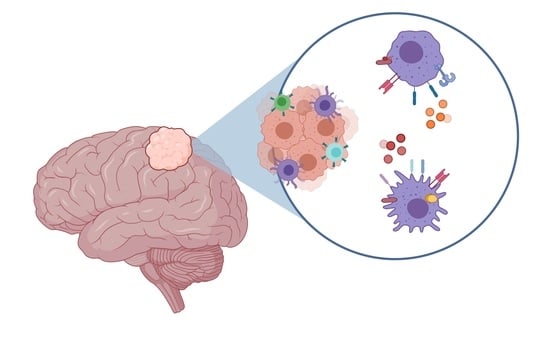
Meningioma–Brain Crosstalk: A Scoping Review
Simple Summary
Abstract
1. Introduction
- (1)
- Attraction and activation of glial cells from the parenchyma to the tumour site;
- (2)
- Reciprocal exchange of substances and cell–cell interactions between glial cells and tumour cells that impact on meningioma growth and behaviour.
2. Methods
(meningioma[MeSH Terms]) AND (astrocytes[MeSH Terms]) OR (meningioma[MeSH Terms]) AND (microglia[MeSH Terms]) OR (meningioma[MeSH Terms]) AND (receptors, chemokine[MeSH Terms]) OR (meningioma[MeSH Terms]) AND (“chemokines”[MeSH Terms]) OR (meningioma[MeSH Terms]) AND (cytokines[MeSH Terms])
3. Results
3.1. Microglia/Macrophage Infiltration in Meningiomas
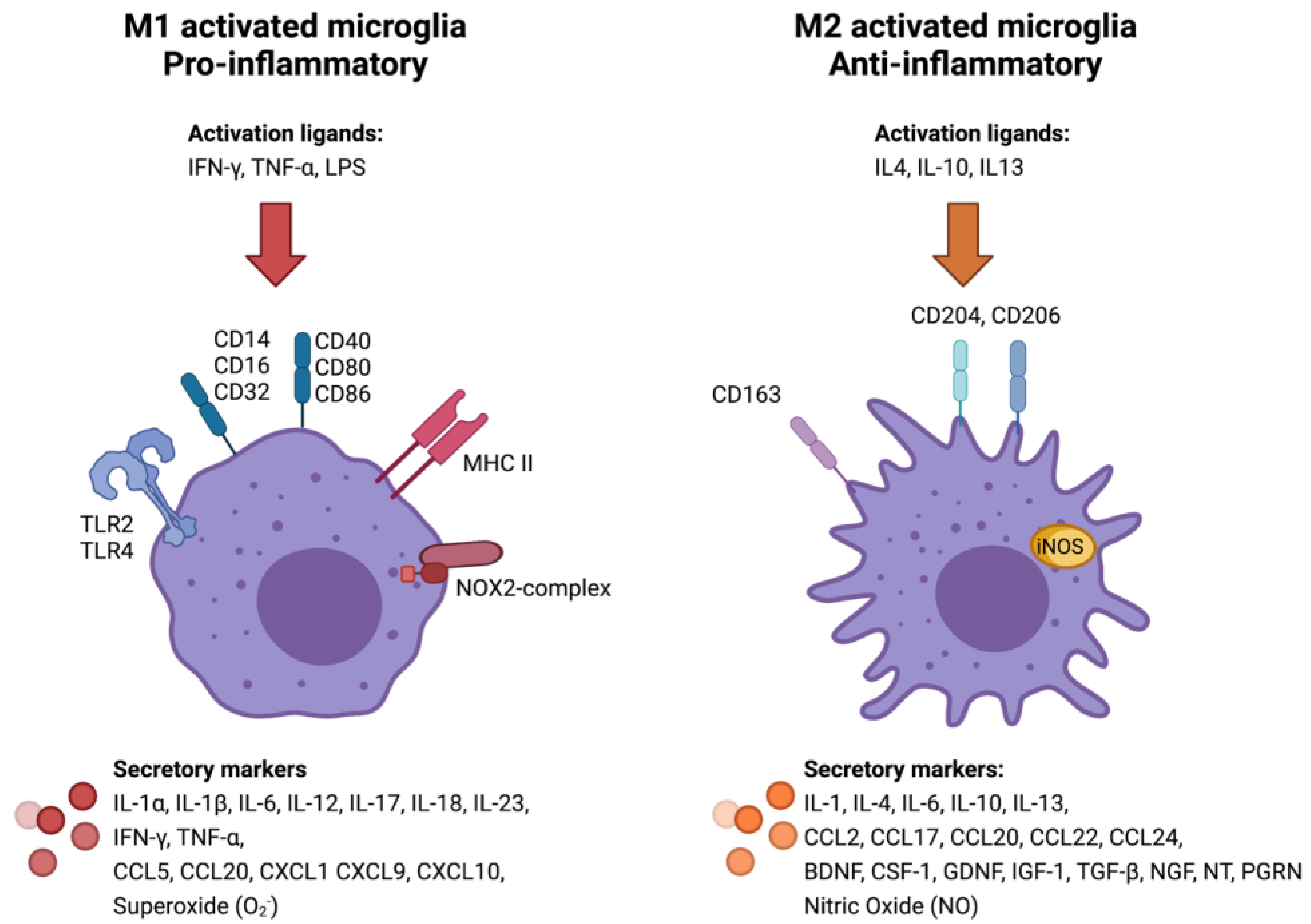
3.2. Classical (M1) or Alternative (M2) Microglia/Macrophage Activation in Meningiomas
3.3. Glial Response at the Tumour–Brain Interface
3.4. Cytokine Expression in Meningiomas
3.5. Microenvironmental Impact on Meningioma Growth and Behaviour
3.6. Sources and Targets of Cytokines in Meningiomas
4. Discussion
4.1. Meningioma–Brain Crosstalk: Current Research Knowledge Base
4.2. Research Gaps and Future Research Recommendations
5. Limitations of This Review
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Supervision
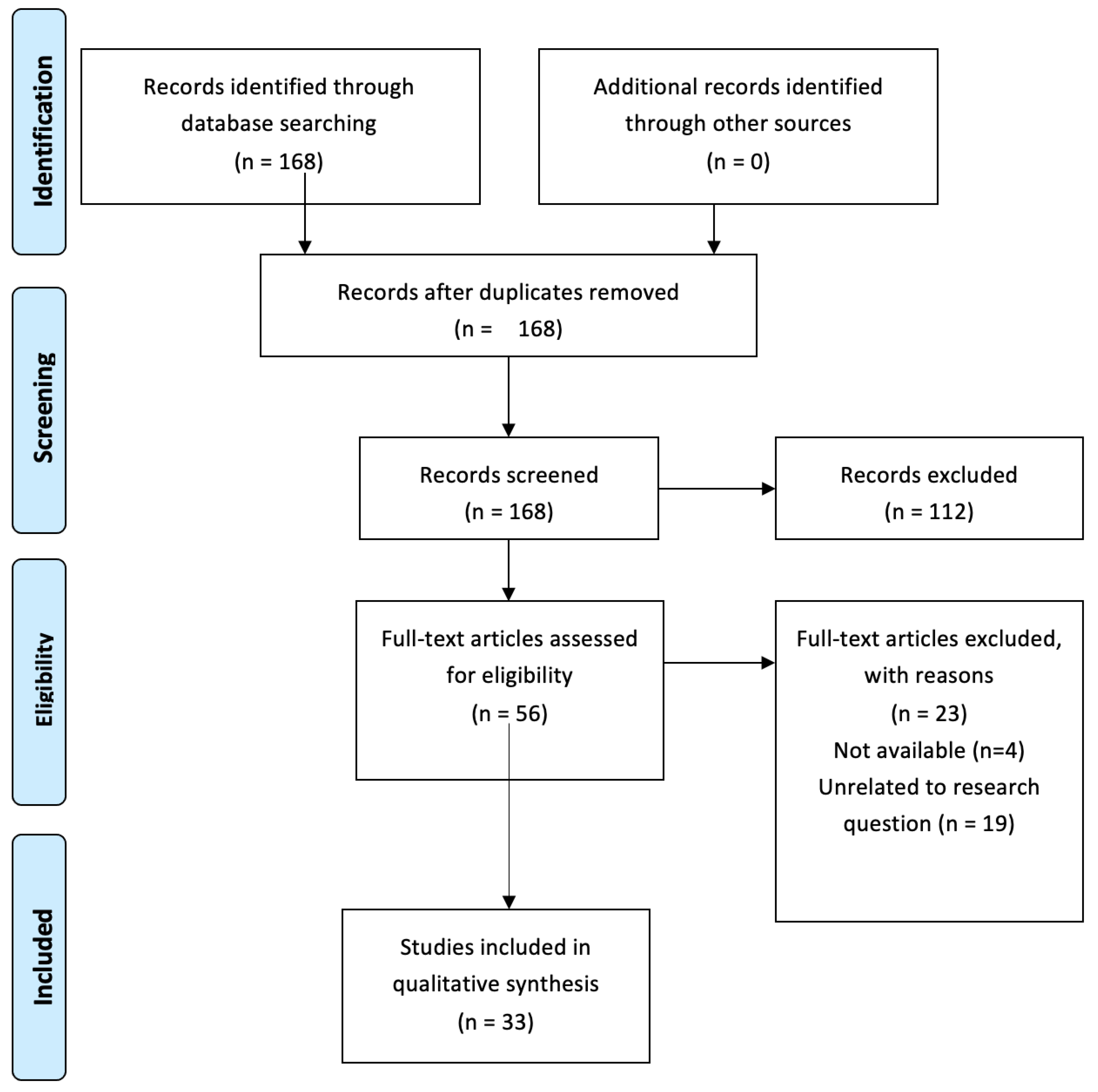
Appendix A. PRISMA Flow Diagram
References
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An Overview of Meningiomas. Futur. Oncol. 2018, 14, 2161–2177. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Preusser, M.; Brastianos, P.K.; Mawrin, C. Advances in Meningioma Genetics: Novel Therapeutic Opportunities. Nat. Rev. Neurol. 2018, 14, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Garzon-Muvdi, T.; Bailey, D.D.; Pernik, M.N.; Pan, E. Basis for Immunotherapy for Treatment of Meningiomas. Front. Neurol. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Whiteside, T.L. The Tumor Microenvironment and Its Role in Promoting Tumor Growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Perspective the Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef]
- Charles, N.A.; Holland, E.C.; Gilbertson, R.; Glass, R.; Kettenmann, H. The Brain Tumor Microenvironment. Glia 2012, 514, 502–514. [Google Scholar] [CrossRef]
- Locarno, C.V.; Simonelli, M.; Carenza, C.; Capucetti, A.; Stanzani, E.; Lorenzi, E.; Persico, P.; Della, S.; Passoni, L.; Mavilio, D.; et al. Immunobiology Role of Myeloid Cells in the Immunosuppressive Microenvironment in Gliomas. Immunobiology 2020, 225, e151853. [Google Scholar] [CrossRef]
- Proctor, D.T.; Huang, J.; Lama, S.; Albakr, A.; Van Marle, G.; Sutherland, G.R. Tumor-associated Macrophage Infiltration in Meningioma. Neuro-Oncol. Adv. 2019, 1, vdz018. [Google Scholar] [CrossRef]
- Bieńkowski, M.; Preusser, M. Prognostic Role of Tumour-infiltrating Inflammatory Cells in Brain Tumours: Literature Review. Curr. Opin. Neurol. 2015, 28, 647–658. [Google Scholar] [CrossRef]
- Strik, H.M.; Stoll, M.; Meyermann, R. Immune Cell Infiltration of Intrinsic and Metastatic Intracranial Tumours. Anticancer Res. 2004, 24, 37–42. [Google Scholar] [PubMed]
- Domingues, P.; González-Tablas, M.; Otero, Á.; Pascual, D.; Miranda, D.; Ruiz, L.; Sousa, P.; Ciudad, J.; Gonçalves, J.M.; Lopes, M.C.; et al. Tumor Infiltrating Immune Cells in Gliomas and Meningiomas. Brain. Behav. Immun. 2016, 53, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Matias, D.; Balça-Silva, J.; da Graça, G.C.; Wanjiru, C.M.; Macharia, L.W.; Nascimento, C.P.; Roque, N.R.; Coelho-Aguiar, J.M.; Pereira, C.M.; Dos Santos, M.F.; et al. Microglia/astrocytes–glioblastoma Crosstalk: Crucial Molecular Mechanisms and Microenvironmental Factors. Front. Cell. Neurosci. 2018, 12, 235. [Google Scholar] [CrossRef]
- BØ, L.; Mørk, S.J.; Nyland, H. An Immunohistochemical Study of Mononuclear Cells in Meningiomas. Neuropathol. Appl. Neurobiol. 1992, 18, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Asai, J.; Suzuki, R.; Fujimoto, T.; Suzuki, T.; Nakagawa, N.; Nagashima, G.; Miyo, T.; Hokaku, H.; Takei, A. Fluorescence Automatic Cell Sorter and Immunohistochemical Investigation of CD68-positive Cells in Meningioma. Clin. Neurol. Neurosurg. 1999, 101, 229–234. [Google Scholar] [CrossRef]
- Morantz, R.A.; Wood, G.W.; Foster, M.; Clark, M.; Gollahon, K. Macrophages in Experimental and Human Brain Tumors. Part 2: Studies of the Macrophage Content of Human Brain Tumors. J. Neurosurg. 1979, 50, 305–311. [Google Scholar] [CrossRef]
- Domingues, P.H.; Teodósio, C.; Otero, Á.; Sousa, P.; Ortiz, J.; de Macias, M.C.G.; Gonçalves, J.M.; Nieto, A.B.; Lopes, M.C.; de Oliveira, C.; et al. Association Between Inflammatory Infiltrates and Isolated Monosomy 22/del(22q) in Meningiomas. PLoS ONE 2013, 8, e74798. [Google Scholar] [CrossRef]
- Sato, K.; Kuratsu, J.; Takeshima, H.; Yoshimura, T.; Ushio, Y. Expression of Monocyte Chemoattractant Protein-1 in Meningioma. J. Neurosurg. 1995, 82, 874–878. [Google Scholar] [CrossRef]
- Nalla, A.K.; Gogineni, V.R.; Gupta, R.; Dinh, D.H.; Rao, J.S. Suppression of uPA and uPAR Blocks Radiation-induced MCP-1 Mediated Recruitment of Endothelial Cells in Meningioma. Cell Signal. 2011, 23, 1299–1310. [Google Scholar] [CrossRef]
- Utz, S.G.; See, P.; Mildenberger, W.; Thion, M.S.; Silvin, A.; Lutz, M.; Ingelfinger, F.; Rayan, N.A.; Lelios, I.; Buttgereit, A.; et al. Early Fate Defines Microglia and Non-parenchymal Brain Macrophage Development. Cell 2020, 181, 557–573.e18. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Van Wageningen, T.A.; Vlaar, E.; Kooij, G.; Jongenelen, C.A.M.; Geurts, J.J.G.; van Dam, A.M. Regulation of Microglial TMEM119 and P2RY12 Immunoreactivity in Multiple Sclerosis White and Grey Matter Lesions is Dependent on Their Inflammatory Environment. Acta Neuropathol. Commun. 2019, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Satoh, J.; Kino, Y.; Asahina, N.; Takitani, M.; Miyoshi, J.; Ishida, T.; Saito, Y. TMEM119 Marks a Subset of Microglia in the Human Brain. Neuropathology 2016, 36, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.A.; Boddeke, H.W.G.M.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Wu, S.-Y. The roles of microglia macrophages in tumor progression of brain cancer and metastatic disease. Front. Biosci. 2017, 22, 4573. [Google Scholar] [CrossRef]
- Graeber, M.B.; Scheithauer, B.W.; Kreutzberg, G.W. Microglia in Brain Tumors. Glia 2002, 40, 252–259. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Guadagno, E.; Presta, I.; Maisano, D.; Donato, A.; Pirrone, C.K.; Cardillo, G.; Corrado, S.D.; Mignogna, C.; Mancuso, T.; Donato, G.; et al. Role of Macrophages in Brain Tumor Growth and Progression. Int. J. Mol. Sci. 2018, 19, 1005. [Google Scholar] [CrossRef]
- Pinton, L.; Solito, S.; Masetto, E.; Vettore, M.; Canè, S.; Della Puppa, A.; Mandruzzato, S. Immunosuppressive Activity of Tumor-Infiltrating Myeloid Cells in Patients with Meningioma. Oncoimmunology 2018, 7, e1440931. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 Polarization and Metabolic States. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Pasqualini, C.; Kozaki, T.; Bruschi, M.; Nguyen, T.H.H.; Minard-Colin, V.; Castel, D.; Grill, J.; Ginhoux, F. Modeling the Interaction Between the Microenvironment and Tumor Cells in Brain Tumors. Neuron 2020, 108, 1025–1044. [Google Scholar] [CrossRef] [PubMed]
- Vilhardt, F.; Haslund-Vinding, J.; Jaquet, V.; McBean, G. Microglia Antioxidant Systems and Redox Signalling. Br. J. Pharmacol. 2017, 174, 1719–1732. [Google Scholar] [CrossRef]
- Haslund-Vinding, J.; McBean, G.; Jaquet, V.; Vilhardt, F. NADPH oxidases in oxidant production by microglia: Activating Receptors, Pharmacology and Association with Disease. Br. J. Pharmacol. 2017, 174, 1733–1749. [Google Scholar] [CrossRef]
- Zeltner, L.; Schittenhelm, J.; Mittelbronn, M.; Roser, F.; Tatagiba, M.; Mawrin, C.; Kim, Y.J.; Bornemann, A. The Astrocytic Response Towards Invasive Meningiomas. Neuropathol. Appl. Neurobiol. 2007, 33, 163–168. [Google Scholar] [CrossRef]
- Grund, S.; Schittenhelm, J.; Roser, F.; Tatagiba, M.; Mawrin, C.; Kim, Y.J.; Bornemann, A. The Microglial/macrophagic Response at the Tumour-brain Border of Invasive Meningiomas. Neuropathol. Appl. Neurobiol. 2009, 35, 82–88. [Google Scholar] [CrossRef]
- Nakasu, S.; Fukami, T.; Jito, J.; Matsuda, M. Microscopic Anatomy of the Brain-meningioma Interface. Brain Tumor Pathol. 2005, 22, 53–57. [Google Scholar] [CrossRef]
- Escalone Zapata, J. Astrocytes in Brain Tumours. Differentiation or Trapping? Histol. Histopathol. 1994, 9, 325–332. [Google Scholar]
- Merlo, A.; Juretic, A.; Zuber, M.; Filgueira, L.; Lüscher, U.; Caetano, V.; Ulrich, J.; Gratzl, O.; Heberer, M.; Spagnoli, G.C. Cytokine Gene Expression in Primary Brain Tumours, Metastases and Meningiomas Suggests Specific Transcription Patterns. Eur. J. Cancer 1993, 29, 2118–2125. [Google Scholar] [CrossRef]
- Levy, E.I.; Paino, J.E.; Sarin, P.S.; Goldstein, A.L.; Caputy, A.J.; Wright, D.C.; Sekhar, L.N. Enzyme-linked Immunosorbent Assay Quantification of Cytokine Concentrations in Human Meningiomas. Neurosurgery 1996, 39, 823–829. [Google Scholar] [CrossRef]
- Boyle-Walsh, E.; Hashim, I.A.; Speirs, V.; Fraser, W.D.; White, M.C. Interleukin-6 (IL-6) Production and Cell Growth of Cultured Human Ameningiomas: Interactions with Interleukin-1β (IL-1β) and Interleukin-4 (IL-4) in vitro. Neurosci. Lett. 1994, 170, 129–132. [Google Scholar] [CrossRef]
- Puri, S.; Joshi, B.H.; Sarkar, C.; Mahapatra, A.K.; Hussain, E.; Sinha, S. Expression and structure of interleukin 4 receptors in primary meningeal tumors. Cancer 2005, 103, 2132–2142. [Google Scholar] [CrossRef]
- Joshi, B.H.; Leland, P.; Asher, A.; Prayson, R.A.; Varricchio, F.; Puri, R.K. In Situ Expression of Interleukin-4 (IL-4) Receptors in Human Brain Tumors and Cytotoxicity of a Recombinant IL-4 Cytotoxin in Primary Glioblastoma Cell Cultures. Cancer Res. 2001, 61, 8058–8061. [Google Scholar]
- Schrell, U.M.H.; Koch, H.U.; Marschalek, R.; Schrauzer, T.; Anders, M.; Adams, E.; Fahlbusch, R. Formation of Autocrine Loops in Human Cerebral Meningioma Tissue by Leukemia Inhibitor Factor, Interleukin-6, and Oncostatin M: Inhibition of Meningioma Cell Growth in vitro by Recombinant Oncostatin M. J. Neurosurg. 1998, 88, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Lilja, A.; Nordborg, C.; Brun, A.; Salford, L.G.; Aman, P. Expression of the IL-6 Family Cytokines in Human Brain Tumors. Int. J. Oncol. 2001, 19, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Adams, E.F.; Rafferty, B.; Fahlbusch, R.; Dingermann, T.; Werner, H. Secretion of Interleukin-6 by Human Meningioma Cells: Possible Autocrine Inhibitory Regulation of Neoplastic Cell Growth. J. Neurosurg. 1994, 81, 394–401. [Google Scholar] [CrossRef]
- Braun, B.; Lange, M.; Oeckler, R.; Mueller, M.M. Expression of G-CSF and GM-CSF in Human Meningiomas Correlates with Increased Tumor Proliferation and Vascularization. J. Neurooncol. 2004, 68, 131–140. [Google Scholar] [CrossRef]
- Halper, J.; Jung, C.; Perry, A.; Suliman, H.; Hill, M.P.; Scheithauer, B. Expression of TGFα in Meningiomas. J. Neurooncol. 1999, 45, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Arnli, M.B.; Backer-Grøndahl, T.; Ytterhus, B.; Granli, U.S.; Lydersen, S.; Gulati, S.; Torp, S.H. Expression and Clinical Value of EGFR in Human Meningiomas. PeerJ 2017, 2017, e3140. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Dicker, A.P.; Whiton, M.; Ivanidze, J.; Hyslop, T.; Hammond, E.H.; Perry, A.; Andrews, D.W.; Kenyon, L. Assessment of Epidermal Growth Factor Receptor (EGFR) Expression in Human Meningioma. Radiat. Oncol. 2010, 5, 46. [Google Scholar] [CrossRef]
- Johnson, M.D.; Federspiel, C.F.; Gold, L.I.; Moses, H.L. Transforming Growth Factor-β and Transforming Growth Factor β-Receptor Expression in Human Meningioma Cells. Am. J. Pathol. 1992, 141, 633–642. [Google Scholar]
- Johnson, M.D. Transforming Growth Factor Beta Family in the Pathogenesis of Meningiomas. World Neurosurg. 2017, 104, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Kataoka, H.; Kawano, H.; Yokogami, K.; Nakano, S.; Goya, T.; Uchino, H.; Koono, M.; Wakisaka, S. Comparative Analysis of Expression of Hepatocyte Growth Factor and its Receptor, c-met, in Gliomas, Meningiomas and Schwannomas in Humans. Cancer Lett. 1998, 124, 149–155. [Google Scholar] [CrossRef]
- Martínez-Rumayor, A.; Arrieta, O.; Guevara, P.; Escobar, E.; Rembao, D.; Salina, C.; Sotelo, J. Coexpression of Hepatocyte Growth Factor/scatter Factor (HGF/SF) and its Receptor cMET Predict Recurrence of Meningiomas. Cancer Lett. 2004, 213, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Bajetto, A.; Porcile, C.; Pattarozzi, A.; Massa, A.; Lunardi, G.; Zona, G.; Dorcaratto, A.; Ravetti, J.L.; Spaziante, R.; et al. CXC Receptor and Chemokine Expression in Human Meningioma: SDF1/CXCR4 Signaling Activates ERK1/2 and Stimulates Meningioma Cell Proliferation. Ann. N. Y. Acad. Sci. 2006, 1090, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Würth, R.; Barbieri, F.; Bajetto, A.; Pattarozzi, A.; Gatti, M.; Porcile, C.; Zona, G.; Ravetti, J.L.; Spaziante, R.; Florio, T. Expression of CXCR7 Chemokine Receptor in Human Meningioma Cells and in Intratumoral Microvasculature. J. Neuroimmunol. 2011, 234, 115–123. [Google Scholar] [CrossRef]
- Tang, T.; Xia, Q.J.; Chen, J.B.; Xi, M.R.; Lei, D. Expression of the CXCL12/SDF-1 Chemokine Receptor CXCR7 in Human Brain Tumours. Asian Pac. J. Cancer Prev. 2012, 13, 5281–5286. [Google Scholar] [CrossRef]
- Razmkhah, M.; Arabpour, F.; Taghipour, M.; Mehrafshan, A.; Chenari, N.; Ghaderi, A. Expression of Chemokines and Chemokine Receptors in Brain Tumor Tissue Derived Cells. Asian Pac. J. Cancer Prev. 2014, 15, 7201–7205. [Google Scholar] [CrossRef]
- Li, G.; Hattermann, K.; Mentlein, R.; Mehdorn, H.M.; Held-Feindt, J. The Transmembrane Chemokines CXCL16 and CX3CL1 and Their Receptors are Expressed in Human Meningiomas. Oncol. Rep. 2013, 29, 563–570. [Google Scholar] [CrossRef]
- Hattermann, K.; Bartsch, K.; Gebhardt, H.H.; Mehdorn, H.M.; Synowitz, M.; Schmitt, A.D.; Mentlein, R.; Held-Feindt, J. “Inverse signaling” of the Transmembrane Chemokine CXCL16 Contributes to Proliferative and Anti-apoptotic Effects in Cultured Human Meningioma Cells. Cell Commun. Signal. 2016, 14, 26. [Google Scholar] [CrossRef]
- Bajetto, A.; Barbieri, F.; Pattarozzi, A.; Dorcaratto, A.; Porcile, C.; Ravetti, J.L.; Zona, G.; Spaziante, R.; Schettini, G.; Florio, T. CXCR4 and SDF1 Expression in Human Meningiomas: A Proliferative role in Tumoral Meningothelial Cells in vitro. Neuro. Oncol. 2007, 9, 3–11. [Google Scholar] [CrossRef]
- Hüttner, A.; Lei, T.; Fahlbusch, R.; Schrell, W.; Adams, E.F. Relationship between cAMP Induced Inhibition of Human Meningioma Cell Proliferation and Autocrine Secretion of Interleukin-6. Life Sci. 1996, 58, 1323–1329. [Google Scholar] [CrossRef]
- Johnson, M.D.; Okediji, E.; Woodard, A. Transforming Growth Factor-beta Effects on Meningioma Cell Proliferation and Signal Transduction Pathways. J. Neurooncol. 2004, 66, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, D.; Chen, Y.; Zhang, Y.; Song, L.; Tian, K.; Yang, Y.; Chen, L.; Weng, J.; Cao, X.; et al. Low Transforming Growth Factor-β3 Expression Predicts Tumor Malignancy in Meningiomas. World Neurosurg. 2019, 125, e353–e360. [Google Scholar] [CrossRef]
- Arıkök, A.T.; Önder, E.; Seçkin, H.; Kaçar, A.; Fesli, R.; Oğuz, A.S.; Alper, M. Osteopontin Expressions Correlate with WHO Grades and Predict Recurrence in Meningiomas. Brain Tumor Pathol. 2014, 31, 94–100. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, S.L.; Tian, X.Y.; Li, B.; Li, Z. Increased Co-expression of Macrophage Migration In-hibitory Factor and Matrix Metalloproteinase 9 is As-sociated with Tumor Recurrence of Meningioma. Int. J. Med. Sci. 2013, 10, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.Y.; Chung, M.H.; Sytwu, H.K.; Lee, H.M.; Chen, K.Y.; Chang, C.; Lin, C.K.; Yen, C.H.; Chen, J.H.; Lin, G.J.; et al. Osteopontin Expression is a Valuable Marker for Prediction of Short-term Recurrence in WHO Grade I Benign Meningiomas. J. Neurooncol. 2010, 100, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Garcia, E.; Guevara, P.; Garcia-Navarrete, R.; Ondarza, R.; Rembao, D.; Sotelo, J. Hepatocyte Growth Factor is Associated with Poor Prognosis of Malignant Gliomas and is a Predictor for Recurrence of Meningioma. Cancer 2002, 94, 3210–3218. [Google Scholar] [CrossRef] [PubMed]
- Kärjä, V.; Sandell, P.J.; Kauppinen, T.; Alafuzoff, I. Does Protein Expression Predict Recurrence of Benign World Health Organization Grade I Meningioma? Hum. Pathol. 2010, 41, 199–207. [Google Scholar] [CrossRef]
- Radin, D.P.; Tsirka, S.E. Interactions between Tumor Cells, Neurons, and Microglia in the Glioma Microenvironment. Int. J. Mol. Sci. 2020, 21, 8476. [Google Scholar] [CrossRef]
- Long, D.M. Vascular Ultrastructure in Human Meningiomas and Schwannomas. J. Neurosurg. 1973, 38, 409–419. [Google Scholar] [CrossRef]
- Ansari, S.F.; Shah, K.J.; Hassaneen, W.; Cohen-Gadol, A.A. Vascularity of Meningiomas. Handb. Clin. Neurol. 2020, 169, 153–165. [Google Scholar] [CrossRef]
- Becerra-Calixto, A.; Cardona-Gómez, G.P. The Role of Astrocytes in Neuroprotection after Brain Stroke: Potential in Cell Therapy. Front. Mol. Neurosci. 2017, 10, 88. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Nordqvist, A.C.; Smurawa, H.; Mathiesen, T. Expression of Matrix Metalloproteinases 2 and 9 in Meningiomas Associated with Different Degrees of Brain Invasiveness and Edema. J. Neurosurg. 2001, 95, 839–844. [Google Scholar] [CrossRef]
- Das, A.; Tan, W.-L.; Smith, D.R. Expression of Extracellular Matrix Markers in Benign Meningiomas. Neuropathology 2003, 23, 275–281. [Google Scholar] [CrossRef]
- Du, Z.; Abedalthagafi, M.; Aizer, A.A.; McHenry, A.R.; Sun, H.H.; Bray, M.A.; Viramontes, O.; Machaidze, R.; Brastianos, P.K.; Reardon, D.A.; et al. Increased Expression of the Immune Modulatory Molecule PDL1 (CD274) in Anaplastic Meningioma. Oncotarget 2015, 6, 4704–4716. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro. Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge Base, Treatment Outcomes, and Uncertainties. A RANO Review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef]
- Maier, A.D.; Bartek, J.J.; Eriksson, F.; Ugleholdt, H.; Juhler, M.; Broholm, H.; Mathiesen, T.I. Clinical and Histopathological Predictors of Outcome in Malignant Meningioma. Neurosurg. Rev. 2020, 43, 643–653. [Google Scholar] [CrossRef]
- Mundt, S.; Greter, M.; Flügel, A.; Becher, B. The CNS Immune Landscape from the Viewpoint of a T Cell. Trends Neurosci. 2019, 42, 667–679. [Google Scholar] [CrossRef]
- Quintana, F.J. Astrocytes to the Rescue! Glia Limitans Astrocytic Endfeet Control CNS Inflammation. J. Clin. Investig. 2017, 127, 2897–2899. [Google Scholar] [CrossRef]
- Joost, E.; Jordão, M.J.C.; Mages, B.; Prinz, M.; Bechmann, I.; Krueger, M. Microglia Contribute to the Glia Limitans Around Arteries, Capillaries and Veins Under Physiological Conditions, in a Model of Neuroinflammation and in Human Brain Tissue. Brain Struct. Funct. 2019, 224, 1301–1314. [Google Scholar] [CrossRef]
- Keane, L.; Cheray, M.; Blomgren, K.; Joseph, B. Multifaceted Microglia-key Players in Primary Brain Tumour Heterogeneity. Nat. Rev. Neurol. 2021, 17, 243–259. [Google Scholar] [CrossRef]
- Polyzoidis, S.; Koletsa, T.; Panagiotidou, S.; Ashkan, K.; Theoharides, T.C. Mast Cells in Meningiomas and Brain Inflammation. J. Neuroinflamm. 2015, 12, 170. [Google Scholar] [CrossRef]
- Han, S.J.; Reis, G.; Kohanbash, G.; Shrivastav, S.; Magill, S.T.; Molinaro, A.M.; McDermott, M.W.; Theodosopoulos, P.V.; Aghi, M.K.; Berger, M.S.; et al. Expression and Prognostic Impact of Immune Modulatory Molecule PD-L1 in Meningioma. J. Neurooncol. 2016, 130, 543–552. [Google Scholar] [CrossRef]
- Li, Y.D.; Veliceasa, D.; Lamano, J.B.; Lamano, J.B.; Kaur, G.; Biyashev, D.; Horbinski, C.M.; Kruser, T.J.; Bloch, O. Systemic and Local Immunosuppression in Patients with High-grade Meningiomas. Cancer Immunol. Immunother. 2019, 68, 999–1009. [Google Scholar] [CrossRef]
- McGranahan, T.; Therkelsen, K.E.; Ahmad, S.; Nagpal, S. Current State of Immunotherapy for Treatment of Glioblastoma. Curr. Treat. Options Oncol. 2019, 20, 24. [Google Scholar] [CrossRef]
- Baker, K.J.; Houston, A.; Brint, E. IL-1 Family Members in Cancer; Two Sides to Every Story. Front. Immunol. 2019, 10, 1197. [Google Scholar] [CrossRef]
- McKelvey, K.J.; Hudson, A.L.; Back, M.; Eade, T.; Diakos, C.I. Radiation, Inflammation and the Immune Response in Cancer. Mamm. Genome 2018, 29, 843–865. [Google Scholar] [CrossRef]
- Choudhury, A.; Raleigh, D.R. Preclinical models of meningioma: Cell culture and animal systems. Handb. Clin. Neurol. 2020, 169, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Linkous, A.; Balamatsias, D.; Snuderl, M.; Edwards, L.; Miyaguchi, K.; Milner, T.; Reich, B.; Cohen-Gould, L.; Storaska, A.; Nakayama, Y.; et al. Modeling Patient-Derived Glioblastoma with Cerebral Organoids. Cell Rep. 2019, 26, 3203–3211.e5. [Google Scholar] [CrossRef] [PubMed]
- Juliano, J.; Gil, O.; Hawkins-Daarud, A.; Noticewala, S.; Rockne, R.C.; Gallaher, J.; Massey, S.C.; Sims, P.A.; Anderson, A.R.A.; Swanson, K.R.; et al. Comparative Dynamics of Microglial and Glioma Cell Motility at the Infiltrative Margin of Brain Tumours. J. R. Soc. Interface 2018, 15, 20170582. [Google Scholar] [CrossRef]
- Mawrin, C. Animal Models of Meningiomas. Chin. Clin. Oncol. 2017, 6, S6. [Google Scholar] [CrossRef]

| Cytokine | n. Positive Meningiomas/Total | Cytokine Receptors | +/n.a. |
|---|---|---|---|
| Type 1 Cytokine Family | |||
| IL-2 | 0/5 [38], 0/9 [39], 0/11 [40] | IL-2R | n.a. |
| IL-3 | 11/11 [40] | IL-3R | n.a. |
| IL-4 | 0/11 [40], 0/5 [38], 0/9 [39] | IL-4R | + [41,42] |
| IL-5 | 0/11 [40], 1/5 [38] | IL-5R | n.a. |
| IL-6 | 11/11 [40], 1/5 [38], 10/10 [43], 9/9 [39], 0/2 [44], 10/10 [45] | IL-6R | + [43] |
| IL-7 | 0/11 [40] | IL-7R | n.a. |
| G-CSF | 28/30 [46] | G-CSFR | + [46] |
| GM-CSF | 1/5 [38], 27/30 [46] | GM-CSFR | + [46] |
| Type 2 Cytokine Family | |||
| IFN-γ | 11/11 [40] | IFN-γR | n.a. |
| IL-10 | 1/5 [38] | IL-10R | n.a. |
| OSM | 2/2 [44], 10/10 [43] | OSM-R | + [43] |
| LIF | 2/2 [44], 10/10 [43] | LIF-R | + [43] |
| TNF Cytokine family | |||
| TNF-α | 3/5 [38], 0/11 [40] | TNFR1 | n.a. |
| TNF-β | 6/11 [40] | TNFR1 | n.a. |
| IL-1 Family | |||
| IL-1α | 0/11 [40], 5/5 [38] | IL-1R | n.a. |
| IL-1β | 5/11 [40], 3/5 [38], 9/9 [39] | IL-1R | n.a. |
| Other Cytokines | |||
| TGF-α | 22/26 [47] | EGFR | + [47,48,49] |
| TGF-β1 | 8/11 [40], 3/5 [38], 3/6 [50] | TGF-βRI-III | + [51] |
| TGF-β2 | 9/11 [40], 5/5 [38], 6/6 [50] | - | - |
| TGF-β3 | 11/11 [40], 5/5 [38], 6/6 [50] | - | - |
| HGF/SF | 5/14 [52] | MET | + [52,53] |
| Chemokines | |||
| IL-8 | 11/11 [40], 14/35 [54] | CXCR1/2 | + [54] |
| CCL2/MCP-1 | 16/16 [18] | CCR2 | n.a. |
| CXCL1/2/3 | 27/27 [54] | CXCR1/2 | + [54] |
| CXCL9 | 10/36 [54] | CXCR3 | + [54] |
| CXCL10 | 15/36 [54] | CXCR3 | + [54] |
| CXCL11 | 22/22 [55] | CXCR3 | + [54] |
| CXCL12/SDF-1 | 22/22 [55], 6/6 [56], 29/55 [54] | CXCR4/CXCR7 | + [25,55,56,57] |
| CXCL13 | 1/35 [54] | CXCR5 | + [54] |
| CXCL16 | 27/27 [58], 28/28 [59] | CXCR6/tmCXCL16 | (+)/+ [58,59] |
| CX3CL1 | 27/27 [58] | CX3CR1 | + [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borch, J.d.S.; Haslund-Vinding, J.; Vilhardt, F.; Maier, A.D.; Mathiesen, T. Meningioma–Brain Crosstalk: A Scoping Review. Cancers 2021, 13, 4267. https://doi.org/10.3390/cancers13174267
Borch JdS, Haslund-Vinding J, Vilhardt F, Maier AD, Mathiesen T. Meningioma–Brain Crosstalk: A Scoping Review. Cancers. 2021; 13(17):4267. https://doi.org/10.3390/cancers13174267
Chicago/Turabian StyleBorch, Josefine de Stricker, Jeppe Haslund-Vinding, Frederik Vilhardt, Andrea Daniela Maier, and Tiit Mathiesen. 2021. "Meningioma–Brain Crosstalk: A Scoping Review" Cancers 13, no. 17: 4267. https://doi.org/10.3390/cancers13174267
APA StyleBorch, J. d. S., Haslund-Vinding, J., Vilhardt, F., Maier, A. D., & Mathiesen, T. (2021). Meningioma–Brain Crosstalk: A Scoping Review. Cancers, 13(17), 4267. https://doi.org/10.3390/cancers13174267