.; Spicka, I.; Shpilberg, O.; Hajek, R.; Dmoszynska, A.; Paiva, B.D.L.; Vidriales, M.-B.; Esteves, G.; et al. Bortezomib, thalidomide and dexamethasone, with or without cyclophosphamide, for patients with previously untreated multiple myeloma: 5-year follow-up. Br. J. Haematol. 2015, 171, 344–354. [Google Scholar] [CrossRef]
- Cohen, O.C.; Rabin, N.; Counsell, N.; Owen, R.G.; Popova, B.; Schofield, O.; Lyons-Lewis, J.; Rawstron, A.; Spence, C.; De Tute, R.M.; et al. Bortezomib Consolidation Following Upfront ASCT for Multiple Myeloma Deepens Disease Response and MRD-Negative Rate without Compromising Response to Subsequent Bortezomib Salvage: Results of a Phase II Study. Blood 2016, 128, 4508. [Google Scholar] [CrossRef]
- De Tute, R.M.; Rawstron, A.C.; Cairns, D.A.; Pawlyn, C.; Davies, F.E.; Collett, C.; Kaiser, M.F.; Jones, J.R.; Waterhouse, A.; Striha, A.; et al. Impact of Minimal Residual Disease in Transplant Ineligible Myeloma Patients: Results from the UK NCRI Myeloma XI Trial. Blood 2016, 128, 245. [Google Scholar] [CrossRef]
- Fukumoto, K.; Fujisawa, M.; Suehara, Y.; Narita, K.-T.; Usui, Y.; Takeuchi, M.; Matsue, K. Prognostic impact of immunophenotypic complete response in patients with multiple myeloma achieving better than complete response. Leuk. Lymphoma 2016, 57, 1–7. [Google Scholar] [CrossRef]
- Solovev, M.; Mendeleeva, L.; Pokrovskaya, O.; Gemdzhian, E.; Galtseva, I.; Davydova, J.; Firsova, M.; Nareyko, M.; Abramova, T.; Savchenko, V. Maintenance Therapy with Bortezomib in Patients with Multiple Myeloma (MM) after ASCT and Minimal Residual Disease (MRD). Haematologica 2017, 102, 531. [Google Scholar]
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.D.L.; Puig, N.; García-Sánchez, O.; Böttcher, S.; Van Der Velden, V.H.J.; Pérez-Morán, J.-J.; Vidriales, M.-B.; Garcia-Sanz, R.; et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef]
- Chakraborty, R.; Muchtar, E.; Kumar, S.K.; Jevremovic, D.; Buadi, F.K.; Dingli, D.; Dispenzieri, A.; Hayman, S.R.; Hogan, W.J.; Kapoor, P.; et al. Impact of Post-Transplant Response and Minimal Residual Disease on Survival in Myeloma with High-Risk Cytogenetics. Biol. Blood Marrow Transplant. 2017, 23, 598–605. [Google Scholar] [CrossRef]
- Popat, R.; De Tute, R.M.; Counsell, N.; De-Silva, D.; Phillips, B.; Cavenagh, J.D.; Adedayo, T.; Braganza, N.; Roddie, C.; Streetly, M. Outcomes of Stratification to ASCT or Not Based on Depth of Response: Results of a Phase 2 Trial Assessing the Impact of Minimal Residual Disease (MRD) in Multiple Myeloma Patients with Deferred ASCT (PADIMAC). Blood 2017, 130, 1864. [Google Scholar]
- Oliva, S.; Bruinink, D.H.O.; Rihova, L.; D’Agostino, M.; Pantani, L.; Capra, A.; van der Holt, B.; Troia, R.; Petrucci, M.T.; Villanova, T.; et al. Minimal residual disease assessment by multiparameter flow cytometry in transplant-eligible myeloma in the EMN02/HOVON 95 MM trial. Blood Cancer J. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Austin, M.; Pawlyn, C.; Woods, H. Sustained MRD Negativity at 12 Months Post-ASCT Predicts Outcomes for Myeloma Patients: A Real World Study. EHA Library 2018, 215626, 2018. [Google Scholar]
- Clark, C.A.; Mosse, C.A.; Chen, H.; Byrne, M.; Chinratanalab, W.; Engelhardt, B.G.; Goodman, S.A.; Harrell, S.L.; Kassim, A.A.; Savani, B.N.; et al. Prospective trial of minimal residual disease assessment by multiparametric flow cytometry for multiple myeloma in the era of bortezomib-based chemotherapy. Bone Marrow Transplant. 2018, 53, 1589–1592. [Google Scholar] [CrossRef]
- Gu, J.; Liu, J.; Chen, M.; Huang, B.; Li, J. Longitudinal Flow Cytometry Identified “Minimal Residual Disease” (MRD) Evolution Patterns for Predicting the Prognosis of Patients with Transplant-Eligible Multiple Myeloma. Biol. Blood Marrow Transplant. 2018, 24, 2568–2574. [Google Scholar] [CrossRef]
- Yong, K.; Hinsley, S.; De Tute, R.M.; Sherratt, D.; Brown, S.R.; Flanagan, L.; Williams, C.; Cavenagh, J.; Kaiser, M.; Rabin, N.K.; et al. Maintenance with Carfilzomib Following Carfilzomib, Cyclophosphamide and Dexamethasone at First Relapse or Primary Refractory Multiple Myeloma (MM) on the Phase 2 Muk Five Study: Effect on Minimal Residual Disease. Blood 2018, 132, 802. [Google Scholar] [CrossRef]
- Shah, G.L.; Seier, K.; Devlin, S.M.; Chung, D.J.; Scordo, M.; Hultcrantz, M.; Korde, N.; Lendvai, N.; Lesokhin, A.M.; Mailankody, S.; et al. Depth of Response and Outcomes in Patients with Multiple Myeloma Undergoing Autologous Stem Cell Transplantation. Blood 2018, 132, 4619. [Google Scholar] [CrossRef]
- Solovev, M.V.; Mendeleeva, L.P.; Firsova, M.V.; Galtseva, I.V.; Davydova, J.; Gemdzhian, E.G.; Savchenko, V.G. Efficacy of Maintenance Therapy Following Auto-HSCT Depending on MRD Status in Patients with Multiple Myeloma. Blood 2018, 132, 3432. [Google Scholar] [CrossRef]
- Facon, T.; Lee, J.H.; Moreau, P.; Niesvizky, R.; Dimopoulos, M.; Hajek, R.; Pour, L.; Jurczyszyn, A.; Qiu, L.; Klippel, Z.; et al. Carfilzomib or bortezomib with melphalan-prednisone for transplant-ineligible patients with newly diagnosed multiple myeloma. Blood 2019, 133, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Avet-Loiseau, H.; Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Corre, J.; Garderet, L.; Karlin, L.; Lambert, J.; Macro, M.; et al. Efficacy of daratumumab (DARA) + bortezomib/thalidomide/dexamethasone (D-VTd) in transplant-eligible newly diagnosed multiple myeloma (TE NDMM) based on minimal residual disease (MRD) status: Analysis of the CASSIOPEIA trial. J. Clin. Oncol. 2019, 37, 8017. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Zhou, X.; Mei, J.; Song, P.; An, Z.; Zhao, Q.; Guo, X.; Wang, X.; Zhai, Y. Achieving minimal residual disease-negative by multiparameter flow cytometry may ameliorate a poor prognosis in MM patients with high-risk cytogenetics: A retrospective single-center analysis. Ann. Hematol. 2019, 98, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Falcone, A.P.; Minervini, M.M.; De Cillis, G.P.; De Waure, C.; Sisti, L.G.; Giambra, V.; Valente, D.; Chiello, V.; Scalzulli, P.R.; et al. Minimal residual disease and log-reduction of plasma cells are associated with superior response after double autologous stem cell transplant in younger patients with multiple myeloma. Cytom. Part. B Clin. Cytom. 2018, 96, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.E.; Wallace, P.K.; Fraser, R.; Fei, M.; Tario, J.D.; Howard, A.; Zhang, Y.; Blackwell, B.; Brunstein, C.G.; Efebera, Y.A.; et al. Minimal Residual Disease (MRD) Assessment before and after Autologous Hematopoietic Cell Transplantation (AutoHCT) and Maintenance for Multiple Myeloma (MM): Results of the Prognostic Immunophenotyping for Myeloma Response (PRIMeR) Study. Biol. Blood Marrow Transplant. 2019, 25, S4–S6. [Google Scholar] [CrossRef]
- Rasche, L.; Alapat, D.; Kumar, M.; Gershner, G.; McDonald, J.; Wardell, C.P.; Samant, R.; Van Hemert, R.; Epstein, J.; Williams, A.F.; et al. Combination of flow cytometry and functional imaging for monitoring of residual disease in myeloma. Leukemia 2018, 33, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Cedena, M.T.; Gomez-Grande, A.; Ríos, R.; Moraleda, J.M.; Cabañas, V.; Moreno, M.J.; López-Jiménez, J.; Martin-Moro, F.; Sanz, A.; et al. Imaging and bone marrow assessments improve minimal residual disease prediction in multiple myeloma. Am. J. Hematol. 2018, 94, 853–861. [Google Scholar] [CrossRef]
- Paiva, B.; Puig, N.; Cedena, M.-T.; Rosiñol, L.; Cordón, L.; Vidriales, M.-B.; Burgos, L.; Flores-Montero, J.; Sanoja-Flores, L.; Lopez-Anglada, L.; et al. Measurable Residual Disease by Next-Generation Flow Cytometry in Multiple Myeloma. J. Clin. Oncol. 2020, 38, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Swedin, A.; Lenhoff, S.; Olofsson, T.; Thuresson, B.; Westin, J. Clinical utility of immunoglobulin heavy chain gene rearrangement identification for tumour cell detection in multiple myeloma. Br. J. Haematol. 1998, 103, 1145–1151. [Google Scholar] [CrossRef]
- Bakkus, M.H.C.; Bouko, Y.; Samson, D.D.; Apperley, J.J.; Thielemans, K.M.; Van Camp, B.; Benner, A.A.; Goldschmidt, H.H.; Moos, M.M.; Cremer, F.F. Post-transplantation tumour load in bone marrow, as assessed by quantitative ASO-PCR, is a prognostic parameter in multiple myeloma. Br. J. Haematol. 2004, 126, 665–674. [Google Scholar] [CrossRef]
- Martínez-Sánchez, P.; Montejano, L.; Sarasquete, M.E.; Garcia-Sanz, R.; Fernández-Redondo, E.; Ayala, R.; Montalbán, M.A.; Martínez, R.; Laraña, J.G.; Alegre, A.; et al. Evaluation of minimal residual disease in multiple myeloma patients by fluorescent-polymerase chain reaction: The prognostic impact of achieving molecular response. Br. J. Haematol. 2008, 142, 766–774. [Google Scholar] [CrossRef]
- Putkonen, M.; Kairisto, V.; Juvonen, V.; Pelliniemi, T.-T.; Rauhala, A.; Itälä-Remes, M.; Remes, K. Depth of response assessed by quantitative ASO-PCR predicts the outcome after stem cell transplantation in multiple myeloma. Eur. J. Haematol. 2010, 85, 416–423. [Google Scholar] [CrossRef]
- Korthals, M.; Sehnke, N.; Kronenwett, R.; Bruns, I.; Mau, J.; Zohren, F.; Haas, R.; Kobbe, G.; Fenk, R. The Level of Minimal Residual Disease in the Bone Marrow of Patients with Multiple Myeloma before High-Dose Therapy and Autologous Blood Stem Cell Transplantation Is an Independent Predictive Parameter. Biol. Blood Marrow Transplant. 2012, 18, 423–431. [Google Scholar] [CrossRef]
- Ferrero, S.; Ladetto, M.; Drandi, D.; Cavallo, F.; Genuardi, E.; Urbano, M.; Caltagirone, S.; Grasso, M.; Rossini, F.; Guglielmelli, T.; et al. Long-term results of the GIMEMA VEL-03-096 trial in MM patients receiving VTD consolidation after ASCT: MRD kinetics’ impact on survival. Leukemia 2014, 29, 689–695. [Google Scholar] [CrossRef]
- Gambella, M.; Omedé, P.; Spada, S.; Muccio, V.E.; Gilestro, M.; Saraci, E.; Grammatico, S.; LaRocca, A.; Conticello, C.; Bernardini, A.; et al. Minimal residual disease by flow cytometry and allelic-specific oligonucleotide real-time quantitative polymerase chain reaction in patients with myeloma receiving lenalidomide maintenance: A pooled analysis. Cancer 2018, 125, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vega, B.; Alonso, R.; Cuenca, I.; Barrio, S.; Ruiz-Heredia, Y.; Marin, C.; Rapado, I.; Jimenez, C.; Aguirre, X.; Ayala, R.M.; et al. Prognostic Impact of Molecular Response Assessed by Next-Generation Sequencing in a Large Cohort of Multiple Myeloma Patients. Blood 2016, 128, 3283. [Google Scholar] [CrossRef]
- Schinke, C.; Hoering, A.; Wang, H.; Carlton, V.; Thanandrarajan, S.; Deshpande, S.; Patel, P.; Molnar, G.; Susanibar, S.; Mohan, M.; et al. The Prognostic Value of the Depth of Response in Multiple Myeloma Depends on the Time of Assessment, Risk Status and Molecular Subtype. Haematologica 2017, 102, e313–e316. [Google Scholar] [CrossRef]
- Martinez-Lopez, J.; Sanchez-Vega, B.; Barrio, S.; Cuenca, I.; Ruiz-Heredia, Y.; Alonso, R.; Rapado, I.; Marin, C.; Cedena, M.-T.; Paiva, B.D.L.; et al. Analytical and clinical validation of a novel in-house deep-sequencing method for minimal residual disease monitoring in a phase II trial for multiple myeloma. Leukemia 2017, 31, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Perrot, A.; Lauwers-Cances, V.; Corre, J.; Robillard, N.; Hulin, C.; Chretien, M.-L.; Dejoie, T.; Maheo, S.; Stoppa, A.-M.; Pegourie, B.; et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood 2018, 132, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Avet-Loiseau, H.; San-Miguel, J.F.; Casneuf, T.; Iida, S.; Lonial, S.; Usmani, S.Z.; Spencer, A.; Moreau, P.; Plesner, T.; Weisel, K.; et al. Evaluation of Sustained Minimal Residual Disease (MRD) Negativity in Relapsed/Refractory Multiple Myeloma (RRMM) Patients (Pts) Treated with Daratumumab in Combination with Lenalidomide Plus Dexamethasone (D-Rd) or Bortezomib Plus Dexamethasone (D-Vd): Analysis of Pollux and Castor. Blood 2018, 132, 3272. [Google Scholar] [CrossRef]
- Bahlis, N.; Facon, T.; Usmani, S.Z.; Kumar, S.K.; Plesner, D.T.; Orlowski, R.Z.; Touzeau, C.; Basu, M.S.; Nahi, H.; Hulin, C.; et al. Daratumumab Plus Lenalidomide and Dexamethasone (D-Rd) Versus Lenalidomide and Dexamethasone (Rd) in Patients with Newly Diagnosed Multiple Myeloma (NDMM) Ineligible for Transplant: Updated Analysis of Maia. Blood 2019, 134, 1875. [Google Scholar] [CrossRef]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef]
- Chari, A.; Martinez-Lopez, J.; Mateos, M.-V.; Bladé, J.; Benboubker, L.; Oriol, A.; Arnulf, B.; Rodriguez-Otero, P.; Pineiro, L.; Jakubowiak, A.; et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood 2019, 134, 421–431. [Google Scholar] [CrossRef]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.P.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef]
- Van De Donk, N.W.C.J.; Richardson, P.G.; Malavasi, F. CD38 antibodies in multiple myeloma: Back to the future. Blood 2018, 131, 13–29. [Google Scholar] [CrossRef]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Mikhael, J. Treatment Options for Triple-class Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Radocha, J.; van de Donk, N.; Weisel, K. Monoclonal Antibodies and Antibody Drug Conjugates in Multiple Myeloma. Cancers 2021, 13, 1571. [Google Scholar] [CrossRef]
- Zhou, X.; Einsele, H.; Danhof, S. Bispecific Antibodies: A New Era of Treatment for Multiple Myeloma. J. Clin. Med. 2020, 9, 2166. [Google Scholar] [CrossRef] [PubMed]
- Mikkilineni, L.; Kochenderfer, J.N. CAR T cell therapies for patients with multiple myeloma. Nat. Rev. Clin. Oncol. 2020, 18, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Heltai, S.; Ghia, P.; Scarfo’, L. Relevance of Minimal R W.; Du, J.; Jiang, H.; Cheng, Z.; Wei, R. Esidual Disease in the Era of Targeted Agents. Cancer J. 2019, 25, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulos, I.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Tsitsilonis, O.E.; Terpos, E. Minimal Residual Disease in Multiple Myeloma: Current Landscape and Future Applications with Immunotherapeutic Approaches. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Mohyuddin, G.R.; Rooney, A.; Balmaceda, N.; Aziz, M.; Sborov, D.W.; McClune, B.; Kumar, S.K. Chimeric antigen receptor T-cell therapy in multiple myeloma: A systematic review and meta-analysis of 950 patients. Blood Adv. 2021, 5, 1097–1101. [Google Scholar] [CrossRef]
- Ramos, C.A.; Savoldo, B.; Torrano, V.; Ballard, B.; Zhang, H.; Dakhova, O.; Liu, E.; Carrum, G.; Kamble, R.T.; Gee, A.P.; et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J. Clin. Investig. 2016, 126, 2588–2596. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti–B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018, 36, 2267–2280. [Google Scholar] [CrossRef]
- Hu, Y.; Yanlei, Z.; Wei, G.; Hong, C.A.; Huang, H. Potent Anti-Tumor Activity of Bcma CAR-T Therapy Against Heavily Treated Multiple Myeloma and Dynamics of Immune Cell Subsets Using Single-Cell Mass Cytometry. Blood 2019, 134, 1859. [Google Scholar] [CrossRef]
- Li, C.; Mei, H.; Hu, Y.; Guo, T.; Liu, L.; Jiang, H.; Tang, L.; Wu, Y.; Ai, L.; Deng, J.; et al. A Bispecific CAR-T Cell Therapy Targeting Bcma and CD38 for Relapsed/Refractory Multiple Myeloma: Updated Results from a Phase 1 Dose-Climbing Trial. Blood 2019, 134, 930. [Google Scholar] [CrossRef]
- Yan, Z.; Cao, J.; Cheng, H.; Qiao, J.; Zhang, H.; Wang, Y.; Shi, M.; Lan, J.; Fei, X.; Jin, L.; et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase 2 trial. Lancet Haematol. 2019, 6, e521–e529. [Google Scholar] [CrossRef]
- Garfall, A.L.; Cohen, A.D.; Lacey, S.F.; Tian, L.; Hwang, W.-T.; Vogl, D.T.; Waxman, A.; Lancaster, E.; Nelson, A.M.; Ferthio, R.; et al. Combination anti-BCMA and anti-CD19 CAR T cells as consolidation of response to prior therapy in multiple myeloma. Blood 2019, 134 (Suppl. 1), 1863. [Google Scholar] [CrossRef]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-Y.; Zhao, W.-H.; Liu, J.; Chen, Y.-X.; Cao, X.-M.; Yang, Y.; Zhang, Y.-L.; Wang, F.-X.; Zhang, P.-Y.; Lei, B.; et al. Long-Term Follow-up of a Phase 1, First-in-Human Open-Label Study of LCAR-B38M, a Structurally Differentiated Chimeric Antigen Receptor T (CAR-T) Cell Therapy Targeting B-Cell Maturation Antigen (BCMA), in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2019, 134, 579. [Google Scholar] [CrossRef]
- Fu, S.W.; Du, J.; Jiang, H.; Cheng, Z.; Wei, R.; Yu, K.; Jiang, S.; He, F.; Fang, H.; Liu, Y.; et al. Efficacy and Safety of CAR-T Therapy with Safety Switch Targeting Bcma for Patients with Relapsed/Refractory Multiple Myeloma in a Phase 1 Clinical Study. Blood 2019, 134, 3154. [Google Scholar] [CrossRef]
- Cowan, A.J.; Pont, M.; Duke Sather, B.; Turtle, C.J.; Till, B.G.; Nagengast, A.M.; Libby, E.N.; Becker, P.S.; Coffey, D.G.; Tuazon, S.A.; et al. Efficacy and safety of fully human BCMA CAR T cells in combination with a gamma secretase inhibitor to increase BCMA surface expression in patients with relapsed or refractory multiple myeloma. Blood 2019, 134 (Suppl. 1), 204. [Google Scholar] [CrossRef]
- Lin, Y.; Raje, N.S.; Berdeja, J.G.; Siegel, D.S.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Massaro, M.; et al. Idecabtagene Vicleucel (ide-cel, bb2121), a BCMA-Directed CAR T Cell Therapy, in Patients with Relapsed and Refractory Multiple Myeloma: Updated Results from Phase 1 CRB-401 Study. Blood 2020, 136, 26–27. [Google Scholar] [CrossRef]
- Hao, S.; Jin, J.; Jiang, S.; Li, Z.; Zhang, W.; Yang, M.; Yu, K.; Wang, W.; Chen, L.; Meng, H.; et al. Two-Year Follow-up of Investigator-Initiated Phase 1 Trials of the Safety and Efficacy of Fully Human Anti-Bcma CAR T Cells (CT053) in Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 27–28. [Google Scholar] [CrossRef]
- Madduri, D.; Berdeja, J.G.; Usmani, M.S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, M.A.K.; Hari, M.P.; Htut, M.; O’Donnell, E.; et al. CARTITUDE-1: Phase 1b/2 Study of Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T Cell Therapy, in Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 22–25. [Google Scholar] [CrossRef]
- Kumar, S.K.; Baz, R.C.; Orlowski, M.R.Z.; Anderson, J.L.D.; Ma, H.; Shrewsbury, M.A.; Croghan, M.K.A.; Bilgi, M.M.; Kansagra, A.; Kapoor, P.; et al. Results from Lummicar-2: A Phase 1b/2 Study of Fully Human B-Cell Maturation Antigen-Specific CAR T Cells (CT053) in Patients with Relapsed and/or Refractory Multiple Myeloma. Blood 2020, 136, 28–29. [Google Scholar] [CrossRef]
- Jiang, H.; Dong, B.; Gao, L.; Liu, L.; Ge, J.; He, A.; Du, J.J.; Li, L.; Lu, J.; Chen, X.; et al. Clinical Results of a Multicenter Study of the First-in-Human Dual BCMA and CD19 Targeted Novel Platform Fast CAR-T Cell Therapy for Patients with Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 25–26. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; San-Miguel, J.; Casneuf, T.; Iida, S.; Lonial, S.; Usmani, S.Z.; Spencer, A.; Moreau, P.; Plesner, T.; Weisel, K.; et al. Evaluation of Sustained Minimal Residual Disease Negativity with Daratumumab-Combination Regimens in Relapsed and/or Refractory Multiple Myeloma: Analysis of POLLUX and CASTOR. J. Clin. Oncol. 2021, 39, 1139–1149. [Google Scholar] [CrossRef]
- Rawstron, A.C.; De Tute, R.M.; Haughton, J.; Owen, R.G. Measuring disease levels in myeloma using flow cytometry in combination with other laboratory techniques: Lessons from the past 20 years at the Leeds Haematological Malignancy Diagnostic Service. Cytom. Part. B Clin. Cytom. 2015, 90, 54–60. [Google Scholar] [CrossRef]
- Mailankody, S.; Korde, N.; Lesokhin, A.M.; Lendvai, N.; Hassoun, H.; Stetler-Stevenson, M.; Landgren, O. Minimal residual disease in multiple myeloma: Bringing the bench to the bedside. Nat. Rev. Clin. Oncol. 2015, 12, 286–295. [Google Scholar] [CrossRef]
- Flores-Montero, J.; De Tute, R.; Paiva, B.D.L.; Perez, J.J.; Böttcher, S.; Wind, H.; Sanoja, L.; Puig, N.; Lecrevisse, Q.; Vidriales, M.-B.; et al. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytom. Part. B Clin. Cytom. 2015, 90, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Lahuerta, J.-J.; Paiva, B.; Vidriales, M.-B.; Cordón, L.; Cedena, M.-T.; Puig, N.; Martinez-Lopez, J.; Rosiñol, L.; Gutierrez, N.; Martín-Ramos, M.-L.; et al. Depth of Response in Multiple Myeloma: A Pooled Analysis of Three PETHEMA/GEM Clinical Trials. J. Clin. Oncol. 2017, 35, 2900–2910. [Google Scholar] [CrossRef] [PubMed]
- Robillard, N.; Béné, M.C.; Moreau, P.; Wuillème, S. A single-tube multiparameter seven-colour flow cytometry strategy for the detection of malignant plasma cells in multiple myeloma. Blood Cancer J. 2013, 3, e134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bruinink, D.H.O.; Oliva, S.; Rihova, L.; Schmitz, A.; Gilestro, M.; Marvelde, J.T.; Kralova, R.; Høholt, H.; Broijl, A.; Johnsen, H.E.; et al. Standardization of flow cytometric minimal residual disease assessment in international clinical trials. A feasibility study from the European Myeloma Network. Haematologica 2020, 106, 1496–1499. [Google Scholar] [CrossRef]
- Roshal, M.; Flores-Montero, J.A.; Gao, Q.; Koeber, M.; Wardrope, J.; Durie, B.G.M.; Dogan, A.; Orfao, A.; Landgren, O. MRD detection in multiple myeloma: Comparison between MSKCC 10-color single-tube and EuroFlow 8-color 2-tube methods. Blood Adv. 2017, 1, 728–732. [Google Scholar] [CrossRef]
- Royston, D.J.; Gao, Q.; Nguyen, N.; Maslak, P.; Dogan, A.; Roshal, M. Single-Tube 10-Fluorochrome Analysis for Efficient Flow Cytometric Evaluation of Minimal Residual Disease in Plasma Cell Myeloma. Am. J. Clin. Pathol. 2016, 146, 41–49. [Google Scholar] [CrossRef]
- Landgren, O.; Gormley, N.; Turley, D.; Owen, R.G.; Rawstron, A.; Paiva, B.D.L.; Barnett, D.; Arroz, M.; Wallace, P.; Durie, B.; et al. Flow cytometry detection of minimal residual disease in multiple myeloma: Lessons learned at FDA-NCI roundtable symposium. Am. J. Hematol. 2014, 89, 1159–1160. [Google Scholar] [CrossRef]
- Oldaker, T.A.; Wallace, P.K.; Barnett, D. Flow cytometry quality requirements for monitoring of minimal disease in plasma cell myeloma. Cytom. Part. B Clin. Cytom. 2015, 90, 40–46. [Google Scholar] [CrossRef]
- Yoroidaka, T.; Narita, K.; Takamatsu, H.; Fujisawa, M.; Nakao, S.; Matsue, K. Comparison of minimal residual disease detection in multiple myeloma between the DuraClone and EuroFlow methods. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Bene, M.C.; Robillard, N.; Moreau, P.; Wuilleme, S. Comparison of the Performance of Surface Alone or Surface Plus Cytoplasmic Approaches for the Assessment of Minimal Residual Disease in Multiparameter Flow Cytometry in Multiple Myeloma. Blood 2019, 134, 1799. [Google Scholar] [CrossRef]
- Dold, S.M.; Riebl, V.; Wider, D.; Follo, M.; Pantic, M.; Ihorst, G.; Duyster, J.; Zeiser, R.; Wäsch, R.; Engelhardt, M. Validated single-tube multiparameter flow cytometry approach for the assessment of minimal residual disease in multiple myeloma. Haematologica 2020, 105, e523. [Google Scholar] [CrossRef] [PubMed]
- Torlakovic, E.E.; Brynes, R.K.; Hyjek, E.; Lee, S.-H.; Kreipe, H.; Kremer, M.; McKenna, R.; Sadahira, Y.; Tzankov, A.; Reis, M.; et al. ICSH guidelines for the standardization of bone marrow immunohistochemistry. Int. J. Lab. Hematol. 2015, 37, 431–449. [Google Scholar] [CrossRef]
- Costa, L.J.; Derman, B.A.; Bal, S.; Sidana, S.; Chhabra, S.; Silbermann, R.; Ye, J.C.; Cook, G.; Cornell, R.F.; Holstein, S.A.; et al. International harmonization in performing and reporting minimal residual disease assessment in multiple myeloma trials. Leukemia 2020, 35, 18–30. [Google Scholar] [CrossRef]
- Chapman, M.A.; Lawrence, M.S.; Keats, J.; Cibulskis, K.; Sougnez, C.; Schinzel, A.C.; Harview, C.; Brunet, J.-P.; Ahmann, G.J.; Adli, M.; et al. Initial genome sequencing and analysis of multiple myeloma. Nature 2011, 471, 467–472. [Google Scholar] [CrossRef]
- Anonymous. ClonoSEQ Cleared for Residual Cancer Testing. Cancer Discov. 2018, 8, OF6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Langerak, A.W.; Groenen, P.J.T.A.; Brüggemann, M.; Beldjord, K.; Bellan, C.; Bonello, L.; Boone, E.; Carter, G.I.; Catherwood, M.; Davi, F.; et al. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia 2012, 26, 2159–2171. [Google Scholar] [CrossRef] [PubMed]
- Puig, N.; Sarasquete, M.E.; Balanzategui, A.; Martínez, J.; Paiva, B.D.L.; García, H.; Fumero, S.; Jiménez, C.; Alcoceba, M.; Chillon, M.C.; et al. Critical evaluation of ASO RQ-PCR for minimal residual disease evaluation in multiple myeloma. A comparative analysis with flow cytometry. Leukemia 2013, 28, 391–397. [Google Scholar] [CrossRef]
- Martinez-Lopez, J.; Lahuerta, J.J.; Pepin, F.; González, M.; Barrio, S.; Ayala, R.; Puig, N.; Montalban, M.A.; Paiva, B.D.L.; Weng, L.; et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood 2014, 123, 3073–3079. [Google Scholar] [CrossRef]
- Kumar, S.K.; Facon, T.; Usmani, S.Z.; Plesner, T.; Orlowski, R.Z.; Touzeau, C.; Basu, S.; Bahlis, N.J.; Goldschmidt, H.; O’Dwyer, M.E.; et al. Updated Analysis of Daratumumab Plus Lenalidomide and Dexamethasone (D-Rd) Versus Lenalidomide and Dexamethasone (Rd) in Patients with Transplant-Ineligible Newly Diagnosed Multiple Myeloma (NDMM): The Phase 3 Maia Study. Blood 2020, 136, 24–26. [Google Scholar] [CrossRef]
- Hillengass, J.; Moulopoulos, L.A.; Delorme, S.; Koutoulidis, V.; Mosebach, J.; Hielscher, T.; Drake, M.; Rajkumar, S.V.; Oestergaard, B.; Abildgaard, N.; et al. Whole-body computed tomography versus conventional skeletal survey in patients with multiple myeloma: A study of the International Myeloma Working Group. Blood Cancer J. 2017, 7, e599. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.; Imwg, O.B.O.T.; Terpos, E.; Comenzo, R.L.; Tosi, P.; Beksac, M.; Sezer, O.; Siegel, D.; Lokhorst, H.; Kumar, S.; et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia 2009, 23, 1545–1556. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Manasanch, E.E.; Landgren, O. Myeloma imaging: Time to move on! Leuk. Lymphoma 2015, 57, 1499–1500. [Google Scholar] [CrossRef]
- Manasanch, E.E. What to do with minimal residual disease testing in myeloma. Hematol. 2014 Am. Soc. Hematol. Program. Book 2019, 2019, 137–141. [Google Scholar] [CrossRef]
- Walker, R.; Barlogie, B.; Haessler, J.; Tricot, G.; Anaissie, E.; Shaughnessy, J.D., Jr.; Epstein, J.; Van Hemert, R.; Erdem, E.; Hoering, A.; et al. Magnetic Resonance Imaging in Multiple Myeloma: Diagnostic and Clinical Implications. J. Clin. Oncol. 2007, 25, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Hillengass, J.; Usmani, S.; Rajkumar, S.V.; Durie, B.G.M.; Mateos, M.-V.; Lonial, S.; Joao, C.; Anderson, K.C.; Garcia-Sanz, R.; Riva, E.; et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019, 20, e302–e312. [Google Scholar] [CrossRef]
- Jamet, B.; Zamagni, E.; Nanni, C.; Bailly, C.; Carlier, T.; Touzeau, C.; Michaud, A.-V.; Moreau, P.; Bodet-Milin, C.; Kraeber-Bodere, F. Functional Imaging for Therapeutic Assessment and Minimal Residual Disease Detection in Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 5406. [Google Scholar] [CrossRef] [PubMed]
- Messiou, C.; Kaiser, M. Whole body diffusion weighted MRI—A new view of myeloma. Br. J. Haematol. 2015, 171, 29–37. [Google Scholar] [CrossRef]
- Zamagni, E.; Tacchetti, P.; Barbato, S.; Cavo, M. Role of Imaging in the Evaluation of Minimal Residual Disease in Multiple Myeloma Patients. J. Clin. Med. 2020, 9, 3519. [Google Scholar] [CrossRef]
- Belotti, A.; Ribolla, R.; Cancelli, V.; Villanacci, A.; Angelini, V.; Ferrari, S.; Peli, A.; Cattaneo, C.; Chiarini, M.; Grazioli, L.; et al. External Validation of Diffusion Weighted Whole Body MRI (DW-MRI) Response Assessment Category (RAC) Criteria Proposed By the Myeloma Response Assessment and Diagnosis System (MY-RADS) Imaging Recommendations: Prognostic Role of Imaging Response after Transplant in Multiple Myeloma and Comparison with MRD Evaluation by Flow Cytometry. Blood 2020, 136, 41–42. [Google Scholar] [CrossRef]
- Gariani, J.; Westerland, O.; Natas, S.; Verma, H.; Cook, G.; Goh, V. Comparison of whole body magnetic resonance imaging (WBMRI) to whole body computed tomography (WBCT) or 18 F-fluorodeoxyglucose positron emission tomography/CT (18 F-FDG PET/CT) in patients with myeloma: Systematic review of diagnostic performance. Crit. Rev. Oncol. 2018, 124, 66–72. [Google Scholar] [CrossRef]
- Zamagni, E.; Patriarca, F.; Nanni, C.; Zannetti, B.; Englaro, E.; Pezzi, A.; Tacchetti, P.; Buttignol, S.; Perrone, G.; Brioli, A.; et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood 2011, 118, 5989–5995. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Mitchell, A.; Waheed, S.; Crowley, J.; Hoering, A.; Petty, N.; Brown, T.; Bartel, T.; Anaissie, E.; Van Rhee, F.; et al. Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy. Blood 2013, 121, 1819–1823. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Caillot, D.; Macro, M.; Karlin, L.; Garderet, L.; Facon, T.; Benboubker, L.; Escoffre-Barbe, M.; Stoppa, A.-M.; et al. Prospective Evaluation of Magnetic Resonance Imaging and [18F]Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography at Diagnosis and Before Maintenance Therapy in Symptomatic Patients With Multiple Myeloma Included in the IFM/DFCI 2009 Trial: Results of the IMAJEM Study. J. Clin. Oncol. 2017, 35, 2911–2918. [Google Scholar] [CrossRef] [PubMed]
- Rasch, S.; Lund, T.; Asmussen, J.; Nielsen, A.L.; Larsen, R.F.; Andersen, M.; Abildgaard, N. Multiple Myeloma Associated Bone Disease. Cancers 2020, 12, 2113. [Google Scholar] [CrossRef]
- Herrmann, K.; Lapa, C.; Wester, H.-J.; Schottelius, M.; Schiepers, C.; Eberlein, U.; Bluemel, C.; Keller, U.; Knop, S.; Kropf, S.; et al. Biodistribution and Radiation Dosimetry for the Chemokine Receptor CXCR4-Targeting Probe 68Ga-Pentixafor. J. Nucl. Med. 2015, 56, 410–416. [Google Scholar] [CrossRef]
- Pandit-Taskar, N. Functional Imaging Methods for Assessment of Minimal Residual Disease in Multiple Myeloma: Current Status and Novel ImmunoPET Based Methods. Semin. Hematol. 2018, 55, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Yokoyama, K.; Imoto, S.; Tojo, A. Role of Circulating Tumor DNA in Hematological Malignancy. Cancers 2021, 13, 2078. [Google Scholar] [CrossRef] [PubMed]
- Sanoja-Flores, L.; Flores-Montero, J.; Puig, N.; Contreras-Sanfeliciano, T.; Pontes, R.; Corral-Mateos, A.; García-Sánchez, O.; Díez-Campelo, M.; De Magalhães, R.J.P.; García-Martín, L.; et al. Blood monitoring of circulating tumor plasma cells by next generation flow in multiple myeloma after therapy. Blood 2019, 134, 2218–2222. [Google Scholar] [CrossRef]
- Mazzotti, C.; Buisson, L.; Maheo, S.; Perrot, A.; Chretien, M.-L.; Leleu, X.; Hulin, C.; Manier, S.; Hébraud, B.; Roussel, M.; et al. Myeloma MRD by deep sequencing from circulating tumor DNA does not correlate with results obtained in the bone marrow. Blood Adv. 2018, 2, 2811–2813. [Google Scholar] [CrossRef] [PubMed]
- Sanoja-Flores, L.; Flores-Montero, J.; Garcés, J.J.; Paiva, B.; Puig, N.; García-Mateo, A.; García-Sánchez, O.; Corral-Mateos, A.; Burgos, L.; Blanco, E.; et al. Next generation flow for minimally-invasive blood characterization of MGUS and multiple myeloma at diagnosis based on circulating tumor plasma cells (CTPC). Blood Cancer J. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Khong, T.; Ramachandran, M.; Chow, A.; Klarica, D.; Mai, L.; Walsh, S.; Broemeling, D.; Marziali, A.; Wiggin, M.; et al. Circulating tumour DNA analysis demonstrates spatial mutational heterogeneity that coincides with disease relapse in myeloma. Leukemia 2016, 31, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Manier, S.; Park, J.; Capelletti, M.; Bustoros, M.; Freeman, S.; Ha, G.; Rhoades, J.; Liu, C.J.; Huynh, D.; Reed, S.; et al. Whole-exome sequencing of cell-free DNA and circulating tumor cells in multiple myeloma. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Morley, R.; Khong, T.; Kalff, A.; Bergin, K.; Hocking, J.; Savvidou, I.; Bowen, K.M.; Ramachandran, M.; Choi, K.; et al. Monitoring tumour burden and therapeutic response through analysis of circulating tumour DNA and extracellular RNA in multiple myeloma patients. Leukemia 2019, 33, 2022–2033. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Sirdesai, S.; Chen, M.; Khong, T.; Spencer, A. Circulating Tumour DNA Analysis for Tumour Genome Characterisation and Monitoring Disease Burden in Extramedullary Multiple Myeloma. Int. J. Mol. Sci. 2018, 19, 1858. [Google Scholar] [CrossRef]
- Carlson, J.J.; Eckert, B.; Zimmerman, M. Cost-effectiveness of next-generation sequencing minimal residual disease testing during maintenance treatment for multiple myeloma. J. Clin. Oncol. 2019, 37, e19529. [Google Scholar] [CrossRef]
- Carlson, M.J.J.; Zimmermann, M.M.; Demaree, A.; Hewitt, T.; Eckert, B.; Nooka, M.A. Cost-Effectiveness of Implementing Clonoseq NGS-MRD Testing Using the Emory MRD Decision Protocol in Multiple Myeloma. Blood 2020, 136, 25–26. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, J.L.D.; Shah, N.; Jagannath, S.; Berdeja, J.G.; Lonial, S.; Raje, N.S.; Siegel, D.S.D.; Lin, Y.; Oriol, A.; et al. Idecabtagene vicleucel (ide-cel; bb2121), a BCMA-targeted CAR T-cell therapy, in patients with relapsed and refractory multiple myeloma (RRMM): Initial KarMMa results. J. Clin. Oncol. 2020, 38, 8503. [Google Scholar] [CrossRef]
- Landgren, O.; Rustad, E.H. Meeting report: Advances in minimal residual disease testing in multiple myeloma. Adv. Cell Gene Ther. 2018, 2, e26. [Google Scholar] [CrossRef][Green Version]
- Ubieto, A.J.; Paiva, B.; Puig, N.; Cedena, M.-T.; Martinez-Lopez, J.; Oriol, A.; Blanchard, M.-J.; Tamayo, R.R.; Sánchez, J.M.; Martinez, R.; et al. Validation of the IMWG standard response criteria in the PETHEMA/GEM2012MENOS65 study: Are these times of change? Blood 2021. [Google Scholar] [CrossRef]
| Laser Line | Robillard | Euroflow | MSKCC | Duraclone | Freiburg | |
|---|---|---|---|---|---|---|
| Violet | CD38H450 | CD138BV421 | CD138BV421 | CD81PB | CD38-nonME PB | CyIg λ AF405 |
| CD27BV510 | CD27BV510 | CD38BV510 | CD45KrO | CD19BV510 | ||
| CD27BV605 | ||||||
| Blue | CyIg λ FITC | CD38MEFITC | CD38MEFITC | CyIg κ FITC | CD81FITC | CyIg κ FITC |
| CD28 + CD56PE | CD56PE | CD56PE | CyIg λ PE | CD27PE | CD27PE | |
| Yellow/ Green | CD138PC5 | CD45PerCP-Cy5.5 | CD45PerCP-Cy5.5 | CD117PC5.5 | CD19PC5.5 | CD56 PerCP-Cy5.5 |
| CD19PE-Cy7 | CD19PE-Cy7 | CD19PE-Cy7 | CD19PC7 | CD200PC7 | CD38PE-Cy7 | |
| Red | CyIg κ APC | CD117APC | CyIg κ APC | CD138APC | CD138APC | CD138APC |
| CD56APC-R700 | ||||||
| CD45APC-H7 | CD81APC-A750 | CyIg κAPC750 | CD45APC-H7 | CD56APC-A750 | CD45APC-H7 | |
| Sensitivity | 10−5 | 10−6 | 10−6 | 10−5 | 10−5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo-Pérez, C.; Sola, M.; Teruel-Montoya, R.; García-Malo, M.D.; Ortuño, F.J.; Vicente, V.; de Arriba, F.; Jerez, A. Minimal Residual Disease in Multiple Myeloma: Something Old, Something New. Cancers 2021, 13, 4332. https://doi.org/10.3390/cancers13174332
Bravo-Pérez C, Sola M, Teruel-Montoya R, García-Malo MD, Ortuño FJ, Vicente V, de Arriba F, Jerez A. Minimal Residual Disease in Multiple Myeloma: Something Old, Something New. Cancers. 2021; 13(17):4332. https://doi.org/10.3390/cancers13174332
Chicago/Turabian StyleBravo-Pérez, Carlos, María Sola, Raúl Teruel-Montoya, María Dolores García-Malo, Francisco José Ortuño, Vicente Vicente, Felipe de Arriba, and Andrés Jerez. 2021. "Minimal Residual Disease in Multiple Myeloma: Something Old, Something New" Cancers 13, no. 17: 4332. https://doi.org/10.3390/cancers13174332
APA StyleBravo-Pérez, C., Sola, M., Teruel-Montoya, R., García-Malo, M. D., Ortuño, F. J., Vicente, V., de Arriba, F., & Jerez, A. (2021). Minimal Residual Disease in Multiple Myeloma: Something Old, Something New. Cancers, 13(17), 4332. https://doi.org/10.3390/cancers13174332






