Longitudinal Monitoring of Simulated Interstitial Fluid Pressure for Pancreatic Ductal Adenocarcinoma Patients Treated with Stereotactic Body Radiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
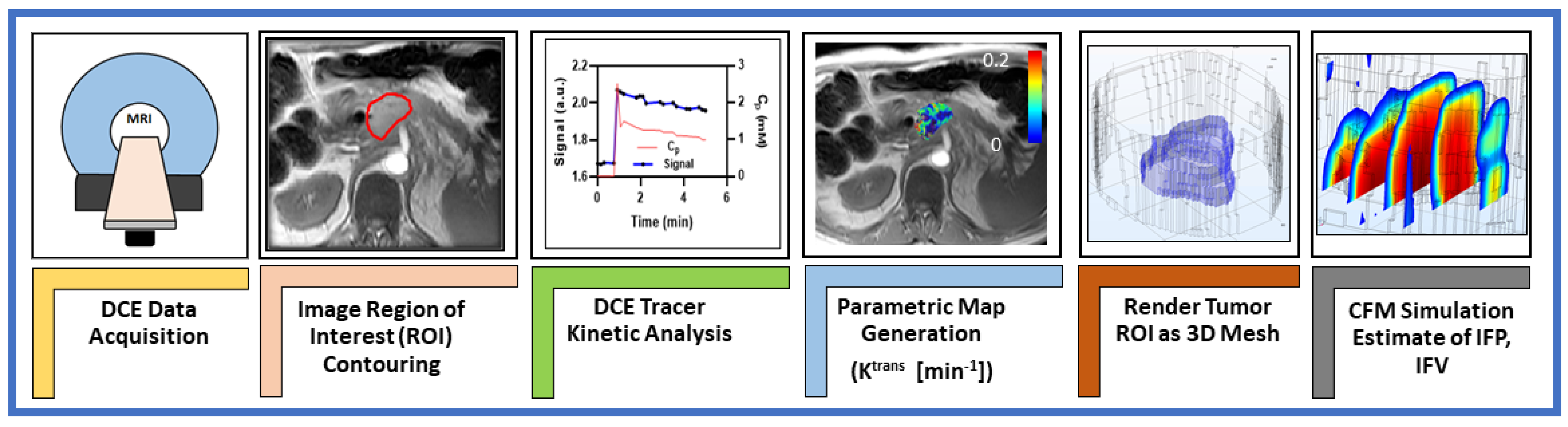
2. Materials and Methods
2.1. Patients
2.2. DCE Data Acquisition
2.3. DCE Data Analysis
2.4. Regions of Interest (ROIs)
2.5. Computational Fluid Modeling Theory and Analysis
2.6. Statistical Analysis
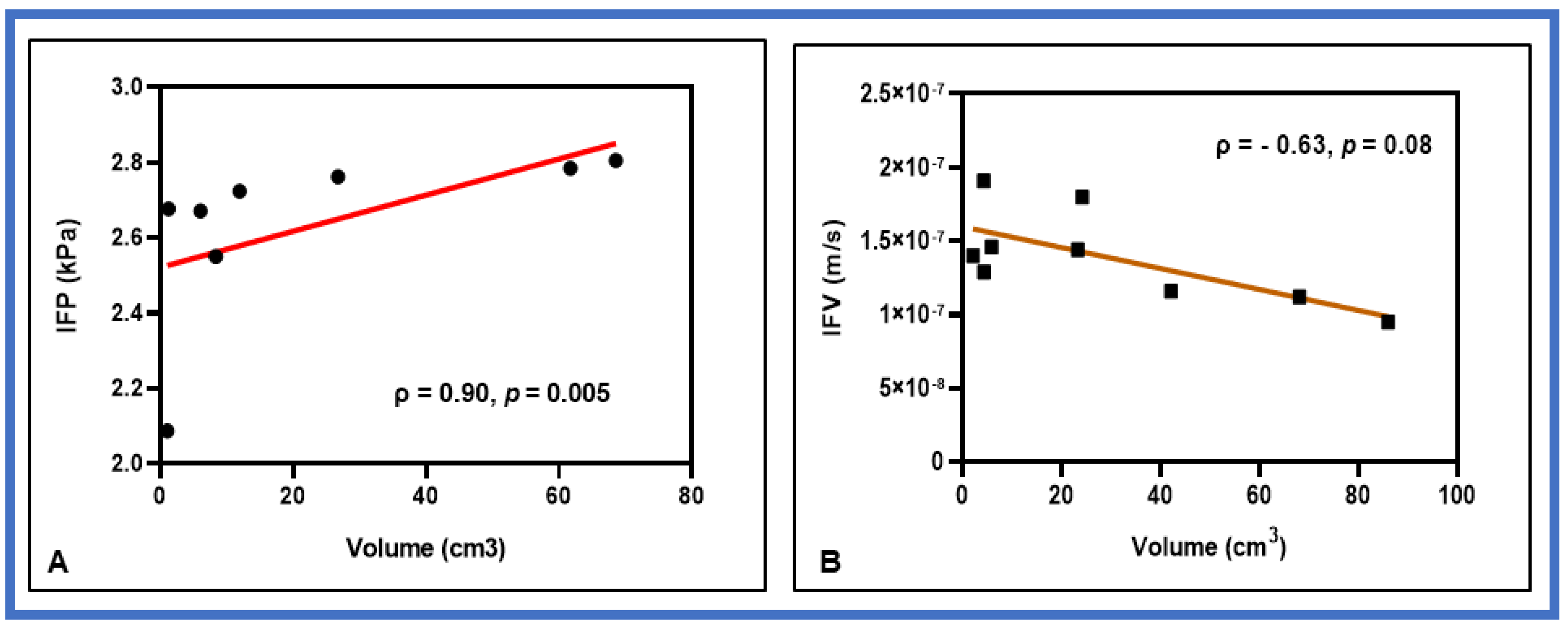
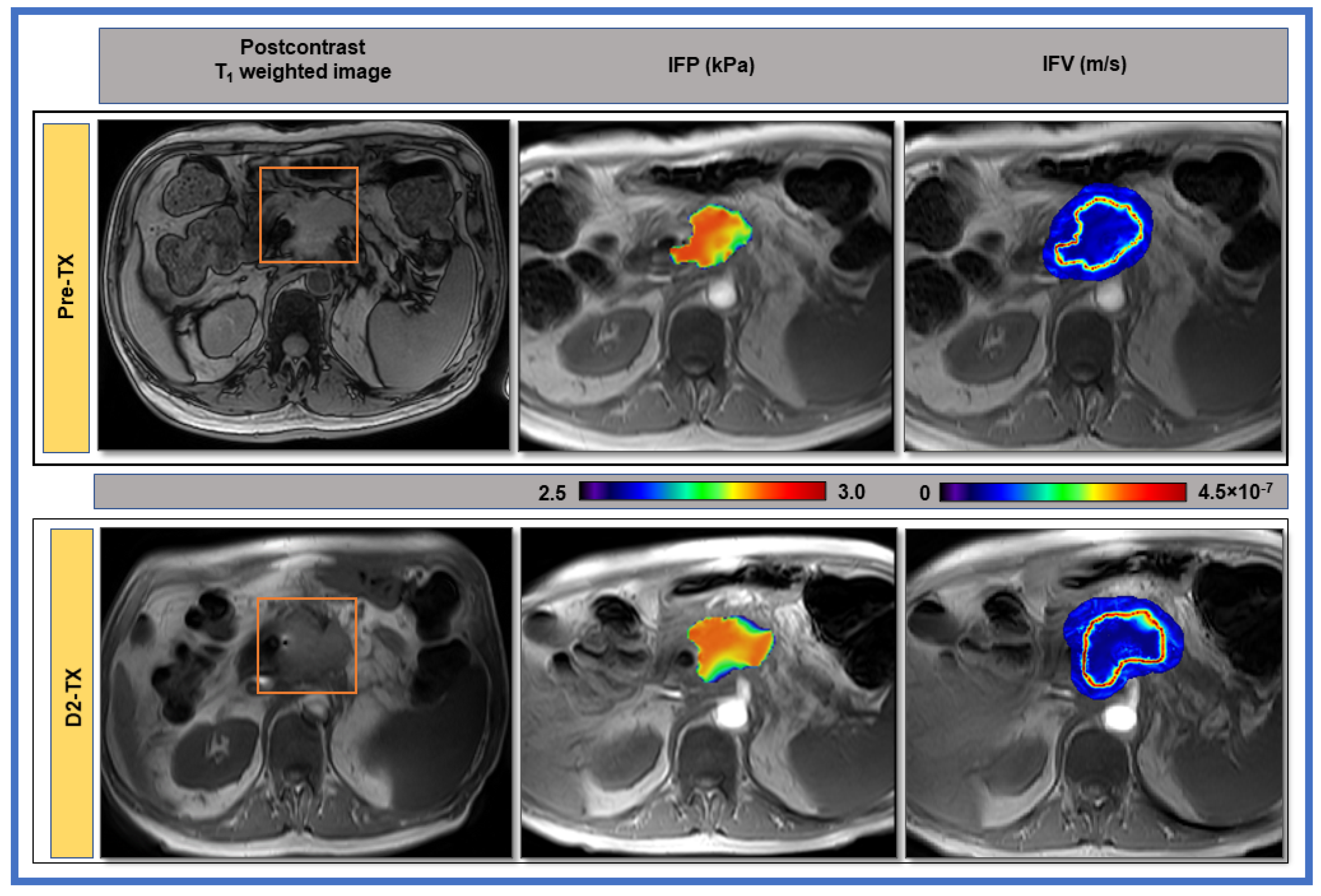
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Orth, M.; Metzger, P.; Gerum, S.; Mayerle, J.; Schneider, G.; Belka, C.; Schnurr, M.; Lauber, K. Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.L.; Zhou, L.; Lu, J.; Wang, Y.Z.; Liu, C.X.; You, L.; Guo, J.C. Stroma-Targeting Therapy in Pancreatic Cancer: One Coin With Two Sides? Front. Oncol. 2020, 10, 576399. [Google Scholar] [CrossRef] [PubMed]
- Nieskoski, M.D.; Marra, K.; Gunn, J.R.; Hoopes, P.J.; Doyley, M.M.; Hasan, T.; Trembly, B.S.; Pogue, B.W. Collagen complexity spatially defines microregions of total tissue pressure in pancreatic cancer. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Martin, J.D.; Stylianopoulos, T. The Role of Mechanical Forces in Tumor Growth and Therapy. Annu. Rev. Biomed. Eng. 2014, 16, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: From mathematical modeling to bench to bedside. Trends Cancer 2018, 4, 292–319. [Google Scholar] [CrossRef]
- Weniger, M.; Honselmann, K.C.; Liss, A.S. The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship. Cancers 2018, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.; Papandreou, I.; Denko, N. Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol. Cancer Res. 2006, 4, 61–70. [Google Scholar] [CrossRef]
- Agarwal, B.; Correa, A.M.; Ho, L. Survival in pancreatic carcinoma based on tumor size. Pancreas 2008, 36, e15–e20. [Google Scholar] [CrossRef]
- Krishnan, S.; Chadha, A.S.; Suh, Y.; Chen, H.C.; Rao, A.; Das, P.; Minsky, B.D.; Mahmood, U.; Delclos, M.E.; Sawakuchi, G.O.; et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int. J. Radiat. Oncol. 2016, 94, 755–765. [Google Scholar] [CrossRef]
- Rudra, S.; Jiang, N.; Rosenberg, S.A.; Olsen, J.R.; Parikh, P.J.; Bassetti, M.F.; Lee, P. High Dose Adaptive MRI Guided Radiation Therapy Improves Overall Survival of Inoperable Pancreatic Cancer. Int. J. Radiat. Oncol. 2017, 99, E184. [Google Scholar] [CrossRef][Green Version]
- Reyngold, M.; Parikh, P.; Crane, C.H. Ablative radiation therapy for locally advanced pancreatic cancer: Techniques and results. Radiat. Oncol. 2019, 14, 95. [Google Scholar] [CrossRef]
- Harrington, K.A.; Shukla-Dave, A.; Paudyal, R.; Do, R.K. MRI of the Pancreas. J. Magn. Reson. Imaging 2021, 53, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Zahra, M.A.; Hollingsworth, K.G.; Sala, E.; Lomas, D.J.; Tan, L.T. Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncol. 2007, 8, 63–74. [Google Scholar] [CrossRef]
- Dalah, E.; Tai, A.; Oshima, K.; Paulson, E.S.; Hall, W.A.; Erickson, B.A.; Li, A. Correlation of ADC With Pathological Treatment Response for Radiation Therapy of Pancreatic Cancer. Int. J. Radiat. Oncol. 2015, 93, S99. [Google Scholar] [CrossRef]
- Haider, M.A.; Sitartchouk, I.; Roberts, T.P.L.; Fyies, A.; Hashmi, A.T.; Milosevic, M. Correlations between dynamic contrast-enhanced magnetic resonance imaging-derived measures of tumor microvasculature and interstitial fluid pressure in patients with cervical cancer. J Magn Reson Imaging 2007, 25, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Mayr, N.A.; Wang, J.Z.; Zhang, D.; Grecula, J.C.; Lo, S.S.; Jaroura, D.; Montebello, J.; Zhang, H.; Li, K.; Lu, L.; et al. Longitudinal changes in tumor perfusion pattern during the radiation therapy course and its clinical impact in cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Zhou, J.; Lindsay, P.; Foltz, W.; Cheung, M.; Siddiqui, I.; Hosni, A.; Amir, A.E.; Kim, J.; Hill, R.P.; et al. Quantifying Reoxygenation in Pancreatic Cancer During Stereotactic Body Radiotherapy. Sci. Rep. 2020, 10, 1638. [Google Scholar] [CrossRef] [PubMed]
- Ewing, J.R.; Bagher-Ebadian, H. Model selection in measures of vascular parameters using dynamic contrast-enhanced MRI: Experimental and clinical applications. NMR Biomed. 2013, 26, 1028–1041. [Google Scholar] [CrossRef]
- Akisik, M.F.; Sandrasegaran, K.; Bu, G.; Lin, C.; Hutchins, G.D.; Chiorean, E.G. Pancreatic cancer: Utility of dynamic contrast-enhanced MR imaging in assessment of antiangiogenic therapy. Radiology 2010, 256, 441–449. [Google Scholar] [CrossRef]
- Kim, H.; Morgan, D.E.; Schexnailder, P.; Navari, R.M.; Williams, G.R.; Bart Rose, J.; Li, Y.; Paluri, R. Accurate Therapeutic Response Assessment of Pancreatic Ductal Adenocarcinoma Using Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Imaging With a Point-of-Care Perfusion Phantom: A Pilot Study. Investig. Radiol. 2019, 54, 16–22. [Google Scholar] [CrossRef]
- Do, R.K.; Reyngold, M.; Paudyal, R.; Oh, J.H.; Konar, A.S.; LoCastro, E.; Goodman, K.A.; Shukla-Dave, A. Diffusion-Weighted and Dynamic Contrast-Enhanced MRI Derived Imaging Metrics for Stereotactic Body Radiotherapy of Pancreatic Ductal Adenocarcinoma: Preliminary Findings. Tomogr. A J. Imaging Res. 2020, 6, 261–271. [Google Scholar] [CrossRef]
- Cao, J.; Pickup, S.; Clendenin, C.; Blouw, B.; Choi, H.; Kang, D.; Rosen, M.; O’Dwyer, P.J.; Zhou, R. Dynamic Contrast-enhanced MRI Detects Responses to Stroma-directed Therapy in Mouse Models of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2019, 25, 2314–2322. [Google Scholar] [CrossRef] [PubMed]
- Starling, E. Starling EH. On the absorption of fluids from connective tissue spaces. J. Physiol. 1896, 19, 312–326. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Boucher, Y.; Ferrone, C.R.; Roberge, S.; Martin, J.D.; Stylianopoulos, T.; Bardeesy, N.; DePinho, R.A.; Padera, T.P.; Munn, L.L.; et al. Compression of Pancreatic Tumor Blood Vessels by Hyaluronan Is Caused by Solid Stress and Not Interstitial Fluid Pressure. Cancer Cell 2014, 26, 14–15. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Hingorani, S.R. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br. J. Cancer 2013, 108, 1–8. [Google Scholar] [CrossRef]
- Ewing, J.R.; Nagaraja, T.N.; Aryal, M.P.; Keenan, K.A.; Elmghirbi, R.; Bagher-Ebadian, H.; Panda, S.; Lu, M.; Mikkelsen, T.; Cabral, G.; et al. Peritumoral tissue compression is predictive of exudate flux in a rat model of cerebral tumor: An MRI study in an embedded tumor. NMR Biomed. 2015, 28, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef]
- Fadnes, H.O.; Reed, R.K.; Aukland, K. Interstitial Fluid Pressure in Rats Measured with a Modified Wick Technique. Microvasc. Res. 1977, 14, 27–36. [Google Scholar] [CrossRef]
- Wiig, H. Comparison of methods for measurement of interstitial fluid pressure in cat skin/subcutis and muscle. Am. J. Physiol. 1985, 249, H929–H944. [Google Scholar] [CrossRef]
- DiResta, G.R.; Lee, J.; Larson, S.M.; Arbit, E. Characterization of neuroblastoma xenograft in rat flank. I. Growth, interstitial fluid pressure, and interstitial fluid velocity distribution profiles. Microvasc. Res. 1993, 46, 158–177. [Google Scholar] [CrossRef]
- Boucher, Y.; Salehi, H.; Witwer, B.; Harsh, G.R.; Jain, R.K. Interstitial fluid pressure in intracranial tumours in patients and in rodents. Brit. J. Cancer 1997, 75, 829–836. [Google Scholar] [CrossRef]
- DuFort, C.C.; DelGiorno, K.E.; Carlson, M.A.; Osgood, R.J.; Zhao, C.M.; Huang, Z.D.; Thompson, C.B.; Connor, R.J.; Thanos, C.D.; Brockenbrough, J.S.; et al. Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase. Biophys. J. 2016, 110, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, R.; Leunig, A.; Leunig, M.; Feyh, J. Importance of increased interstitial fluid pressure in therapy of malignant tumors of the head-neck area. Laryngorhinootologie 1993, 72, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, S.D.; Nelson, L. Interstitial fluid pressure in breast cancer, benign breast conditions, and breast parenchyma. Ann. Surg. Oncol. 1994, 1, 333–338. [Google Scholar] [CrossRef]
- Milosevic, M.; Fyles, A.; Hedley, D.; Pintilie, M.; Levin, W.; Manchul, L.; Hill, R. Interstitial fluid pressure predicts survival in patients with cervix cancer independent of clinical prognostic factors and tumor—Oxygen measurements. Cancer Res. 2001, 61, 6400–6405. [Google Scholar] [PubMed]
- Yeo, S.G.; Kim, J.S.; Cho, M.J.; Kim, K.H.; Kim, J.S. Interstitial Fluid Pressure as a Prognostic Factor in Cervical Cancer Following Radiation Therapy. Clin. Cancer Res. 2009, 15, 6201–6207. [Google Scholar] [CrossRef] [PubMed]
- Fyles, A.; Pintilie, M.; Hedley, D.; Bristow, R.; Wouters, B.; Hill, R.; Milosevic, M. High interstitial fluid pressure (IFP) and hypoxia as biomarkers of cisplatin chemoradiation response in advanced cervix cancer. J. Clin. Oncol. 2009, 27, 5584. [Google Scholar] [CrossRef]
- Wegner, C.S.; Hauge, A.; Gaustad, J.V.; Andersen, L.M.K.; Simonsen, T.G.; Galappathi, K.; Rofstad, E.K. Dynamic contrast-enhanced MRI of the microenvironment of pancreatic adenocarcinoma xenografts. Acta Oncol 2017, 56, 1754–1762. [Google Scholar] [CrossRef]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Mapping Physical Tumor Microenvironment and Drug Delivery. Clin. Cancer Res. 2019, 25, 2024–2026. [Google Scholar] [CrossRef]
- Elmghirbi, R.; Nagaraja, T.N.; Brown, S.L.; Keenan, K.A.; Panda, S.; Cabral, G.; Bagher-Ebadian, H.; Divine, G.W.; Lee, I.Y.; Ewing, J.R. Toward a noninvasive estimate of interstitial fluid pressure by dynamic contrast-enhanced MRI in a rat model of cerebral tumor. Magn. Reson. Med. 2018, 80, 2040–2052. [Google Scholar] [CrossRef]
- Baxter, L.T.; Jain, R.K. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc. Res. 1989, 37, 77–104. [Google Scholar] [CrossRef]
- Zhao, J.B.; Salmon, H.; Sarntinoranont, M. Effect of heterogeneous vasculature on interstitial transport within a solid tumor. Microvasc. Res. 2007, 73, 224–236. [Google Scholar] [CrossRef]
- Pishko, G.L.; Astary, G.W.; Mareci, T.H.; Sarntinoranont, M. Sensitivity Analysis of an Image-Based Solid Tumor Computational Model with Heterogeneous Vasculature and Porosity. Ann. Biomed. Eng. 2011, 39, 2360–2373. [Google Scholar] [CrossRef]
- LoCastro, E.; Paudyal, R.; Mazaheri, Y.; Hatzoglou, V.; Oh, J.H.; Lu, Y.G.; Konar, A.S.; vom Eigen, K.; Ho, A.; Ewing, J.R.; et al. Computational Modeling of Interstitial Fluid Pressure and Velocity in Head and Neck Cancer Based on Dynamic Contrast-Enhanced Magnetic Resonance Imaging: Feasibility Analysis. Tomography 2020, 6, 129–138. [Google Scholar] [CrossRef]
- Swinburne, N.; LoCastro, E.; Paudyal, R.; Oh, J.H.; Taunk, N.K.; Shah, A.; Beal, K.; Vachha, B.; Young, R.J.; Holodny, A.I.; et al. Computational Modeling of Interstitial Fluid Pressure and Velocity in Non-small Cell Lung Cancer Brain Metastases Treated With Stereotactic Radiosurgery. Front. Neurol 2020, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Baxter, L.T. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: Significance of elevated interstitial pressure. Cancer Res. 1988, 48, 7022–7032. [Google Scholar] [PubMed]
- Pishko, G.L.; Astary, G.W.; Mareci, T.H.; Sarntinoranont, M. A Computational Model of Interstitial Transport in Murine Sarcoma with Heterogeneous Vasculature: A Sensitivity Analysis. In Proceedings of the Summer Bioengineering Conference, Lake Tahoe, CA, USA, 17–21 June 2009; pp. 1135–1136. [Google Scholar]
- Bhandari, A.; Bansal, A.; Singh, A.; Sinha, N. Perfusion kinetics in human brain tumor with DCE-MRI derived model and CFD analysis. J. Biomech. 2017, 59, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tofts, P.S.; Brix, G.; Buckley, D.L.; Evelhoch, J.L.; Henderson, E.; Knopp, M.V.; Larsson, H.B.; Lee, T.Y.; Mayr, N.A.; Parker, G.J.; et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J. Magn. Reson. Imaging 1999, 10, 223–232. [Google Scholar] [CrossRef]
- Kedem, O.; Katchalsky, A. A physical interpretation of the phenomenological coefficients of membrane permeability. J. Gen. Physiol. 1961, 45, 143–179. [Google Scholar] [CrossRef]
- Steuperaert, M.; Debbaut, C.; Carlier, C.; De Wever, O.; Descamps, B.; Vanhove, C.; Ceelen, W.; Segers, P. A 3D CFD model of the interstitial fluid pressure and drug distribution in heterogeneous tumor nodules during intraperitoneal chemotherapy. Drug Deliv. 2019, 26, 404–415. [Google Scholar] [CrossRef]
- Jain, R.K. Transport of molecules in the tumor interstitium: A review. Cancer Res. 1987, 47, 3039–3051. [Google Scholar]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Paudyal, R.; Konar, A.S.; Obuchowski, N.A.; Hatzoglou, V.; Chenevert, T.L.; Malyarenko, D.I.; Swanson, S.D.; LoCastro, E.; Jambawalikar, S.; Liu, M.Z.; et al. Repeatability of Quantitative Diffusion-Weighted Imaging Metrics in Phantoms, Head-and-Neck and Thyroid Cancers: Preliminary Findings. Tomography 2019, 5, 15–25. [Google Scholar] [CrossRef]
- Dullien, F. Porous Media: Fluid Transport and Pore Structure; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Baxter, L.T.; Jain, R.K. Vascular-Permeability and Interstitial Diffusion in Superfused Tissues—A Two-Dimensional Model. Microvasc. Res. 1988, 36, 108–115. [Google Scholar] [CrossRef]
- Padera, T.P.; Kadambi, A.; di Tomaso, E.; Carreira, C.M.; Brown, E.B.; Boucher, Y.; Choi, N.C.; Mathisen, D.; Wain, J.; Mark, E.J.; et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 2002, 296, 1883–1886. [Google Scholar] [CrossRef]
- Magdoom, K.N.; Pishko, G.L.; Rice, L.; Pampo, C.; Siemann, D.W.; Sarntinoranont, M. MRI-Based Computational Model of Heterogeneous Tracer Transport following Local Infusion into a Mouse Hind Limb Tumor. PLoS ONE 2014, 9, e89594. [Google Scholar] [CrossRef] [PubMed]
- Stohrer, M.; Boucher, Y.; Stangassinger, M.; Jain, R.K. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000, 60, 4251–4255. [Google Scholar] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Park, H.J.; Griffin, R.J.; Hui, S.; Levitt, S.H.; Song, C.W. Radiation-induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat. Res. 2012, 177, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.L.; Nagaraja, T.N.; Aryal, M.P.; Panda, S.; Cabral, G.; Keenan, K.A.; Elmghirbi, R.; Mikkelsen, T.; Hearshen, D.; Knight, R.A. MRI-tracked tumor vascular changes in the hours after single-fraction irradiation. Radiat. Res. 2015, 183, 713–721. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Tumor microvasculature and microenvironment: Targets for anti-angiogenesis and normalization. Microvasc. Res. 2007, 74, 72–84. [Google Scholar] [CrossRef]
- Znati, C.A.; Rosenstein, M.; Boucher, Y.; Epperly, M.W.; Bloomer, W.D.; Jain, R.K. Effect of radiation on interstitial fluid pressure and oxygenation in a human tumor xenograft. Cancer Res. 1996, 56, 964–968. [Google Scholar] [PubMed]
- Ferretti, S.; Allegrini, P.R.; Becquet, M.M.; McSheehy, P.M. Tumor interstitial fluid pressure as an early-response marker for anticancer therapeutics. Neoplasia 2009, 11, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Bulow, R.; Simon, P.; Thiel, R.; Thamm, P.; Messner, P.; Lerch, M.M.; Mayerle, J.; Volzke, H.; Hosten, N.; Kuhn, J.P. Anatomic variants of the pancreatic duct and their clinical relevance: An MR-guided study in the general population. Eur. Radiol. 2014, 24, 3142–3149. [Google Scholar] [CrossRef]
- Whisenant, J.G.; Sorace, A.G.; McIntyre, J.O.; Kang, H.; Sánchez, V.; Loveless, M.E.; Yankeelov, T.E. Evaluating treatment response using DW-MRI and DCE-MRI in trastuzumab responsive and resistant HER2-overexpressing human breast cancer xenografts. Transl. Oncol. 2014, 7, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Yotnda, P.; Wu, D.L.; Swanson, A.M. Hypoxic Tumors and Their Effect on Immune Cells and Cancer Therapy. Methods Mol. Biol. 2010, 651, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10, 562. [Google Scholar] [CrossRef]
- Garcia-Barros, M.; Paris, F.; Cordon-Cardo, C.; Lyden, D.; Rafii, S.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003, 300, 1155–1159. [Google Scholar] [CrossRef]
| Parameter | Pre-TX | D1-TX | D2-TX | p-value |
|---|---|---|---|---|
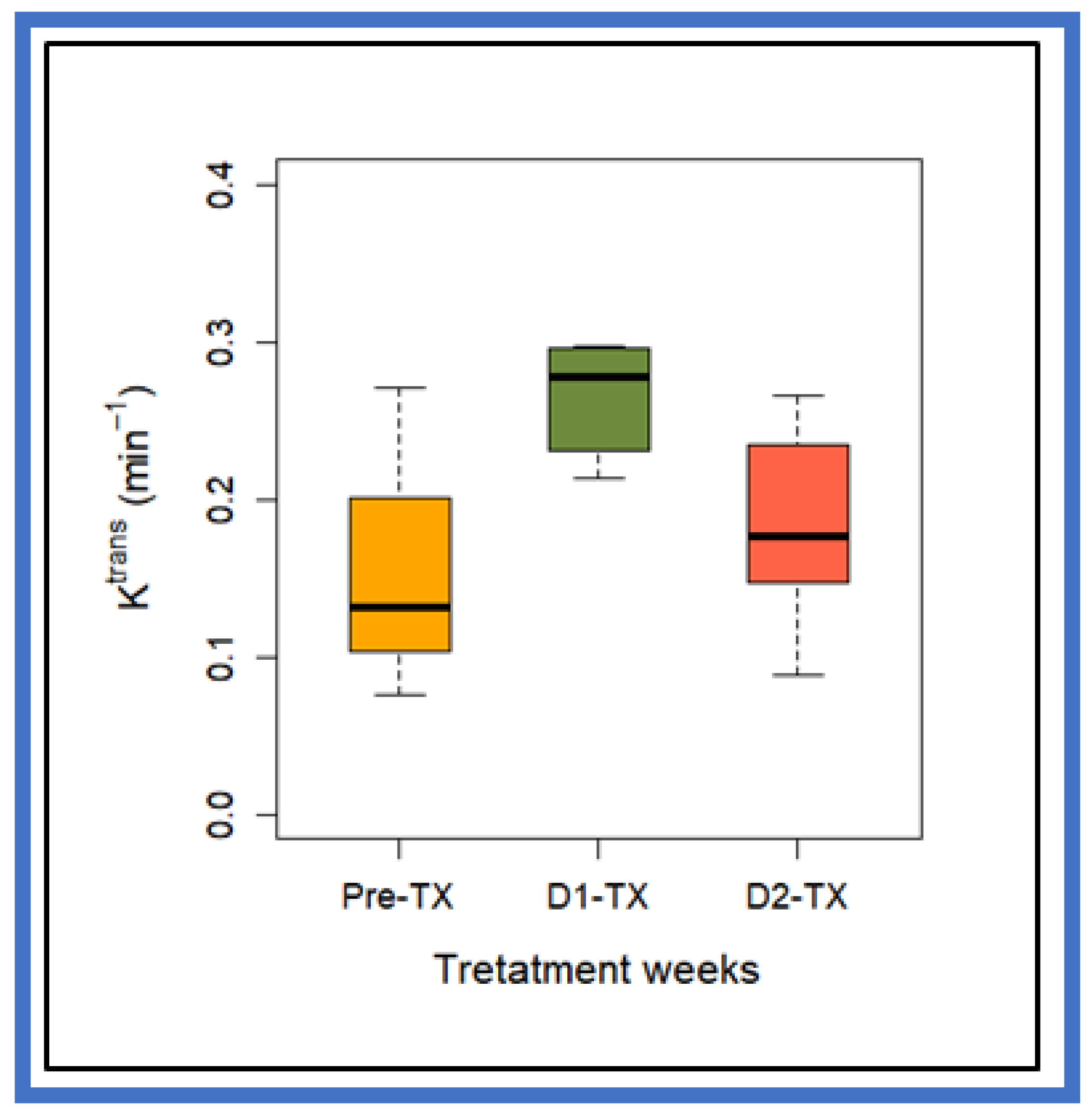
| Ktrans (min−1) | 0.14 ± 0.06 | 0.27 ± 0.035 | 0.19 ± 0.06 | p = 0.005 for Pre-TX vs. D1-TX, p = 0.01 for D2-TX vs. D1-TX, and p = 0.27 for Pre-TX vs. D2-TX |
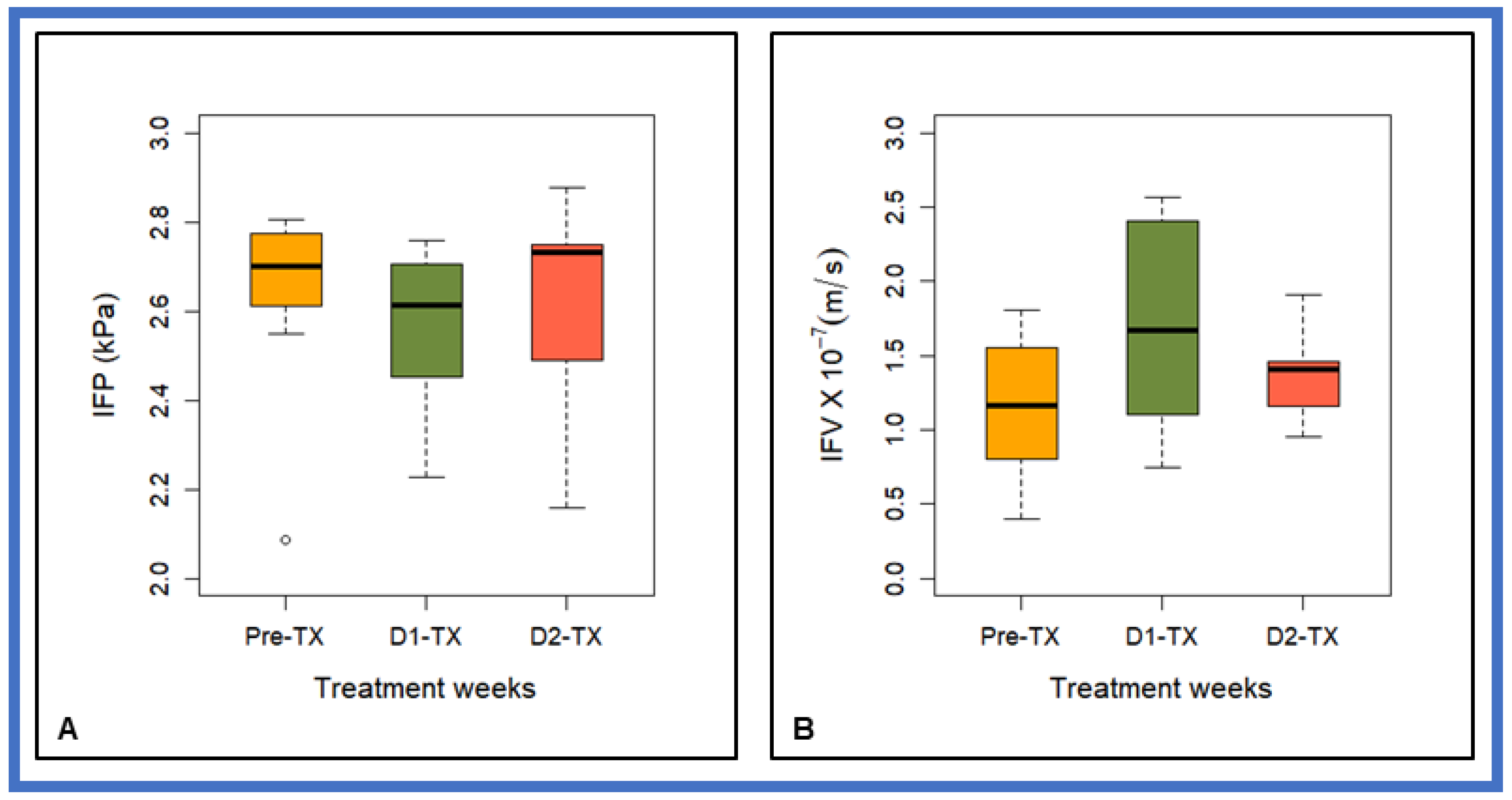
| IFP (kPa) | 2.63 ± 0.23 | 2.57 ± 0.19 | 2.60 ± 0.25 | >0.05 (for all TX) |
| IFV (m/s) | (1.15 ± 0.50) ×10−7 | (1.71 ± 0.75) ×10−7 | (1.39 ± 0.31) ×10−7 | p = 0.08 for Pre-TX vs. D1-TX p > 0.05 for all others TX |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paudyal, R.; LoCastro, E.; Reyngold, M.; Do, R.K.; Konar, A.S.; Oh, J.H.; Dave, A.; Yu, K.; Goodman, K.A.; Shukla-Dave, A. Longitudinal Monitoring of Simulated Interstitial Fluid Pressure for Pancreatic Ductal Adenocarcinoma Patients Treated with Stereotactic Body Radiotherapy. Cancers 2021, 13, 4319. https://doi.org/10.3390/cancers13174319
Paudyal R, LoCastro E, Reyngold M, Do RK, Konar AS, Oh JH, Dave A, Yu K, Goodman KA, Shukla-Dave A. Longitudinal Monitoring of Simulated Interstitial Fluid Pressure for Pancreatic Ductal Adenocarcinoma Patients Treated with Stereotactic Body Radiotherapy. Cancers. 2021; 13(17):4319. https://doi.org/10.3390/cancers13174319
Chicago/Turabian StylePaudyal, Ramesh, Eve LoCastro, Marsha Reyngold, Richard Kinh Do, Amaresha Shridhar Konar, Jung Hun Oh, Abhay Dave, Kenneth Yu, Karyn A. Goodman, and Amita Shukla-Dave. 2021. "Longitudinal Monitoring of Simulated Interstitial Fluid Pressure for Pancreatic Ductal Adenocarcinoma Patients Treated with Stereotactic Body Radiotherapy" Cancers 13, no. 17: 4319. https://doi.org/10.3390/cancers13174319
APA StylePaudyal, R., LoCastro, E., Reyngold, M., Do, R. K., Konar, A. S., Oh, J. H., Dave, A., Yu, K., Goodman, K. A., & Shukla-Dave, A. (2021). Longitudinal Monitoring of Simulated Interstitial Fluid Pressure for Pancreatic Ductal Adenocarcinoma Patients Treated with Stereotactic Body Radiotherapy. Cancers, 13(17), 4319. https://doi.org/10.3390/cancers13174319







