Dynamic 11C-Methionine PET-CT: Prognostic Factors for Disease Progression and Survival in Patients with Suspected Glioma Recurrence
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. PET-CT Imaging: Acquisition Protocol
2.3. PET-CT Imaging: Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Semiquantitative Analysis (n = 67)
3.3. Kinetic Analyses (n = 46)
3.4. Univariate and Multivariate Survival Analysis
3.5. Subgroup Analysis of Patients with HGG (n = 40)
4. Discussion
4.1. Detection of Glioma Recurrence and the Role of MET-PET
4.2. Static MET-PET Parameters
4.3. Kinetic MET-PET Parameters
4.4. Future Perspectives
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamborn, K.R.; Yung, W.K.A.; Chang, S.M.; Wen, P.Y.; Cloughesy, T.F.; DeAngelis, L.; Robins, H.I.; Lieberman, F.S.; Fine, H.A.; Fink, K.L.; et al. Progression-free survival: An important end point in evaluating therapy for recurrent high-grade gliomas. Neuro-Oncology 2008, 10, 162–170. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Berger, M.S. Maximizing safe resection of low- and high-grade glioma. J. Neuro-Oncol. 2016, 130, 269–282. [Google Scholar] [CrossRef]
- Berendsen, S.; Van Bodegraven, E.; Seute, T.; Spliet, W.G.M.; Geurts, M.; Hendrikse, J.; Schoysman, L.; Huiszoon, W.B.; Varkila, M.; Rouss, S.; et al. Adverse prognosis of glioblastoma contacting the subventricular zone: Biological correlates. PLoS ONE 2019, 14, e0222717. [Google Scholar] [CrossRef]
- Grabowski, M.M.; Recinos, P.; Nowacki, A.S.; Schroeder, J.L.; Angelov, L.; Barnett, G.H.; Vogelbaum, M.A. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma. J. Neurosurg. 2014, 121, 1115–1123. [Google Scholar] [CrossRef]
- Henker, C.; Hiepel, M.C.; Kriesen, T.; Scherer, M.; Glass, A.; Herold-Mende, C.; Bendszus, M.; Langner, S.; Weber, M.-A.; Schneider, B.; et al. Volumetric assessment of glioblastoma and its predictive value for survival. Acta Neurochir. 2019, 161, 1723–1732. [Google Scholar] [CrossRef]
- Treglia, G.; Sadeghi, R.; Del Sole, A.; Giovanella, L. Diagnostic performance of PET/CT with tracers other than F-18-FDG in oncology: An evidence-based review. Clin. Transl. Oncol. 2014, 16, 770–775. [Google Scholar] [CrossRef]
- Pike, V.W. PET radiotracers: Crossing the blood–brain barrier and surviving metabolism. Trends Pharmacol. Sci. 2009, 30, 431–440. [Google Scholar] [CrossRef]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; la Fougère, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncology 2016, 18, 1199–1208. [Google Scholar] [CrossRef]
- Kläsner, B.D.; Krause, B.J.; Beer, A.J.; Drzezga, A. PET imaging of gliomas using novel tracers: A sleeping beauty waiting to be kissed. Expert Rev. Anticancer. Ther. 2010, 10, 609–613. [Google Scholar] [CrossRef]
- Herholz, K. Brain Tumors: An Update on Clinical PET Research in Gliomas. Semin. Nucl. Med. 2017, 47, 5–17. [Google Scholar] [CrossRef]
- Hutterer, M.; Nowosielski, M.; Putzer, D.; Jansen, N.L.; Seiz, M.; Schocke, M.; McCoy, M.; Göbel, G.; la Fougère, C.; Virgolini, I.J.; et al. [18F]-fluoro-ethyl-l-tyrosine PET: A valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro-Oncology 2013, 15, 341–351. [Google Scholar] [CrossRef] [PubMed]
- la Fougère, C.; Suchorska, B.; Bartenstein, P.; Kreth, F.-W.; Tonn, J.-C. Molecular imaging of gliomas with PET: Opportunities and limitations. Neuro-Oncology 2011, 13, 806–819. [Google Scholar] [CrossRef]
- Verger, A.; Arbizu, J.; Law, I. Role of amino-acid PET in high-grade gliomas: Limitations and perspectives. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 254–266. [Google Scholar] [CrossRef]
- Holzgreve, A.; Albert, N.; Galldiks, N.; Suchorska, B. Use of PET Imaging in Neuro-Oncological Surgery. Cancers 2021, 13, 2093. [Google Scholar] [CrossRef]
- Treglia, G.; Muoio, B.; Trevisi, G.; Mattoli, M.V.; Albano, D.; Bertagna, F.; Giovanella, L. Diagnostic Performance and Prognostic Value of PET/CT with Different Tracers for Brain Tumors: A Systematic Review of Published Meta-Analyses. Int. J. Mol. Sci. 2019, 20, 4669. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, X.; Wei, G.; Qiu, C.; Qu, H.; Zhou, X. Prognostic Value of MTV, SUVmax and the T/N Ratio of PET/CT in Patients with Glioma: A Systematic Review and Meta-Analysis. J. Cancer 2019, 10, 1707–1716. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Jang, S. Prognostic Value of the Metabolic and Volumetric Parameters of 11C-Methionine Positron-Emission Tomography for Gliomas: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2018, 39, 1629–1634. [Google Scholar] [CrossRef]
- De Witte, O.; Goldberg, I.; Wikler, D.; Rorive, S.; Damhaut, P.; Monclus, M.; Salmon, I.; Brotchi, J.; Goldman, S. Positron emission tomography with injection of methionine as a prognostic factor in glioma. J. Neurosurg. 2001, 95, 746–750. [Google Scholar] [CrossRef]
- Galldiks, N.; Dunkl, V.; Kracht, L.W.; Vollmar, S.; Jacobs, A.H.; Fink, G.R.; Schroeter, M. Volumetry of [11C]-Methionine Positron Emission Tomographic Uptake as a Prognostic Marker before Treatment of Patients with Malignant Glioma. Mol. Imaging 2012, 11, 516–527. [Google Scholar] [CrossRef]
- Poetsch, N.; Woehrer, A.; Gesperger, J.; Furtner, J.; Haug, A.R.; Wilhelm, D.; Widhalm, G.; Karanikas, G.; Weber, M.; Rausch, I.; et al. Visual and semiquantitative 11C-methionine PET: An independent prognostic factor for survival of newly diagnosed and treatment-naïve gliomas. Neuro-Oncology 2017, 20, 411–419. [Google Scholar] [CrossRef]
- Van Laere, K.; Ceyssens, S.; Van Calenbergh, F.; De Groot, T.; Menten, J.; Flamen, P.; Bormans, G.; Mortelmans, L. Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: Sensitivity, inter-observer variability and prognostic value. Eur. J. Nucl. Med. Mol. Imaging 2004, 32, 39–51. [Google Scholar] [CrossRef]
- Takano, K.; Kinoshita, M.; Arita, H.; Okita, Y.; Chiba, Y.; Kagawa, N.; Fujimoto, Y.; Kishima, H.; Kanemura, Y.; Nonaka, M.; et al. Diagnostic and Prognostic Value of11C-Methionine PET for Nonenhancing Gliomas. Am. J. Neuroradiol. 2015, 37, 44–50. [Google Scholar] [CrossRef]
- Ribom, D.; Eriksson, A.; Hartman, M.; Engler, H.; Nilsson, A.; Långström, B.; Bolander, H.; Bergström, M.; Smits, A. Positron Emission Tomography (11)C-methionine and Survival in Patients with Low-Grade Gliomas. Cancer 2001, 92, 1541–1549. [Google Scholar] [CrossRef]
- Bauer, E.K.; Stoffels, G.; Blau, T.; Reifenberger, G.; Felsberg, J.; Werner, J.-M.; Lohmann, P.; Rosen, J.; Ceccon, G.; Tscherpel, C.; et al. Prediction of survival in patients with IDH-wildtype astrocytic gliomas using dynamic O-(2-[18F]-fluoroethyl)-l-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1486–1495. [Google Scholar] [CrossRef]
- Suchorska, B.; Jansen, N.L.; Linn, J.; Kretzschmar, H.; Janssen, H.; Eigenbrod, S.; Simon, M.; Pöpperl, G.; Kreth, F.W.; la Fougère, C.; et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology 2015, 84, 710–719. [Google Scholar] [CrossRef]
- Fleischmann, D.F.; Unterrainer, M.; Bartenstein, P.; Belka, C.; Albert, N.L.; Niyazi, M. 18F-FET PET prior to recurrent high-grade glioma re-irradiation—additional prognostic value of dynamic time-to-peak analysis and early static summation images? J. Neuro-Oncol. 2017, 132, 277–286. [Google Scholar] [CrossRef]
- Niyazi, M.; Jansen, N.L.; Rottler, M.; Ganswindt, U.; Belka, C. Recurrence pattern analysis after re-irradiation with bevacizumab in recurrent malignant glioma patients. Radiat. Oncol. 2014, 9, 299. [Google Scholar] [CrossRef]
- Zaragori, T.; Ginet, M.; Marie, P.-Y.; Roch, V.; Grignon, R.; Gauchotte, G.; Rech, F.; Blonski, M.; Lamiral, Z.; Taillandier, L.; et al. Use of static and dynamic [18F]-F-DOPA PET parameters for detecting patients with glioma recurrence or progression. EJNMMI Res. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Nomura, Y.; Asano, Y.; Shinoda, J.; Yano, H.; Ikegame, Y.; Kawasaki, T.; Nakayama, N.; Maruyama, T.; Muragaki, Y.; Iwama, T. Characteristics of time-activity curves obtained from dynamic 11C-methionine PET in common primary brain tumors. J. Neuro-Oncol. 2018, 138, 649–658. [Google Scholar] [CrossRef]
- Aki, T.; Nakayama, N.; Yonezawa, S.; Takenaka, S.; Miwa, K.; Asano, Y.; Shinoda, J.; Yano, H.; Iwama, T. Evaluation of brain tumors using dynamic 11C-methionine-PET. J. Neuro-Oncol. 2012, 109, 115–122. [Google Scholar] [CrossRef]
- Yano, H.; Ohe, N.; Nakayama, N.; Nomura, Y.-I.; Miwa, K.; Shinoda, J.; Iwama, T. Dynamic study of methionine positron emission tomography in patients with glioblastoma with oligodendroglial components. Brain Tumor Pathol. 2015, 32, 253–260. [Google Scholar] [CrossRef]
- Moulin-Romsée, G.; D’Hondt, E.; De Groot, T.; Goffin, J.; Sciot, R.; Mortelmans, L.; Menten, J.; Bormans, G.; Van Laere, K. Non-invasive grading of brain tumours using dynamic amino acid PET imaging: Does it work for 11C-Methionine? Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 2082–2087. [Google Scholar] [CrossRef]
- Weller, M.; Bent, M.V.D.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Le Rhun, E.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef]
- Bartenstein, P.; Boecker, H.; Brust, P.; Coenen, H.H.; Drzezga, A.; Grünwald, F.; Krause, B.J.; Kuwert, T.; Sabri, O.; Tatsch, K.; et al. PET- und SPECT-Untersuchungen von Hirn tumoren mit radioaktiv markierten Aminosäuren. Nuklearmedizin 2011, 50, 167–173. [Google Scholar] [CrossRef]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; La Fougère, C.; Langen, K.-J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 540–557. [Google Scholar] [CrossRef]
- Kelly, C.; Majewska, P.; Ioannidis, S.; Raza, M.H.; Williams, M. Estimating progression-free survival in patients with glioblastoma using routinely collected data. J. Neuro-Oncol. 2017, 135, 621–627. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Chung, C.; Pope, W.B.; Boxerman, J.L.; Kaufmann, T.J. Pseudoprogression, radionecrosis, inflammation or true tumor progression? Challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J. Neuro-Oncol. 2017, 134, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Karunanithi, S.; Sharma, P.; Kumar, A.; Khangembam, B.C.; Bandopadhyaya, G.P.; Kumar, R.; Gupta, D.K.; Malhotra, A.; Bal, C. 18F-FDOPA PET/CT for detection of recurrence in patients with glioma: Prospective comparison with 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Czernin, J.; Cloughesy, T.; Lai, A.; Pomykala, K.L.; Benz, M.R.; Buck, A.K.; Phelps, M.E.; Chen, W. Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro-Oncology 2013, 16, 603–609. [Google Scholar] [CrossRef]
- Xu, W.; Gao, L.; Shao, A.; Zheng, J.; Zhang, J. The performance of 11C-Methionine PET in the differential diagnosis of glioma recurrence. Oncotarget 2017, 8, 91030–91039. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zheng, J.; Xu, W.; Weng, J.; Gao, L.; Tao, L.; Liang, F.; Zhang, J. Accuracy of 18 F-FDOPA Positron Emission Tomography and 18 F-FET Positron Emission Tomography for Differentiating Radiation Necrosis from Brain Tumor Recurrence. World Neurosurg. 2018, 114, e1211–e1224. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.-Y.; Min, J.-J.; Bom, H.-S.; Kim, I.-Y.; Lim, S.-H.; Kwon, S.Y. Prognostic value of post-treatment metabolic tumor volume from 11C-methionine PET/CT in recurrent malignant glioma. Neurosurg. Rev. 2016, 40, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Jansen, N.L.; Suchorska, B.; Wenter, V.; Eigenbrod, S.; Schmid-Tannwald, C.; Zwergal, A.; Niyazi, M.; Drexler, M.; Bartenstein, P.; Schnell, O.; et al. Dynamic 18F-FET PET in Newly Diagnosed Astrocytic Low-Grade Glioma Identifies High-Risk Patients. J. Nucl. Med. 2013, 55, 198–203. [Google Scholar] [CrossRef]
- Calcagni, M.L.; Galli, G.; Giordano, A.; Taralli, S.; Anile, C.; Niesen, A.; Baum, R.P. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine (F-18 FET) PET for Glioma Grading. Clin. Nucl. Med. 2011, 36, 841–847. [Google Scholar] [CrossRef]
- Galldiks, N.; Stoffels, G.; Filss, C.; Rapp, M.; Blau, T.; Tscherpel, C.; Ceccon, G.; Dunkl, V.; Weinzierl, M.R.; Stoffel, M.; et al. The use of dynamic O-(2-18F-fluoroethyl)-L-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro-Oncology 2015, 17, 1293–1300. [Google Scholar] [CrossRef]
- Bustany, P.; Chatel, M.; Derlon, J.M.; Darcel, F.; Sgouropoulos, P.; Soussaline, F.; Syrota, A. Brain tumor protein synthesis and histological grades: A study by positron emission tomography (PET) with C11-L-Methionine. J. Neuro-Oncol. 1986, 3, 397–404. [Google Scholar] [CrossRef]
- Derlon, J.M.; Bourdet, C.; Bustany, P.; Chatel, M.; Theron, J.; Darcel, F.; Syrota, A. [11C]L-methionine uptake in gliomas. Neurosurgery 1989, 25, 720–728. [Google Scholar] [CrossRef]
- Knudsen, G.M.; Pettigrew, K.D.; Patlak, C.S.; Hertz, M.M.; Paulson, O.B. Asymmetrical Transport of Amino Acids across the Blood—Brain Barrier in Humans. Br. J. Pharmacol. 1990, 10, 698–706. [Google Scholar] [CrossRef]
- Souba, W.W.; Pacitti, A.J. Review: How Amino Acids Get Into Cells: Mechanisms, Models, Menus, and Mediators. J. Parenter. Enter. Nutr. 1992, 16, 569–578. [Google Scholar] [CrossRef]
- Chung, J.-K.; Kim, Y.K.; Kim, S.-K.; Lee, Y.J.; Paek, S.; Yeo, J.S.; Jeong, J.M.; Lee, D.S.; Jung, H.W.; Lee, M.C. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur. J. Nucl. Med. Mol. Imaging 2001, 29, 176–182. [Google Scholar] [CrossRef]
- Kato, T.; Shinoda, J.; Nakayama, N.; Miwa, K.; Okumura, A.; Yano, H.; Yoshimura, S.; Maruyama, T.; Muragaki, Y.; Iwama, T. Metabolic Assessment of Gliomas Using11C-Methionine, [18F] Fluorodeoxyglucose, and11C-Choline Positron-Emission Tomography. Am. J. Neuroradiol. 2008, 29, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Kracht, L.W.; Friese, M.; Herholz, K.; Schroeder, R.; Bauer, B.; Jacobs, A.; Heiss, W.-D. Methyl-[11C]-l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 868–873. [Google Scholar] [CrossRef]
- Nojiri, T.; Nariai, T.; Aoyagi, M.; Senda, M.; Ishii, K.; Ishiwata, K.; Ohno, K. Contributions of biological tumor parameters to the incorporation rate of l-[methyl-11C] methionine into astrocytomas and oligodendrogliomas. J. Neuro-Oncol. 2008, 93, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Torii, K.; Shiomi, S.; Kawabe, J.; Tsuyuguchi, N.; Sunada, I.; Hara, M. Correlation of amino-acid uptake using methionine PET and histological classifications in various gliomas. Ann. Nucl. Med. 2005, 19, 677–683. [Google Scholar] [CrossRef]
- Beppu, T.; Sato, Y.; Sasaki, T.; Terasaki, K.; Yamashita, F.; Sasaki, M.; Ogasawara, K. Comparisons Between PET With 11C-Methyl-l-Methionine and Arterial Spin Labeling Perfusion Imaging in Recurrent Glioblastomas Treated with Bevacizumab. Clin. Nucl. Med. 2019, 44, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.; Debeir, O.; Decaestecker, C.; Metens, T.; Lebrun, L.; Leurquin-Sterk, G.; Trotta, N.; Goldman, S.; Van Simaeys, G. Voxelwise Principal Component Analysis of Dynamic [S-Methyl-11C]Methionine PET Data in Glioma Patients. Cancers 2021, 13, 2342. [Google Scholar] [CrossRef] [PubMed]
- Bergström, M.; Ericson, K.; Hagenfeldt, L.; Mosskin, M.; von Holst, H.; Norén, G.; Eriksson, L.; Ehrin, E.; Johnström, P. PET Study of Methionine Accumulation in Glioma and Normal Brain Tissue. J. Comput. Assist. Tomogr. 1987, 11, 208–213. [Google Scholar] [CrossRef]
- Ericson, D.K.; Blomqvist, G.; Bergström, M.; Eriksson, L.; Stone-Elander, S. Application of a Kinetic Model on the Methionine Accumulation in Intracranial Tumours Studied with Positron Emission Tomography. Acta Radiol. 1987, 28, 505–509. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
| Characteristics | Number |
|---|---|
| patients | 67 |
| gender (M/F) | 40/27 |
| mean age ± SD (yrs) | 48.4 ± 15.3 |
| histopathology of primary tumour (n(%)) | |
| pilocytic astrocitoma | 1 (1.5%) |
| diffuse astrocitoma | 12 (18%) |
| anaplastic astrocitoma | 2 (3%) |
| ependimoma | 1 (1.5%) |
| anaplastic ependimoma | 2 (3%) |
| oligoastrocitoma | 1 (1.5%) |
| anaplastic oligoastrocitoma | 1 (1.5%) |
| oligodendroglioma | 15 (22.3%) |
| anaplastic oligodendroglioma | 7 (10.4%) |
| gliomatosi cerebri | 1 (1.5%) |
| diffuse midline glioma | 1 (1.5%) |
| glioblastoma | 23 (34.3%) |
| WHO grade of primary tumour (n(%)) | |
| I | 1 (1.5%) |
| II | 29 (43.3%) |
| III | 13 (19.4%) |
| IV | 24 (35.8%) |
| surgical approach of primary tumour (n(%)) | |
| biopsy | 8 (11.9%) |
| surgical resection | 59 (88.1%) |
| first-line therapy following resection/biopsy (n(%)) | |
| no therapy | 12 (18%) |
| TMZ alone | 8 (11.9%) |
| radiotherapy alone | 7 (10.4%) |
| radiotherapy + TMZ | 39 (58.2%) |
| n.a. | 1 (1.5%) |
| recurrence (n(%)) | 46 (68.7%) |
| second therapy (after recurrence) (n(%)) | |
| surgery | 5 (11%) |
| surgery + radiotherapy + TMZ | 5 (11%) |
| surgery + TMZ | 3 (6.5%) |
| TMZ alone | 13 (28.2%) |
| radiotherapy alone | 6 (13%) |
| radiotherapy + TMZ | 2 (4.3%) |
| no therapy | 4 (8.7%) |
| n.a. | 8 (17.4%) |
| death (n(%)) | 23 (34.3%) |
| A | B | |||||
|---|---|---|---|---|---|---|
| Static MET-PET Parameters | Recurrence within 6 Months (n = 38) | Nonrecurrence within 6 Months (n = 29) | p-Value | Deceased within 12 Months (n = 16) | Alive after 12 Months (n = 51) | p-Value |
| SUVmax | 3.66 (2.76–5.10) | 2.50 (2.09–3.23) | 0.001 | 3.73 (3.05–5.25) | 2.85 (2.13–3.69) | 0.032 |
| SUVmean | 1.84 (1.36–2.29) | 1.25 (1.02–1.61) | 0.002 | 1.88 (1.60–2.28) | 1.45 (1.04–1.97) | 0.036 |
| T/B | 3.46 (2.51–4.59) | 2.30 (1.84–2.86) | <0.001 | 3.95 (2.89–5.08) | 2.47 (2.02–3.40) | 0.006 |
| Static MET-PET Parameters | ROC Cut-Off | AUC | Younden’s Index | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy | |
|---|---|---|---|---|---|---|---|---|---|
| A | SUVmax | 3.15 | 0.727 | 0.407 | 69.23 | 71.43 | 77.14 | 62.50 | 0.701 |
| SUVmean | 1.64 | 0.722 | 0.417 | 66.67 | 75.00 | 78.79 | 61.76 | 0.701 | |
| T/B | 2.47 | 0.787 | 0.473 | 79.49 | 67.86 | 77.50 | 70.37 | 0.746 | |
| B | SUVmax | 2.52 | 0.604 | 2.41 | 86.36 | 37.78 | 40.43 | 85.00 | 0.687 |
| SUVmean | 1.21 | 0.611 | 2.19 | 86.36 | 35.56 | 39.58 | 84.21 | 0.687 | |
| T/B | 2.42 | 0.664 | 3.53 | 86.36 | 48.89 | 45.24 | 88.00 | 0.701 | |
| Characteristics | Number |
|---|---|
| Patients | 46 |
| Gender (M/F) | 29/17 |
| Mean age ± SD (yrs) | 48.7 ± 15.7 |
| WHO grade of primary tumour (n(%)) | |
| I | 1 (2.2%) |
| II | 18 (39.1%) |
| III | 7 (15.2%) |
| IV | 20 (43.5%) |
| MET-PET positive (n(%)) | 30 (65.2%) |
| MET-PET negative (n(%)) | 16 (34.8%) |
| SUVmax (median (IQR)) | 3.35 (2.32–4.58) |
| SUVmean (median (IQR)) | 1.67 (1.03–2.04) |
| T/B (median (IQR)) | 2.88 (2.30–3.91) |
| Time–activity curve type A (increasing) (n(%)) | 31 (67.4%) |
| Time–activity curve type B (decreasing) (n(%)) | 15 (32.6%) |
| Slope (median (IQR)) | 9.66 × 10−5 (−2.45 × 10−4–1.33 × 10−4) |
| TTP (median (IQR)) in seconds | 30 (20–35) |
| SUVmaxTTP (median (IQR)) | 11.5 (8.59–14.3) |
| A | B | |||||
|---|---|---|---|---|---|---|
| Kinetic MET-PET Parameters | Recurrence within 6 Months (n = 27) | Nonrecurrence within 6 Months (n = 19) | p-Value | Deceased within 12 Months (n = 14) | Alive after 12 Months (n = 32) | p-Value |
| Slope | 9.87 × 10−5 (−2.91 × 10−5–1.36 × 10−4) | 9.59 × 10−5 (1.00 × 10−6–1.15 × 10−4) | n.s. | 1.03 × 10−4 (−2.55 × 10−4–2.07 × 10−4) | 9.45 × 10−5 (−2.6 × 10−5–1.21 × 10−4) | n.s. |
| TTP | 30 (20–35) | 30 (22.5–42.5) | n.s. | 25 (20–30) | 32.5 (25–45) | n.s. |
| SUVmaxTTP | 12.80 (9.52–15.60) | 9.25 (7.12–12.70) | 0.02 | 13.09 (10.20–17.40) | 10.20 (7.99–13.00) | 0.01 |
| Kinetic MET-PET Parameters | ROC Cut-Off | AUC | Younden’s Index | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy | |
|---|---|---|---|---|---|---|---|---|---|
| A | Slope | −5.65 × 10−5 | 0.521 | 0.250 | 84.21 | 40.74 | 50.00 | 78.57 | 0.587 |
| TTP | 40 | 0.519 | 0.131 | 31.58 | 81.48 | 54.55 | 62.86 | 0.609 | |
| SUVmaxTTP | 10.66 | 0.678 | 0.335 | 70.37 | 63.16 | 73.08 | 60.00 | 0.674 | |
| B | Slope | −6.64 × 10−4 | 0.424 | 0.071 | 100.00 | 7.14 | 71.11 | 100.00 | 0.717 |
| TTP | 30 | 0.660 | 0.299 | 65.62 | 64.29 | 80.77 | 45.00 | 0.696 | |
| SUVmaxTTP | 12.76 | 0.710 | 0.433 | 71.43 | 71.88 | 52.63 | 85.19 | 0.783 | |
| A | B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | ||||||||
| Variables | Number of Patients | HR (95% CI) | p | HR (95% CI) | p | Variables | Number of Patients | HR (95% CI) | p | HR (95% CI) | p |
| Age | Age | ||||||||||
| >70 | 4 | 0.74 (0.23–2.41) | 0.61 | >70 | 4 | 0.41 (0.12–1.39) | 0.1 | ||||
| ≤70 | 63 | ≤70 | 63 | ||||||||
| Gender | Gender | ||||||||||
| F | 27 | 0.84 (0.44–1.58) | 0.58 | F | 27 | 0.79 (0.34–1.82) | 0.5 | ||||
| M | 40 | M | 40 | ||||||||
| Grade at diagnosis | Grade at diagnosis | ||||||||||
| LGG | 30 | 1.77 (0.92–3.41) | 0.09 | LGG | 30 | 11.10 (2.59–47.57) | 0.001 | 0.92 (0.10–8.16) | 0.9 | ||
| HGG | 37 | HGG | 37 | ||||||||
| Grade at recurrence | Grade at recurrence | ||||||||||
| HGG | 40 | 0.52 (0.26–1.03) | 0.06 | HGG | 40 | 0.11 (0.03–0.48) | 0.003 | 0.98 (0.10–8.17) | 0.9 | ||
| LGG | 27 | LGG | 27 | ||||||||
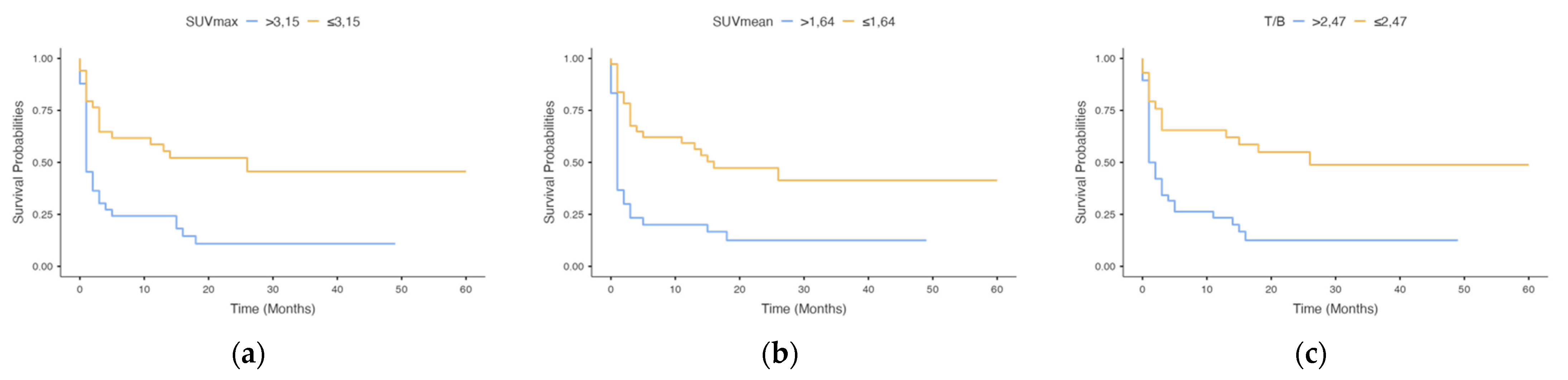
| SUVmax | SUVmax | ||||||||||
| >3.15 | 33 | 0.36 (0.19–0.70) | 0.002 | 0.35 (0.05–2.22) | 0.264 | >2.52 | 46 | 0.45 (0.15–1.34) | 0.1 | ||
| ≤3.15 | 34 | ≤2.52 | 21 | ||||||||
| SUVmean | SUVmean | ||||||||||
| >1.64 | 30 | 0.28 (0.15–0.55) | <0.001 | 0.76 (0.16–3.52) | 0.726 | >1.21 | 46 | 0.41 (0.14–1.22) | 0.1 | ||
| 1.64 | 37 | ≤1.21 | 21 | ||||||||
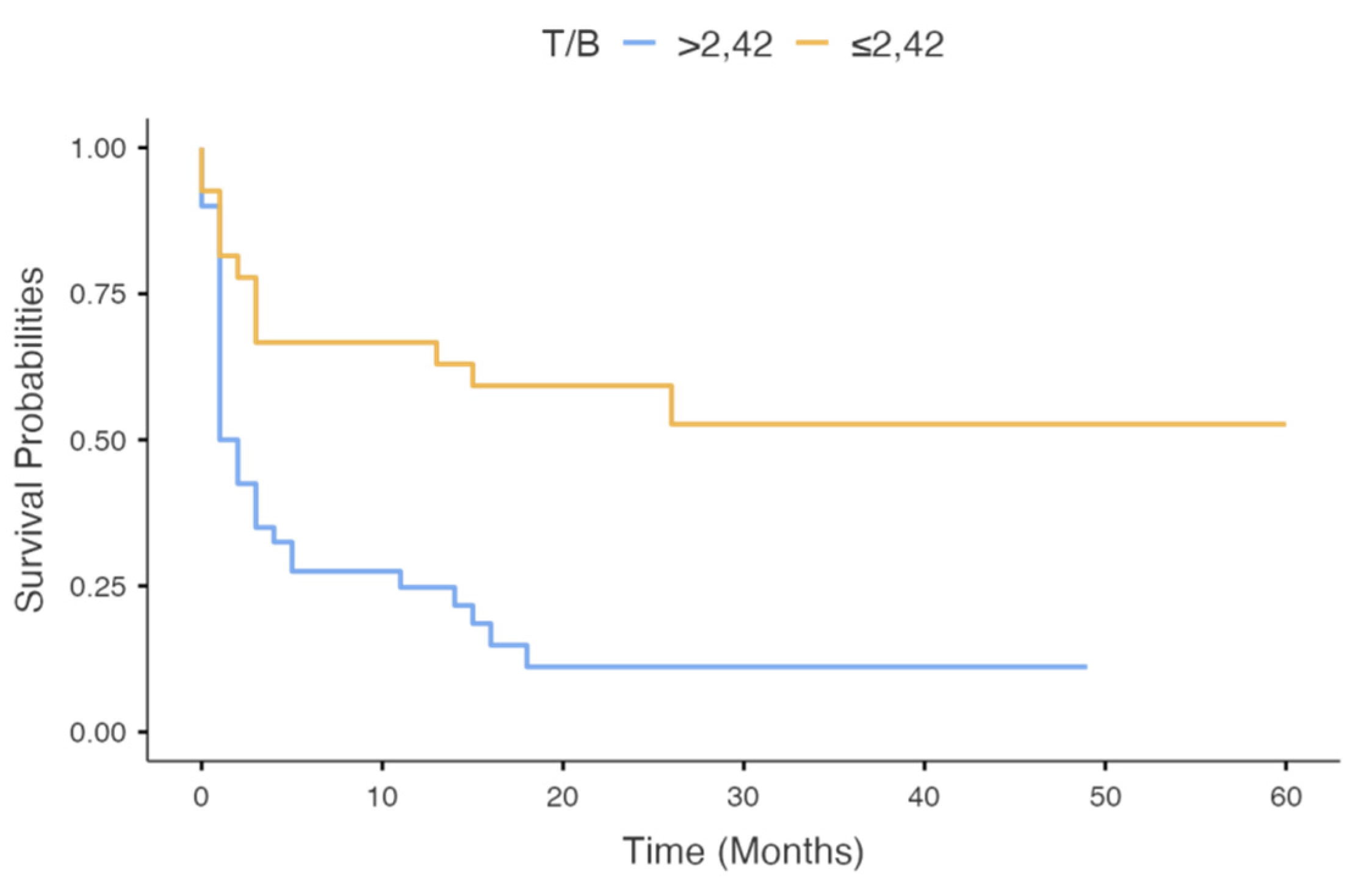
| T/B | T/B | ||||||||||
| >2.47 | 38 | 0.32 (0.15–0.66) | 0.002 | 0.53 (0.18–1.54) | 0.244 | >2.42 | 40 | 0.38 (0.14–1.03) | 0.06 | ||
| ≤2.47 | 29 | ≤2.42 | 27 | ||||||||
| Curve Type | Curve Type | ||||||||||
| A | 31 | 1.89 (0.88–4.09) | 0.1 | A | 31 | 0.80 (0.25–2.57) | 0.7 | ||||
| B | 15 | B | 15 | ||||||||
| Slope | Slope | ||||||||||
| ≤−0.0000565 | 15 | 0.53 (0.25–1.14) | 0.1 | ≤−0.000664 | 2 | 0.64 (0.08–4.90) | 0.6 | ||||
| >−0.0000565 | 31 | >−0–000664 | 44 | ||||||||
| TTP | TTP | ||||||||||
| ≤40 | 36 | 0.82 (0.31–2.17) | 0.68 | ≤30 | 27 | 0.31 (0.09–1.12) | 0.07 | ||||
| >40 | 10 | >30 | 19 | ||||||||
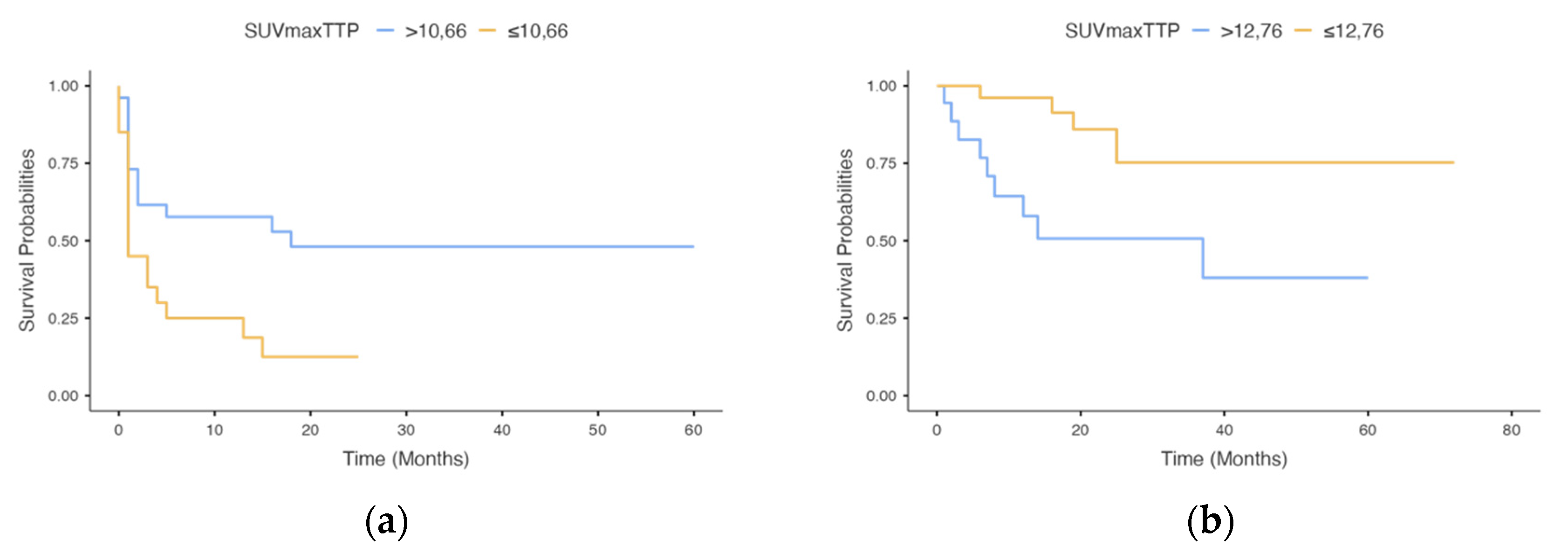
| SUVmaxTTP | SUVmaxTTP | ||||||||||
| >10.66 | 25 | 0.41 (0.18–0.93) | 0.032 | 0.30 (0.09–1.04) | 0.038 | >12.76 | 18 | 0.28 (0.09–0.83) | 0.022 | 0.30 (0.09–1.04) | 0.058 |
| ≤10.66 | 21 | ≤12.76 | 28 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattoli, M.V.; Trevisi, G.; Scolozzi, V.; Capotosti, A.; Cocciolillo, F.; Marini, I.; Mare, V.; Indovina, L.; Caulo, M.; Saponiero, A.; et al. Dynamic 11C-Methionine PET-CT: Prognostic Factors for Disease Progression and Survival in Patients with Suspected Glioma Recurrence. Cancers 2021, 13, 4777. https://doi.org/10.3390/cancers13194777
Mattoli MV, Trevisi G, Scolozzi V, Capotosti A, Cocciolillo F, Marini I, Mare V, Indovina L, Caulo M, Saponiero A, et al. Dynamic 11C-Methionine PET-CT: Prognostic Factors for Disease Progression and Survival in Patients with Suspected Glioma Recurrence. Cancers. 2021; 13(19):4777. https://doi.org/10.3390/cancers13194777
Chicago/Turabian StyleMattoli, Maria Vittoria, Gianluca Trevisi, Valentina Scolozzi, Amedeo Capotosti, Fabrizio Cocciolillo, Irene Marini, Valerio Mare, Luca Indovina, Massimo Caulo, Antonella Saponiero, and et al. 2021. "Dynamic 11C-Methionine PET-CT: Prognostic Factors for Disease Progression and Survival in Patients with Suspected Glioma Recurrence" Cancers 13, no. 19: 4777. https://doi.org/10.3390/cancers13194777
APA StyleMattoli, M. V., Trevisi, G., Scolozzi, V., Capotosti, A., Cocciolillo, F., Marini, I., Mare, V., Indovina, L., Caulo, M., Saponiero, A., Balducci, M., Taralli, S., & Calcagni, M. L. (2021). Dynamic 11C-Methionine PET-CT: Prognostic Factors for Disease Progression and Survival in Patients with Suspected Glioma Recurrence. Cancers, 13(19), 4777. https://doi.org/10.3390/cancers13194777






