1. Introduction
CD200 is a single-pass, type I membrane glycoprotein belonging to the immunoglobulin superfamily [
1]. Thymocytes, B lymphocytes, a subset of T lymphocytes, endothelial cells, some dendritic cells, neurons, kidney glomeruli and syncytiothrophoblasts express CD200, while the expression of its receptor (CD200R) is more restricted and involves primarily myeloid leukocytes, such as macrophages, dendritic cells and mast cells, as well as B lymphocytes and a subset of T lymphocytes [
2].
The anti-CD200 monoclonal antibody identifies a surface membrane antigen that has recently emerged as a useful tool to better discriminate among neoplasias of mature B lymphocytes [
3]. In fact, CD200 is particularly helpful in distinguishing some disease entities, such as chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL), whose clinical behavior and prognosis is quite different [
3,
4,
5]. For this reason, CD200 has now gained a relevant role in the monoclonal antibody panels to be tested in the diagnostic work-up of lymphoid chronic leukemias by means of flow cytometry [
4].
It is well-established that CD200 is a molecule with a negative immunoregulatory function and is overexpressed on cells of various tumors including CLL [
6,
7,
8]. CLL cells can influence T-cell function via expression of cell-surface molecules and soluble factors [
9,
10], and it has also been demonstrated that CD200 is functionally involved in modulating the CLL microenvironment, which is crucial for the survival and proliferation of CLL cells [
11,
12,
13]. Regarding the possible prognostic role of the intensity of CD200 expression in CLL, to date only a limited number of studies have been conducted, and results have not been definitive [
14]. CD200 is also emerging as a potential therapeutic target in CLL [
15,
16,
17], and promising results have been recently obtained by a first-in-human study investigating samalizumab, a recombinant humanized monoclonal anti-CD200 antibody, suggesting that CD200 could represent a novel target for immune checkpoint inhibitor drugs [
18].
Interestingly, CD200 is not only present as a membrane-bound molecule but also in its soluble forms (sCD200), which can be shed from the CLL cell surface by stimulation with phorbol 12-myristate 13-acetate and TLR7 agonists in vitro [
12]. There is also some evidence that serum levels of sCD200 could be related to disease progression in patients with CLL [
19,
20,
21]. Moreover, as postulated for the membrane-bound counterpart, sCD200 may exert immunoregulatory functions. Released sCD200 is able to engage CD200R1, inducing intracellular signaling, and it has been reported that sCD200 is able to promote CLL cells’ growth in immunodeficient mice, in a process that critically involves T cells [
20]. Of note, a subpopulation of CD4+ T cells have previously been shown to express CD200R [
22], and CD200+ CLL cells and CD200RCD4+ T cells seem to colocalize in the tumor microenvironment.
With the aim of verifying whether sCD200 tested at diagnosis in CLL patients is correlated with clinical and biological features of the disease and is able to predict the prognosis, we performed the present study on a large cohort of CLL patients consecutively seen at our institutions.
2. Materials and Methods
Patients with CLL or small lymphocytic lymphoma (SLL), consecutively diagnosed at our institutions (Turin University; Fondazione Gemelli, University of Rome; Cancer Referral Center of Basilicata, Rionero in Vulture), and for whom stored serum samples collected at diagnosis were available, were included in the study. We analyzed sera from 272 patients and from 78 age- and sex-matched healthy donors (HD), used as normal controls. For 12 patients with CLL/SLL, a post-treatment sample was also available. CLL/SLL diagnosis and post-treatment response evaluation were performed according to iwCLL-NCI criteria [
23]. Clinical data were collected from electronic medical records at each Institution.
This retrospective study was performed according to the informed consent procedure approved by the local internal review board (Protocol no. 20140040750—18.11.2014), and it conforms to the provisions of the Declaration of Helsinki.
The concentration of CD200 (OX-2 membrane glycoprotein) in human serum was detected by enzyme-linked immunosorbent assay technology (ELISA) manufactured by Wuhan Fine Biotech Co., Ltd. (Wuhan, Hubei, China) (Catalogue number: EH0077). The analysis was carried out by Labospace S.r.l. (Via Virgilio Ranzato, 12, 20128 Milano, Italy) following the procedure described in the kit manual. All the serum samples were diluted with the buffer solution with the proper dilution factor and were analyzed in duplicate. The ELISA technology was employed by the following assay principle. The captured antibody (mouse monoclonal) was pre-coated onto 96-well plates, and the biotin-conjugated rabbit polyclonal antibody was used for detection. The standards, the test samples and the biotin-conjugated detection antibody were added to the wells subsequently and washed with the wash buffer. Horseradish peroxidase (HRP)–streptavidin complex was added, and unbound conjugates were washed away with wash buffer. The 3.3′, 5.5′-tetramethylbenzidine (TMB) substrates were used to detect HRP enzymatic reaction. The reaction between the TMB substrate and the HRP led to a blue-colored reaction product. Then, the reaction was quenched by the addition of the acidic stop solution, obtaining a yellow final product. The intensity of the yellow color was proportional to the CD200 amount captured on the plate. The O.D. absorbance was registered, in correspondence with the typical wavelength of 450 nm, by using a microplate reader (Victor 3V Mod. 1420 S.N. 4206516, Perkin Elmer, Shelton, CT, USA). The concentration value (pg/mL) of the CD200 was calculated by the Software WorkOut 2.5.
Descriptive statistics were used to summarize patients’ characteristics. Patient groups were compared using the Mann–Whitney test. The correlation between variables was assessed by the nonparametric Spearman’s rank correlation test. The difference between pre- and post-treatment sCD200 value was assessed with the Wilcoxon matched-pairs signed rank test. Time-to-first treatment (TTFT) was defined as the time interval between the date of CLL diagnosis and the date of first treatment or last follow-up. Overall survival (OS) was defined as time from diagnosis to death for any cause or last follow-up. TTFT and OS were estimated using the Kaplan–Meier method, and differences between groups were evaluated with the log-rank test. Multivariate Cox proportional hazards regression models were fitted to assess associations between patients’ characteristics and time-dependent variables. For the multivariate analysis, covariates were selected based on the significance in univariate analysis. Statistical analyses were performed using the IBM SPSS Statistics software version 22.0 for Windows (IBM Corp., Armonk, NY, USA). A p value < 0.05 was considered significant.
3. Results
Clinical and biological features of patients at study entry are summarized in
Table 1. For the entire cohort, median follow-up was 106 months. Median TTFT and OS were 74 months and 299 months, respectively.
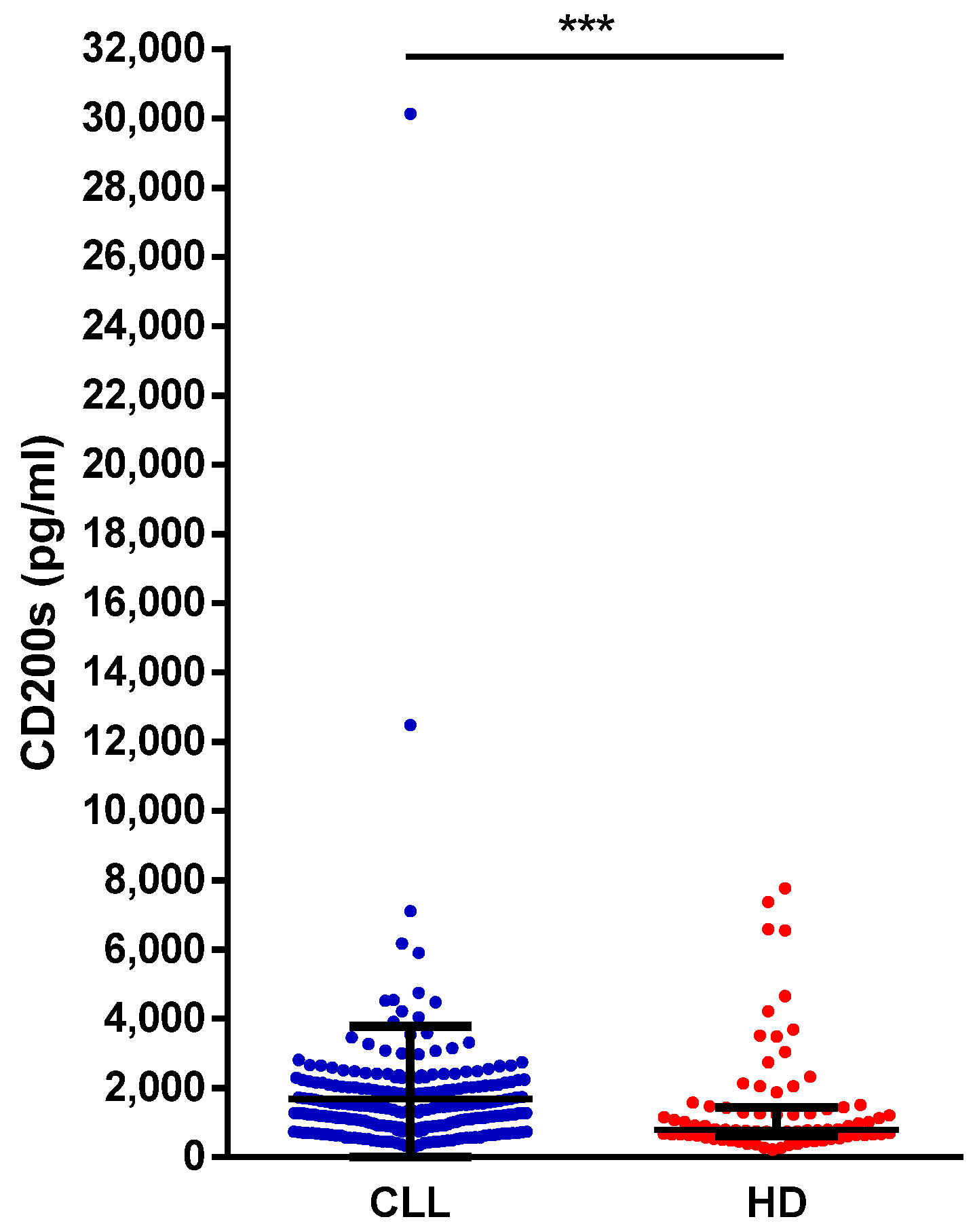
The HD and CLL cohorts were balanced in terms of age and sex distribution (HD, median age 63 years, range 42–100, 58% males; CLL, median age 66 years, range 33–90, 58% males). A significantly higher concentration of sCD200 was found in CLL patients compared to HD (median, 1281 pg/mL vs. 799 pg/mL;
p = 0.0002) (
Figure 1).
In our cohort, sCD200 was significantly higher in patients ≥66 vs. <66 years old (median sCD200, 1560 pg/mL vs. 1193 pg/mL; p = 0.0001), in those with Binet stage C vs. A/B (2055 pg/mL vs. 1274 pg/mL; p = 0.0045), in those with unmutated vs. mutated IgVH (1601 pg/mL vs. 1131 pg/mL; p < 0.0001), and in those with unfavorable (del11q or del17p) vs. favorable (normal or del13q or tris12) FISH (1897 pg/mL vs. 1239 pg/mL; p = 0.0077). On the contrary, gender, bulky disease, whole blood cell or lymphocyte count, β2-microglobulin serum levels and presence of autoimmune complications did not significantly correlate with sCD200 levels.
We selected the median value for sCD200 serum concentration in the whole CLL cohort—1281 pg/mL—as the cutoff to discriminate patients with low and high sCD200. TTFT was shorter in patients with high sCD200 concentration (median TTFT, 61 vs. 150 months;
p = 0.0006) (
Figure 2). Finally, as shown in
Figure 3, when patients were grouped according to sCD200 ≥ 1281 pg/mL or <1281 pg/mL, an impact on OS was also shown (median OS, 222 vs. 299 months;
p = 0.0044).
Time-to-first treatment was significantly longer in patients with low sCD200 compared to high sCD200 (median TTFT 150 vs. 61 months, p = 0.0006).
Overall survival was significantly longer in patients with low sCD200 compared to high sCD200 (median OS 299 vs. 222 months, p = 0.0044).
A total of 152 patients in our cohort received CLL-directed treatment (chemotherapy or chemo-immunotherapy, n = 123; anti-CD20 monoclonal antibody alone, n = 3; targeted agent, n = 26, among which ibrutinib ± anti-CD20 monoclonal antibody, n = 18; venetoclax + anti-CD20 monoclonal antibody, n = 4; rituximab and idelalisib, n = 3; zanubrutinib, n = 1) and had response data available. Overall, 42 patients were categorized as a CR (27%), 77 as a PR (51%), 6 as a nPR (4%) and 27 did not respond to therapy (no response, NR; 18%). Baseline sCD200 values appeared to have an impact on response to therapy (median sCD200 in CR vs. PR/NR patients, 1308 pg/mL vs. 1590 pg/mL; p 0.0468), and this difference seemed to increase when the analysis was restricted only to patients who had received chemotherapy or chemo-immunotherapy (1244 pg/mL vs. 1602 pg/mL; p = 0.0193). On the contrary, we did not find any association between baseline sCD200 values and response to targeted agents.
Of interest, we evaluated sCD200 serum concentration in 12 patients before and after frontline therapy (FCR,
n = 9; FC,
n = 1, BR,
n = 2). A significant decrease in sCD200 after therapy was observed (
p = 0.0093). Specifically, 8 out of 12 patients (4 CR, 1 nPR, 3 PR) had a sCD200 decrease, while in the remaining 4 patients, sCD200 concentration remained unchanged or slightly increased (1 CR, 2 PR, 1 SD) (
Figure 4,
Table 2).
In a multivariate model considering only variables that were deemed significant in univariate analysis, Binet stage C, unmutated IgVH, unfavorable FISH and high sCD200 maintained their significant impact on TTFT. However, when predictors for OS were evaluated, high sCD200 was not significant (
Table 3).
4. Discussion
CD200 is overexpressed on the surface of neoplastic cells from patients with CLL and other malignancies, delivering immunoregulatory functions [
15]. A soluble form of CD200 has also been identified [
11], which is shed from the CLL cell surface via proteolytic cleavage [
14]. In this process, called ectodomain shedding, a cell surface protein is cleaved near its transmembrane domain, releasing biologically active soluble ectodomains [
13].
Wong and colleagues previously evaluated the prognostic impact of sCD200 on a cohort of 82 patients with CLL, demonstrating that levels of sCD200 were greater in CLL patients as compared with healthy controls [
16]. In addition, in their cohort, higher sCD200 levels correlated with tumor burden, advanced stages of the disease (Rai III and IV), more courses of treatment received (using the requirement for multiple treatment as a surrogate for worst prognosis) and higher serum β
2-microglobulin levels. Of interest, patients with normal karyotype or del13q were treated more often if they also had high plasma levels of sCD200 [
20].
In our larger cohort of patients, we confirmed that CLL patients have significantly higher levels of sCD200 in serum than normal subjects. Furthermore, an association between higher sCD200 levels and poor clinical and biological prognostic factors (older age, more advanced clinical stage, unmutated IgVH and unfavorable cytogenetics abnormalities) was identified. Unfortunately, the absence of data regarding TP53 mutation status prevented us from drawing conclusions regarding a possible correlation between sCD200 and this unfavorable molecular characteristic.
It is quite surprising that bulky disease or lymphocytosis were not associated with higher levels of sCD200 in our study. The discrepancies between the findings from our series and Wong’s may be attributable to the retrospective nature of the studies and to the differences in terms of patients’ characteristics between the two cohorts. The mean age of patients in the paper by Wong and colleagues was 61 years, slightly inferior to that observed in our cohort, which was 66 years. Notably, both cohorts are constituted by younger patients, with the mean age of patients diagnosed with CLL in Western countries being 71 years, probably representing a selected group of patients referred to University or tertiary care Centers. In our study, 72% of patients had early-stage CLL (Rai 0 or Binet A), as compared to Wong’s, in which Rai stage III/IV accounted for 64.6% of cases. Additionally, patients with a normal karyotype constituted 40% of the population in our study, but only 18.6% in Wong’s. This may be the reason of the greater number of patients with normal FISH in our cohort of patients with respect to that normally found.
Of relevance, to our knowledge, this is the first paper showing a prognostic impact of baseline sCD200 in CLL, in terms of TTFT and OS, but also in terms of the quality of the response achieved after chemotherapy or chemo-immunotherapy. Interestingly, the response to targeted therapy did not seem to be influenced by the levels of sCD200 at baseline, and this may be attributable to the small number of patients receiving targeted therapy in our study and to the different kinds of response expected with this therapeutic approach compared to standard chemo-immunotherapy. It is also conceivable that the excellent efficacy of targeted treatment could somehow overcome the negative prognostic impact of baseline elevated sCD200 levels. Of note, in our series, the majority of patients treated with targeted therapies received a BTK inhibitor-based regimen, whereas only four patients were treated with venetoclax, a compound that can induce deeper responses. A possible prognostic value of sCD200 in the setting of patients treated with targeted therapies certainly needs to be confirmed in a larger cohort of patients.
Despite the limited number of tested patients, the observation that sCD200 decreases in parallel with the CLL tumor burden reduction after treatment is also interesting and is worth confirming based on a larger series of samples.
5. Conclusions
Our study supports a relevant role for CD200 not only as a diagnostic tool, when evaluated in terms of surface expression, but also as a prognostic indicator, when evaluated as a soluble factor. This outlines the role of ectodomain shedding with the release of proteins exhibiting functions similar to their cell surface counterpart. In fact, both the membrane and soluble forms of CD200 can engage the CD200 receptor, which in turn can result in increased tumor growth, by means of a negative impact on tumor immunosurveillance.
Author Contributions
Conceptualization, G.D., C.V., A.S. and L.L.; methodology, F.D., T.S., L.V., M.C., A.S.; formal analysis, C.V.; investigation, G.P., G.M., O.V., S.D., V.D.F.; data curation, D.L., F.P., A.T., V.G., R.J., F.C., C.M., V.V.; writing—original draft preparation, G.D., C.V. and L.L.; writing—review and editing, G.D., D.G.E., C.V., A.S., L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Cancer Referral Center of Basilicata, Rionero in Vulture, Italy (protocol code 20140040750—18.11.2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (G.D.) upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mathews, L.; Farrar, W. CD200 (CD200 molecule). Atlas Genet. Cytogenet. Oncol. Haematol. 2009, 13, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Barclay, A.N.; Clark, M.J.; McCaughan, G.W. Neuronal/lymphoid membrane glycoprotein MRC OX-2 is a member of the immunoglobulin superfamily with a light-chain-like structure. Biochem. Soc. Symp. 1986, 51, 149–157. [Google Scholar]
- D’Arena, G.; De Feo, V.; Pietrantuono, G.; Seneca, E.; Mansueto, G.; Villani, O.; La Rocca, F.; D’Auria, F.; Statuto, T.; Valvano, L.; et al. CD200 and chronic lymphocytic leukemia: Biological and clinical relevance. Front. Oncol. 2020, 10, 584427. [Google Scholar] [CrossRef] [PubMed]
- D’Arena, G.; Vitale, C.; Rossi, G.; Coscia, M.; Omedè, P.; D’Auria, F.; Statuto, T.; Valvano, L.; Ciolli, S.; Gilestro, M.; et al. CD200 included in a 4-marker modified Matuets score provides optimal sensitivity and specificity for the diagnosis of chronic lymphocytic leukemia. Hematol. Oncol. 2019, 36, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, G.A.; Parrinello, N.; Fargione, G.; Cardillo, K.; Chiarenza, A.; Berretta, S.; Conticello, C.; Villari, L.; Di Raimondo, F. CD200 expression may help in differential diagnosis between mantle cell lymphoma and B-cell chronic lymphocytic leukemia. Leuk. Res. 2009, 145, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Kretz-Rommel, A.F.; Qin, N.; Dakappagari, E.P.; Ravey, E.P.; McWhirter, J.; Oltean, D.; Frederickson, S.; Maruyama, T.; Wild, M.A.; Nolan, M.J.; et al. CD200 expression on tumor cells suppress antitumor immunity: New approaches to cancer immunotherapy. J. Immunol. 2007, 178, 5595–5602. [Google Scholar] [CrossRef] [Green Version]
- Moreaux, J.; Hose, D.; Reme, T.; Jourdan, E.; Hundemer, M.; Legouffe, E.; Moine, P.; Bourin, P.; Moos, M.; Corre, J.; et al. CD200 is a new prognostic factor in multiple myeloma. Blood 2006, 108, 4194–4197. [Google Scholar] [CrossRef]
- Tonks, A.; Hills, R.; White, P.; Rosie, B.; Mills, K.I.; Burnett, A.K.; Darley, R.L. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia 2007, 21, 566–568. [Google Scholar] [CrossRef]
- Gorgun, G.; Holderried, T.A.W.; Zahrieh, D.; Neuberg, D.; Gribben, J.G. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J. Clin. Investig. 2005, 115, 1797–1805. [Google Scholar] [CrossRef]
- Ramsay, A.G.; Johnson, A.J.; Lee, A.M.; Gorgun, G.; Le Dieu, R.; Blum, W.; Byrd, J.C.; Gribben, J.G. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J. Clin. Investig. 2008, 118, 2427–2437. [Google Scholar] [CrossRef]
- Herishanu, Y.; Perez-Galan, P.; Liu, D.; Biancotto, A.; Pittaluga, S.; Vire, B.; Gibellini, F.; Njuguna, N.; Lee, E.; Stennett, L.; et al. The lymph node microenvironment promotes Bcell receptor signaling, NF-kB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 2011, 117, 563–574. [Google Scholar] [CrossRef]
- Svanberg, R.; Janum, S.; Pattern, P.E.M.; Ramsay, A.G.; Niemann, C.U. Targeting the tumor microenvironment in chronic lymphocytic leukemia. Haematologica 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Bartlett, A.H.; Chen, Y.; Park, P.W. Molecular and cellular mechanisms of ectodomain shedding. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2010, 29, 925–937. [Google Scholar] [CrossRef] [Green Version]
- D’Arena, G.; Valvano, L.; Vitale, C.; Coscia, M.; Statuto, T.; Bellesi, S.; Lamorte, D.; Musto, P.; Laurenti, L.; D’Auria, F. CD200 and prognosis in chronic lymphocytic leukemia: Conflicting results. Leuk. Res. 2019, 83, 106169. [Google Scholar] [CrossRef] [PubMed]
- Gorczynski, R.M. CD200 and its receptors as targets for immunoregulation. Curr. Opin. Investig. Drugs 2005, 6, 483–488. [Google Scholar]
- Moreaux, J.; Veyrune, J.L.; Reme, T.; De Vos, J.; Klein, B. CD200: A putative therapeutic target in cancer. Biochem. Biophys. Res. Commun. 2008, 366, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallasch, C.P.; Ulbrich, S.; Brinker, R.; Hallek, M.; Uger, R.A.; Wendtner, C.M. Disruption of T cell suppression in chronic lymphocytic leukemia by CD200 blockade. Leuk. Res. 2009, 33, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, D.; Lanasa, M.C.; Farcer, C.; Pandey, M.; Whelden, M.; Faas, S.J.; Ulery, T.; Kukreja, A.; Li, L.; Bedrosian, C.L.; et al. Phase I study of samalizumab in chronic lymphocytic leukemia and multiple myeloma: Blockade of the immune checkpoint CD200. J. Immunother. Cancer 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.K.; Zhu, F.; Khatri, I.; Huo, Q.; Spaner, D.E.; Gorczynski, R.M. Characterization of CD200 ectodomain shedding. PLoS ONE 2016, 11, e0152073. [Google Scholar] [CrossRef]
- Wong, K.K.; Brenneman, F.; Chesney, A.; Spaner, D.E.; Gorczynski, R.M. Soluble CD200 is critical to engraft chronic lymphocytic leukemia cells in immunocompromised mice. Cancer Res. 2012, 72, 4931–4942. [Google Scholar] [CrossRef] [Green Version]
- Twito, T.; Chen, Z.; Khatri, I.; Wong, K.; Spaner, D.; Gorczynski, R.M. Ectodomain shedding of CD200 from the B-CLL surface is regulated by ADAM28 expression. Leuk. Res. 2013, 37, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Khatri, I.; Shaha, S.; Spaner, D.E.; Gorczynski, R.M. The role of CD200 in immunity to B cell lymphoma. J. Leukoc. Biol. 2010, 88, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Dohner, G.; Dohner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood J. Am. Soc. Hematol. 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).