Distinct Molecular Mechanisms of Altered HLA Class II Expression in Malignant Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Melanoma Cell Lines, Cell Culture and Treatment
2.2. qPCR Analysis
2.3. Monoclonal Antibodies
2.4. Flow Cytometry
2.5. Analysis of CIITA Methylation Pattern
2.6. Immunohistochemistry of Tissue Microarrays (TMAs)
2.7. Statistical Analysis
2.8. Bioinformatics Evaluation of Clinical Relevance
3. Results
3.1. HLA Class II Expression in Melanoma Cells
3.2. Correlation of Heterogeneous HLA Class II Surface Antigen Expression with Altered APM Component Expression
3.3. Distinct Responsiveness of Melanoma Cells to IFN-γ
3.4. Upregulation of HLA Class II Surface Expression upon Treatment of Melanoma Cells with Epigenetic Drugs
3.5. CIITA Expression as a Major Regulator of the HLA Class II Surface Expression
3.6. Correlation of HLA Class II APM Components and Immune Cell Infiltration with Clinical Relevance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delamarre, L.; Mellman, I.; Yadav, M. Neo approaches to cancer vaccines. Science 2015, 348, 760–761. [Google Scholar] [CrossRef]
- Kalaora, S.; Barnea, E.; Merhavi-Shoham, E.; Qutob, N.; Teer, J.K.; Shimony, N.; Schachter, J.; Rosenberg, S.A.; Besser, M.J.; Admon, A.; et al. Use of HLA peptidomics and whole exome sequencing to identify human immunogenic neo-antigens. Oncotarget 2016, 7, 5110–5117. [Google Scholar] [CrossRef]
- Weizman, E.; Cohen, C.J. Engineering T-Cell Specificity Genetically to Generate Anti-melanoma Reactivity. Methods Mol. Biol. 2015. [Google Scholar] [CrossRef]
- Valpione, S.; Campana, L. Immunotherapy for advanced melanoma: Future directions. Immunotherapy 2016, 8, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Kelderman, S.; Schumacher, T.; Haanen, J.B. Acquired and intrinsic resistance in cancer immunotherapy. Mol. Oncol. 2014, 8, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Aptsiauri, N.; Carretero, R.; Garcia-Lora, A.; Real, L.M.; Cabrera, T.; Garrido, F. Regressing and progressing metastatic lesions: Resistance to immunotherapy is predetermined by irreversible HLA class I antigen alterations. Cancer Immunol. Immunother. 2008, 57, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Carretero, R.; Wang, E.; Rodriguez, A.I.; Reinboth, J.; Ascierto, M.L.; Engle, A.M.; Liu, H.; Camacho, F.M.; Marincola, F.M.; Garrido, F.; et al. Regression of melanoma metastases after immunotherapy is associated with activation of antigen presentation and interferon-mediated rejection genes. Int. J. Cancer 2011, 131, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Tjin, E.P.M.; Konijnenberg, D.; Krebbers, G.; Mallo, H.; Drijfhout, J.W.; Franken, K.L.M.C.; Van Der Horst, C.M.A.M.; Bos, J.D.; Nieweg, O.E.; Kroon, B.B.R.; et al. T-Cell Immune Function in Tumor, Skin, and Peripheral Blood of Advanced Stage Melanoma Patients: Implications for Immunotherapy. Clin. Cancer Res. 2011, 17, 5736–5747. [Google Scholar] [CrossRef]
- Del Campo, A.B.; Kyte, J.A.; Carretero, J.; Zinchencko, S.; Méndez, R.; González-Aseguinolaza, G.; Ruiz-Cabello, F.; Aamdal, S.; Gaudernack, G.; Garrido, F.; et al. Immune escape of cancer cells with beta2-microglobulin loss over the course of metastatic melanoma. Int. J. Cancer 2013, 134, 102–113. [Google Scholar] [CrossRef]
- Seliger, B.; Maeurer, M.; Ferrone, S. Antigen-processing machinery breakdown and tumor growth. Immunol. Today 2000, 21, 455–464. [Google Scholar] [CrossRef]
- Abuzahra, F.; Heise, R.; Joussen, S.; Dreuw, A.; Merk, H.; Zwadlo-Klarwasser, G.; Baron, J.M. Adjuvant interferon alfa treatment for patients with malignant melanoma stimulates transporter proteins associated with antigen processing and proteasome activator 28. Lancet Oncol. 2004, 5, 250. [Google Scholar] [CrossRef]
- Seliger, B.; Ritz, U.; Abele, R.; Bock, M.; Tampé, R.; Sutter, G.; Drexler, I.; Huber, C.; Ferrone, S. Immune escape of melanoma: First evidence of structural alterations in two distinct components of the MHC class I antigen processing pathway. Cancer Res. 2001, 61, 8647–8650. [Google Scholar] [PubMed]
- Donia, M.; Andersen, R.; Kjeldsen, J.W.; Fagone, P.; Munir, S.; Nicoletti, F.; Andersen, M.H.; Straten, P.T.; Svane, I.M. Aberrant Expression of MHC Class II in Melanoma Attracts Inflammatory Tumor-Specific CD4+ T- Cells, Which Dampen CD8+ T-cell Antitumor Reactivity. Cancer Res. 2015, 75, 3747–3759. [Google Scholar] [CrossRef]
- Johnson, D.B.; Estrada, M.V.; Salgado, R.; Sanchez, V.; Doxie, D.; Opalenik, S.R.; Vilgelm, A.; Feld, E.; Johnson, A.S.; Greenplate, A.R.; et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat. Commun. 2016, 7, 10582. [Google Scholar] [CrossRef]
- Seliger, B.; Kloor, M.; Ferrone, S. HLA class II antigen-processing pathway in tumors: Molecular defects and clinical relevance. OncoImmunology 2017, 6, e1171447. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.R.; Phelan, J.J.; Michielsen, A.J.; Maguire, A.A.; Dunne, C.; Martin, P.; Noonan, S.; Tosetto, M.; Geraghty, R.; Fennelly, D.; et al. Characterising the prognostic potential of HLA-DR during colorectal cancer development. Cancer Immunol. Immunother. 2020, 69, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.L.; Matynia, A.P.; Factor, R.E.; Varley, K.E. Spatially-resolved quantification of proteins in triple negative breast cancers reveals differences in the immune microenvironment associated with prognosis. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Rodig, S.J.; Gusenleitner, D.; Jackson, D.G.; Gjini, E.; Giobbie-Hurder, A.; Jin, C.; Chang, H.; Lovitch, S.B.; Horak, C.; Weber, J.S.; et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci. Transl. Med. 2018, 10, eaar3342. [Google Scholar] [CrossRef]
- Hirschberg, H.; Braathen, L.R.; Thorsby, E. Antigen Presentation by Vascular Endothelial Cells and Epidermal Langerhans Cells: The Role of HLA-DR. Immunol. Rev. 1982, 66, 57–77. [Google Scholar] [CrossRef]
- Morris, A.C.; Beresford, G.W.; Mooney, M.R.; Boss, J.M. Kinetics of a Gamma Interferon Response: Expression and Assembly of CIITA Promoter IV and Inhibition by Methylation. Mol. Cell. Biol. 2002, 22, 4781–4791. [Google Scholar] [CrossRef]
- Boss, J.M.; Jensen, P.E. Transcriptional regulation of the MHC class II antigen presentation pathway. Curr. Opin. Immunol. 2003, 15, 105–111. [Google Scholar] [CrossRef]
- Elsen, P.J.V.D.; Holling, T.M.; Kuipers, H.F.; van der Stoep, N. Transcriptional regulation of antigen presentation. Curr. Opin. Immunol. 2004, 16, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Beresford, G.W.; Boss, J.M. CIITA coordinates multiple histone acetylation modifications at the HLA-DRA promoter. Nat. Immunol. 2001, 2, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Boss, J.M. Regulation of transcription of MHC class II genes. Curr. Opin. Immunol. 1997, 9, 107–113. [Google Scholar] [CrossRef]
- Masternak, K.; Mühlethaler-Mottet, A.; Villard, J.; Zufferey, M.; Steimle, V.; Reith, W. CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genome Res. 2000, 14, 1156–1166. [Google Scholar]
- Mühlethaler-Mottet, A.; Otten, L.A.; Steimle, V.; Mach, B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 1997, 16, 2851–2860. [Google Scholar] [CrossRef]
- Choi, N.M.; Boss, J.M. Multiple Histone Methyl and Acetyltransferase Complex Components Bind the HLA-DRA Gene. PLoS ONE 2012, 7, e37554. [Google Scholar] [CrossRef]
- Majumder, P.; Boss, J.M. DNA methylation dysregulates and silences the HLA-DQ locus by altering chromatin architecture. Genes Immun. 2011, 12, 291–299. [Google Scholar] [CrossRef]
- Mehta, N.T.; Truax, A.D.; Boyd, N.H.; Greer, S.F. Early epigenetic events regulate the adaptive immune response gene CIITA. Epigenetics 2011, 6, 516–525. [Google Scholar] [CrossRef]
- Serrano, A.; Castro-Vega, I.; Redondo, M. Role of Gene Methylation in Antitumor Immune Response: Implication for Tumor Progression. Cancers 2011, 3, 1672–1690. [Google Scholar] [CrossRef]
- Truax, A.D.; Koues, O.I.; Mentel, M.K.; Greer, S.F. The 19S ATPase S6a (S6′/TBP1) Regulates the Transcription Initiation of Class II Transactivator. J. Mol. Biol. 2010, 395, 254–269. [Google Scholar] [CrossRef]
- Wright, K.L.; Ting, J.P.-Y. Epigenetic regulation of MHC-II and CIITA genes. Trends Immunol. 2006, 27, 405–412. [Google Scholar] [CrossRef]
- Piskurich, J.F.; Linhoff, M.W.; Wang, Y.; Ting, J.P.-Y. Two Distinct Gamma Interferon-Inducible Promoters of the Major Histocompatibility Complex Class II Transactivator Gene Are Differentially Regulated by STAT1, Interferon Regulatory Factor 1, and Transforming Growth Factor β. Mol. Cell. Biol. 1999, 19, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Shallak, M.; Celesti, F.; Accolla, R.S. Unveiling the Hidden Treasury: CIITA-Driven MHC Class II Expression in Tumor Cells to Dig up the Relevant Repertoire of Tumor Antigens for Optimal Stimulation of Tumor Specific CD4+ T Helper Cells. Cancers 2020, 12, 3181. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Michaux, J.; Pak, H.; Huber, F.; Joseph, E.L.M.; Ramia, E.; Stevenson, B.J.; Linnebacher, M.; Accolla, R.S.; Bassani-Sternberg, M. CIITA-Transduced Glioblastoma Cells Uncover a Rich Repertoire of Clinically Relevant Tumor-Associated HLA-II Antigens. Mol. Cell. Proteom. 2021, 20, 100032. [Google Scholar] [CrossRef]
- Taramelli, D.; Fossati, G.; Mazzocchi, A.; Delia, D.; Ferrone, S.; Parmiani, G. Classes I and II HLA and melanoma-associated antigen expression and modulation on melanoma cells isolated from primary and metastatic lesions. Cancer Res. 1986, 46, 433–439. [Google Scholar] [PubMed]
- Garrido, F.; Cabrera, T.; Concha, A.; Glew, S.; Ruiz-Cabello, F.; Stern, P.L. Natural history of HLA expression during tumour development. Immunol. Today 1993, 14, 491–499. [Google Scholar] [CrossRef]
- Ma, X.C.; Hattori, T.; Kushima, R.; Terata, N.; Kodama, M. Expression of Hla-Class II Antigen in Gastric Carcinomas: Its relationship to histopathological grade, lymphocyte infiltration and five-year survival rate. Acta Oncol. 1994, 33, 187–190. [Google Scholar] [CrossRef]
- Sadanaga, N.; Kuwano, H.; Watanabe, M.; Maekawa, S.; Mori, M.; Sugimachi, K. Local immune response to tumor invasion in esophageal squamous cell carcinoma: The expression of human leukocyte antigen-DR and lymphocyte infiltration. Cancer 1994, 74, 586–591. [Google Scholar] [CrossRef]
- López-Nevot, M.A.; Garcia, E.; Romero, C.; Oliva, M.R.; Serrano, S.; Garrido, F. Phenotypic and genetic analysis of HLA class I and HLA-DR antigen expression on human melanomas. Exp. Clin. Immunogenet. 1988, 5, 203–212. [Google Scholar]
- Mostafa, A.; Codner, D.; Hirasawa, K.; Komatsu, Y.; Young, M.N.; Steimle, V.; Drover, S. Activation of ERα Signaling Differentially Modulates IFN-γ Induced HLA-Class II Expression in Breast Cancer Cells. PLoS ONE 2014, 9, e87377. [Google Scholar] [CrossRef]
- Van Duinen, S.G.; Ruiter, D.J.; Broecker, E.B.; Van Der Velde, E.A.; Sorg, C.; Welvaart, K.; Ferrone, S. Level of HLA antigens in locoregional metastases and clinical course of the disease in patients with melanoma. Cancer Res. 1988, 48, 1019–1025. [Google Scholar]
- Zaloudik, J.; Moore, M.; Ghosh, A.K.; Mechl, Z.; Rejthar, A. DNA content and MHC class II antigen expression in malignant mel-anoma: Clinical course. J. Clin. Pathol. 1988, 41, 1078–1084. [Google Scholar] [CrossRef]
- Degenhardt, Y.; Huang, J.; Greshock, J.; Horiates, G.; Nathanson, K.; Yang, X.; Herlyn, M.; Weber, B. DistinctMHCgene expression patterns during progression of melanoma. Genes Chromosom. Cancer 2009, 49, 144–154. [Google Scholar] [CrossRef]
- Michel, S.; Linnebacher, M.; Alcaniz, J.; Voss, M.; Wagner, R.; Dippold, W.; Becker, C.; Doeberitz, M.V.K.; Ferrone, S.; Kloor, M. Lack of HLA class II antigen expression in microsatellite unstable colorectal carcinomas is caused by mutations in HLA class II regulatory genes. Int. J. Cancer 2010, 127, 889–898. [Google Scholar] [CrossRef]
- Iizuka, N.; Oka, M. CIITA methylation and decreased levels of HLA-DR in tumour progression. Br. J. Cancer 2004, 91, 813–815. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morimoto, Y.; Toyota, M.; Satoh, A.; Murai, M.; Mita, H.; Suzuki, H.; Takamura, Y.; Ikeda, H.; Ishida, T.; Sato, N.; et al. Inactivation of class II transactivator by DNA methylation and histone deacetylation associated with absence of HLA-DR induction by interferon-γ in haematopoietic tumour cells. Br. J. Cancer 2004, 90, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Rimondi, A.P.; Buzzoni, D.; Luppi, L.; Nastruzzi, C.; Orlando, P.; Gambari, R. Hypomethylation of the human HLA-DR alpha gene in breast carcinomas and autologous metastases. Clin. Exp. Metastasis 1989, 7, 417–426. [Google Scholar] [CrossRef]
- Redondo, M.; Ruiz-Cabello, F.; Concha, A.; Hortas, M.L.; Serrano, A.; Morell, M.; Garrido, F. Differential expression of MHC class II genes in lung tumour cell lines. Eur. J. Immunogenetics 1998, 25, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Truax, A.D.; Thakkar, M.; Greer, S.F. Dysregulated Recruitment of the Histone Methyltransferase EZH2 to the Class II Transactivator (CIITA) Promoter IV in Breast Cancer Cells. PLoS ONE 2012, 7, e36013. [Google Scholar] [CrossRef] [PubMed]
- Codolo, G.; Toffoletto, M.; Chemello, F.; Coletta, S.; Teixidor, G.S.; Battaggia, G.; Munari, G.; Fassan, M.; Cagnin, S.; De Bernard, M. Helicobacter pylori Dampens HLA-II Expression on Macrophages via the Up-Regulation of miRNAs Targeting CIITA. Front. Immunol. 2020, 10, 2923. [Google Scholar] [CrossRef]
- Forero, A.; Li, Y.; Chen, D.; Grizzle, W.E.; Updike, K.L.; Merz, N.D.; Downs-Kelly, E.; Burwell, T.C.; Vaklavas, C.; Buchsbaum, D.J.; et al. Expression of the MHC Class II Pathway in Triple-Negative Breast Cancer Tumor Cells Is Associated with a Good Prognosis and Infiltrating Lymphocytes. Cancer Immunol. Res. 2016, 4, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Rodeck, U.; Herlyn, M.; Menssen, H.D.; Furlanetto, R.W.; Koprowsk, H. Metastatic but not primary melanoma cell lines grow in vitro independently of exogenous growth factors. Int. J. Cancer 1987, 40, 687–690. [Google Scholar] [CrossRef]
- Wulfänger, J.; Biehl, K.; Tetzner, A.; Wild, P.; Ikenberg, K.; Meyer, S.; Seliger, B. Heterogeneous expression and functional relevance of the ubiquitin carboxyl-terminal hydrolase L1 in melanoma. Int. J. Cancer 2013, 133, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Surmann, E.-M.; Voigt, A.Y.; Michel, S.; Bauer, K.; Reuschenbach, M.; Ferrone, S.; Doeberitz, M.V.K.; Kloor, M. Association of high CD4-positive T cell infiltration with mutations in HLA class II-regulatory genes in microsatellite-unstable colorectal cancer. Cancer Immunol. Immunother. 2015, 64, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Fuchs, T.J.; Bosserhoff, A.K.; Hofstädter, F.; Pauer, A.; Roth, V.; Buhmann, J.M.; Moll, I.; Anagnostou, N.; Brandner, J.M.; et al. A Seven-Marker Signature and Clinical Outcome in Malignant Melanoma: A Large-Scale Tissue-Microarray Study with Two Independent Patient Cohorts. PLoS ONE 2012, 7, e38222. [Google Scholar] [CrossRef]
- Wickenhauser, C.; Bethmann, D.; Kappler, M.; Eckert, A.; Steven, A.; Bukur, J.; Fox, B.; Beer, J.; Seliger, B. Tumor Microenvironment, HLA Class I and APM Expression in HPV-Negative Oral Squamous Cell Carcinoma. Cancers 2021, 13, 620. [Google Scholar] [CrossRef]
- Amaldi, I.; Reith, W.; Berte, C.; Mach, B. Induction of HLA class II genes by IFN-gamma is transcriptional and requires a trans-acting protein. J. Immunol. 1989, 142, 999–1004. [Google Scholar]
- Campoli, M.; Ferrone, S. HLA antigen changes in malignant cells: Epigenetic mechanisms and biologic significance. Oncogene 2008, 27, 5869–5885. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Luo, Y. Histone Acetylation and the Regulation of Major Histocompatibility Class II Gene Expression. Adv. Protein Chem. Struct. Biol. 2017, 106, 71–111. [Google Scholar] [CrossRef]
- Van den Elsen, P.J.; van der Stoep, N.; Vietor, H.E.; Wilson, L.; van Zutphen, M.; Gobin, S.J. Lack of CIITA expression is central to the absence of antigen presentation functions of trophoblast cells and is caused by methylation of the IFN-gamma inducible pro-moter (PIV) of CIITA. Hum. Immunol. 2000, 61, 850–862. [Google Scholar] [CrossRef]
- Satoh, A.; Toyota, M.; Ikeda, H.; Morimoto, Y.; Akino, K.; Mita, H.; Suzuki, H.; Sasaki, Y.; Kanaseki, T.; Takamura, Y.; et al. Epigenetic inactivation of class II transactivator (CIITA) is associated with the absence of interferon-γ-induced HLA-DR expression in colorectal and gastric cancer cells. Oncogene 2004, 23, 8876–8886. [Google Scholar] [CrossRef][Green Version]
- Van der Stoep, N.; Biesta, P.; Quinten, E.; van den Elsen, P.J. Lack of IFN-gamma-mediated induction of the class II transactivator (CIITA) through promoter methylation is predominantly found in developmental tumor cell lines. Int. J. Cancer 2002, 97, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Linnemann, C.; Van Buuren, M.M.; Bies, L.; Verdegaal, E.M.E.; Schotte, R.; Calis, J.J.A.; Behjati, S.; Velds, A.; Hilkmann, H.; El Atmioui, D.; et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat. Med. 2015, 21, 81–85. [Google Scholar] [CrossRef]
- Sconocchia, G.; Eppenberger-Castori, S.; Zlobec, I.; Karamitopoulou, E.; Arriga, R.; Coppola, A.; Caratelli, S.; Spagnoli, G.C.; Lauro, D.; Lugli, A.; et al. HLA Class II Antigen Expression in Colorectal Carcinoma Tumors as a Favorable Prognostic Marker. Neoplasia 2014, 16, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Cioni, B.; Jordanova, E.S.; Hooijberg, E.; Van Der Linden, R.; De Menezes, R.X.; Tan, K.; Willems, S.; Ma, J.B.W.E.; Broeks, A.; Bergman, A.M.; et al. HLA class II expression on tumor cells and low numbers of tumor-associated macrophages predict clinical outcome in oropharyngeal cancer. Head Neck 2018, 41, 463–478. [Google Scholar] [CrossRef]
- Axelrod, M.; Cook, R.S.; Johnson, D.B.; Balko, J.M. Biological Consequences of MHC-II Expression by Tumor Cells in Cancer. Clin. Cancer Res. 2019, 25, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.T.; Fernandez, D.R.; Murphy, S.P.; Braziel, R.M.; Campo, E.; Chan, W.C.; Delabie, J.; Gascoyne, R.D.; Staudt, L.M.; Jaffe, E.S.; et al. Decreased major histocompatibility complex class II expression in diffuse large B-cell lymphoma does not correlate with CpG methylation of class II transactivator promoters III and IV. Leuk. Lymphoma 2009, 50, 1875–1878. [Google Scholar] [CrossRef]
- Solheim, O.; Johansen, T.F.; Cappelen, J.; Unsgård, G.; Selbekk, T. Transsellar Ultrasound in Pituitary Surgery With a Designated Probe. Oper. Neurosurg. 2015, 12, 128–134. [Google Scholar] [CrossRef]
- Toffalori, C.; Zito, L.; Gambacorta, V.; Riba, M.; Oliveira, G.; Bucci, G.; Barcella, M.; Spinelli, O.; Greco, R.; Crucitti, L.; et al. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat. Med. 2019, 25, 603–611. [Google Scholar] [CrossRef]
- Barbaro, A.D.L.; De Ambrosis, A.; Banelli, B.; Pira, G.L.; Aresu, O.; Romani, M.; Ferrini, S.; Accolla, R.S. Methylation of CIITA promoter IV causes loss of HLA-II inducibility by IFN- in promyelocytic cells. Int. Immunol. 2008, 20, 1457–1466. [Google Scholar] [CrossRef]
- Langford, G.A.; Ainsworth, L.; Marcus, G.J.; Shrestha, J. Photoperiod Entrainment of Testosterone, Luteinizing Hormone, Follicle-Stimulating Hormone, and Prolactin Cycles in Rams in Relation to Testis Size and Semen Quality1. Biol. Reprod. 1987, 37, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, T.; Mendez, R.; Del Campo, A.; Aptsiauri, N.; Martin, J.; Orozco, G.; Pawelec, G.; Schadendorf, D.; Ruiz-Cabello, F.; Garrido, F. Patterns of constitutive and IFN-γ inducible expression of HLA class II molecules in human melanoma cell lines. Immunogenetics 2006, 59, 123–133. [Google Scholar] [CrossRef]
- Turner, T.B.; Meza-Perez, S.; Londoño, A.; Katre, A.; Peabody, J.E.; Smith, H.J.; Forero, A.; Norian, L.A.; Jr, J.M.S.; Buchsbaum, D.J.; et al. Epigenetic modifiers upregulate MHC II and impede ovarian cancer tumor growth. Oncotarget 2017, 8, 44159–44170. [Google Scholar] [CrossRef] [PubMed]
- Steimle, V.; Otten, L.A.; Zufferey, M.; Mach, B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome). Cell 1993, 75, 135–146. [Google Scholar] [CrossRef]
- Yavorski, J.M.; Blanck, G. MHC class II associated stomach cancer mutations correlate with lack of subsequent tumor development. Mol. Clin. Oncol. 2017, 7, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Ramia, E.; Chiaravalli, A.M.; Eddine, F.B.N.; Tedeschi, A.; Sessa, F.; Accolla, R.S.; Forlani, G. CIITA-related block of HLA class II expression, upregulation of HLA class I, and heterogeneous expression of immune checkpoints in hepatocarcinomas: Implications for new therapeutic approaches. OncoImmunology 2019, 8, 1548243. [Google Scholar] [CrossRef]
- Watts, U.M.; Macdonald, C.; Bailey, C.L.; Meegan, J.M.; Peters, C.J.; McKee, K.T. Experimental Infection of Phlebotomus Papatasi with Sand Fly Fever Sicilian Virus. Am. J. Trop. Med. Hyg. 1988, 39, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Cycon, K.A.; Mulvaney, K.; Rimsza, L.M.; Persky, D.; Murphy, S.P. Histone deacetylase inhibitors activate CIITA and MHC class II antigen expression in diffuse large B-cell lymphoma. Immunology 2013, 140, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Vinyals, A.; Alía, P.; Broceño, C.; Chen, F.; Adrover, M.; Gelpi, C.; Price, J.E.; Fabra, À. Differential expression of MHC class II molecules in highly metastatic breast cancer cells is mediated by the regulation of the CIITA transcription: Implication of CIITA in tumor and metastasis development. Int. J. Biochem. Cell Biol. 2006, 38, 544–562. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Chang, W.-A.; Lin, E.-S.; Chen, Y.-J.; Kuo, P.-L. Expressions of HLA Class II Genes in Cutaneous Melanoma Were Associated with Clinical Outcome: Bioinformatics Approaches and Systematic Analysis of Public Microarray and RNA-Seq Datasets. Diagnostics 2019, 9, 59. [Google Scholar] [CrossRef] [PubMed]
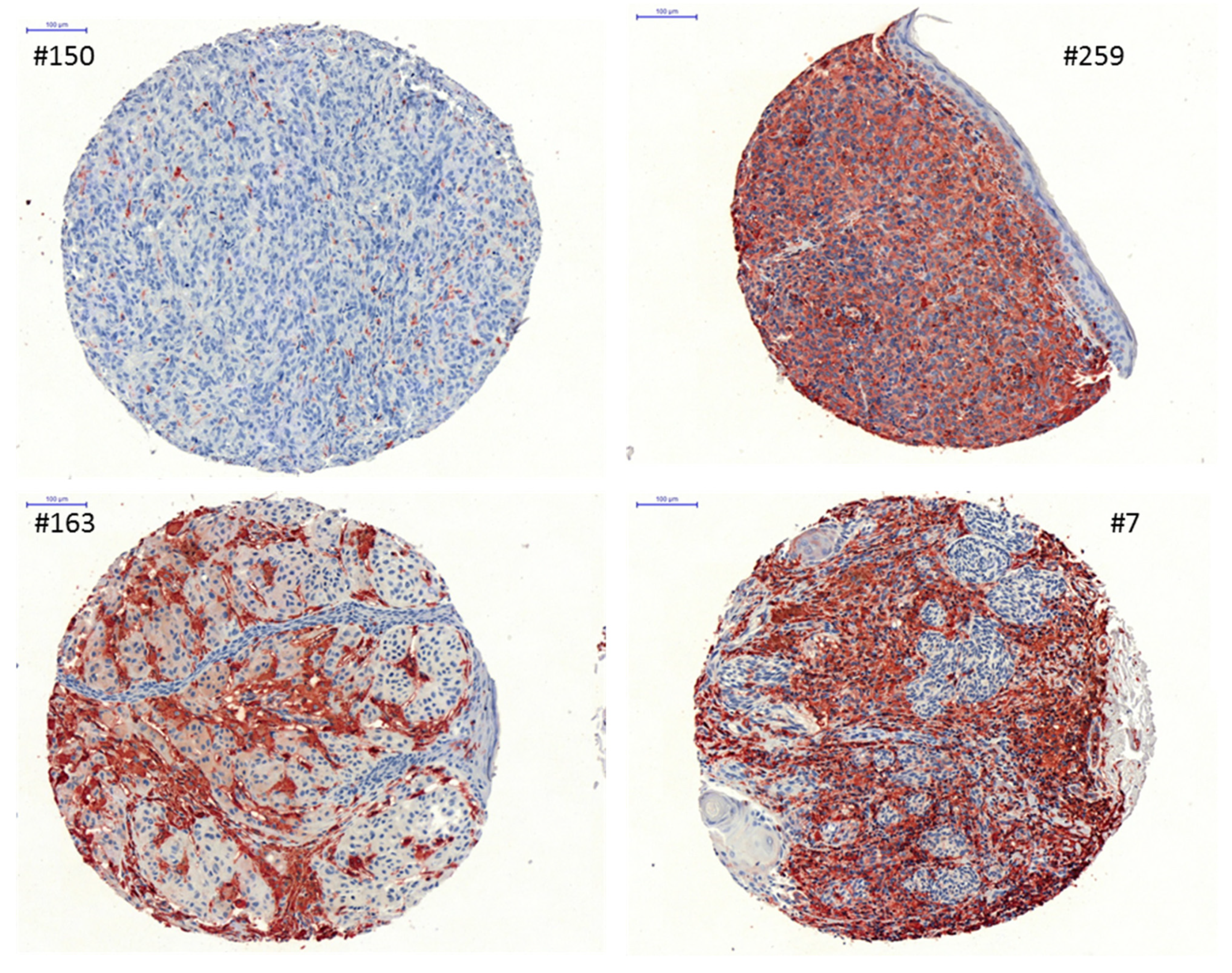
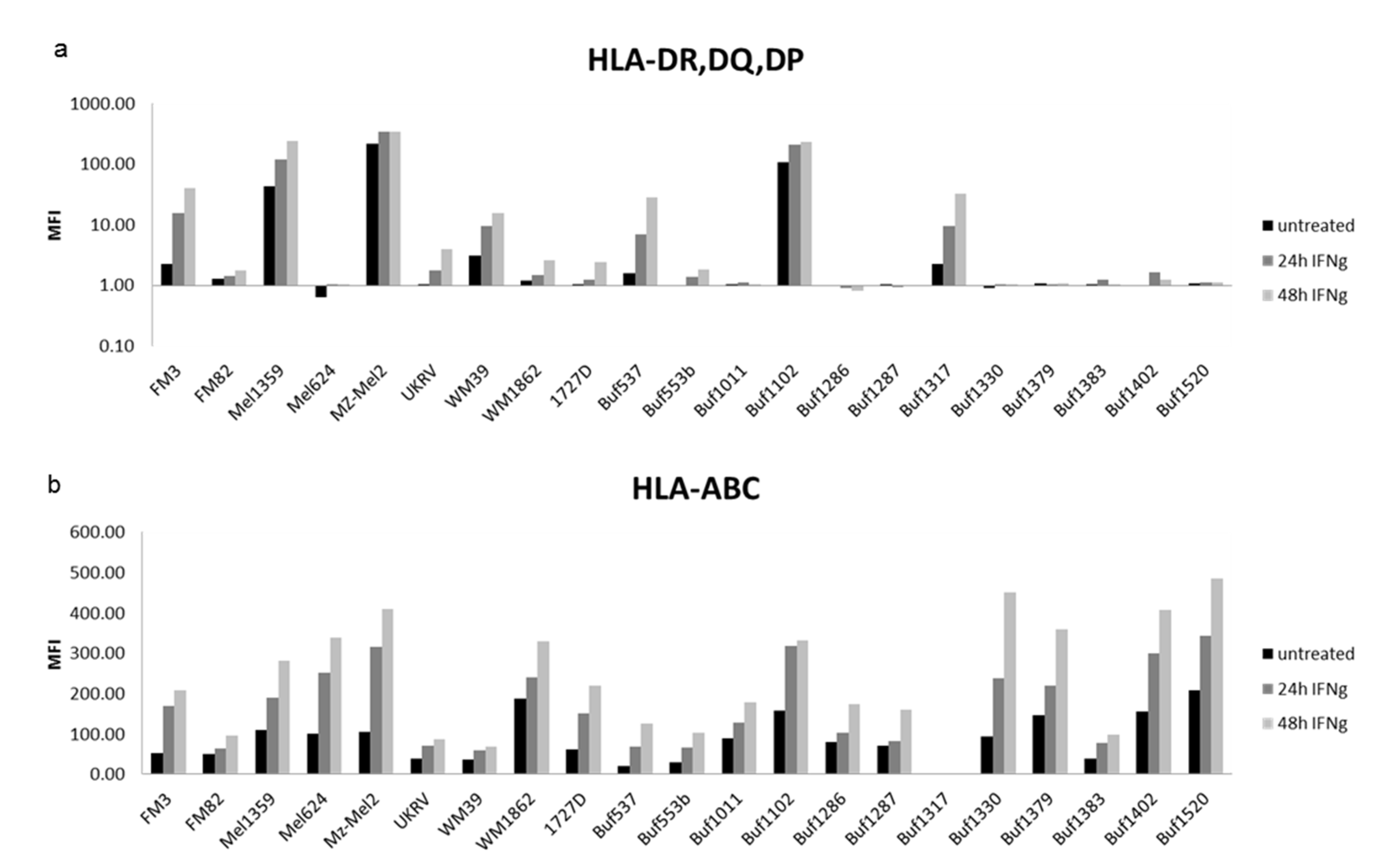
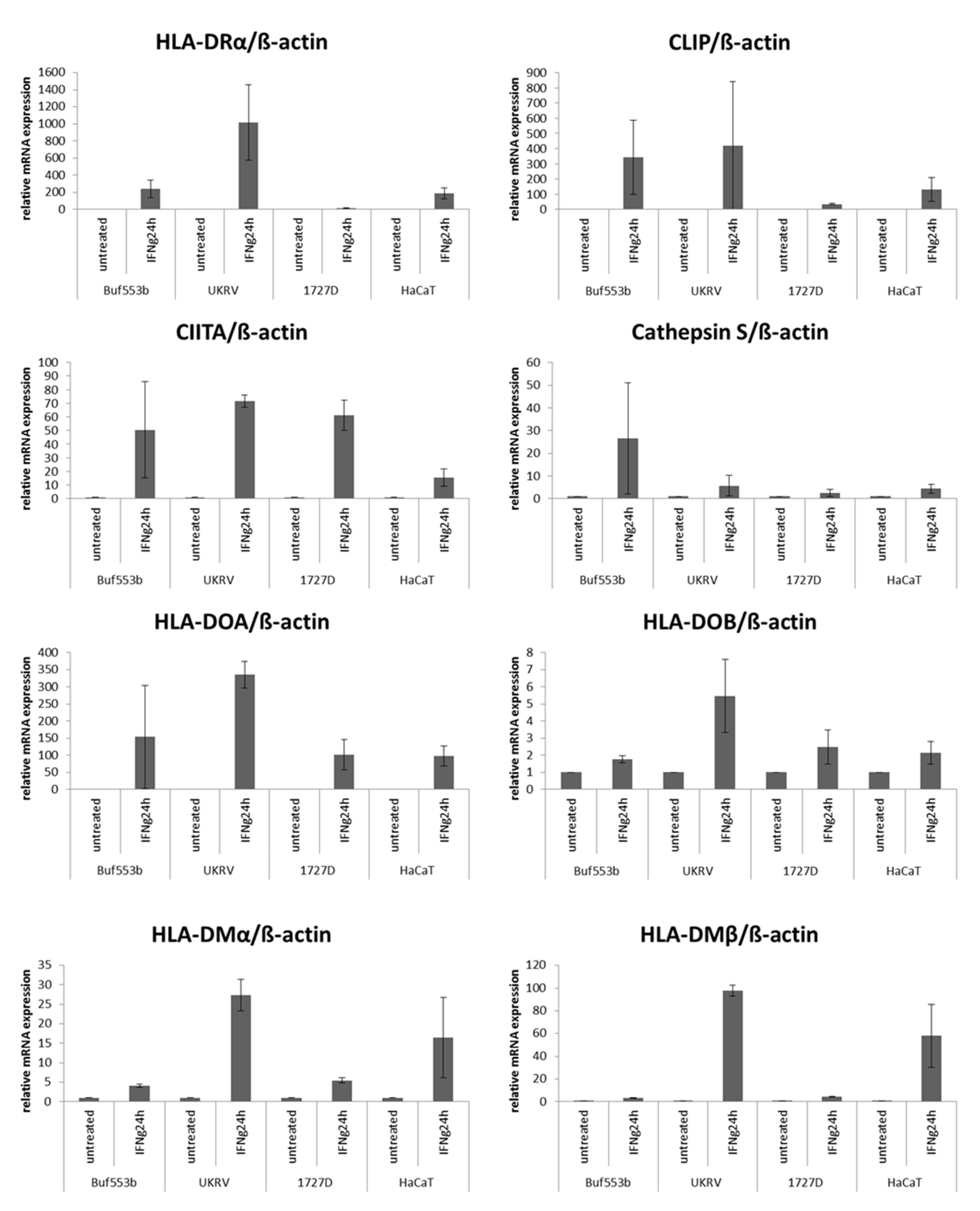
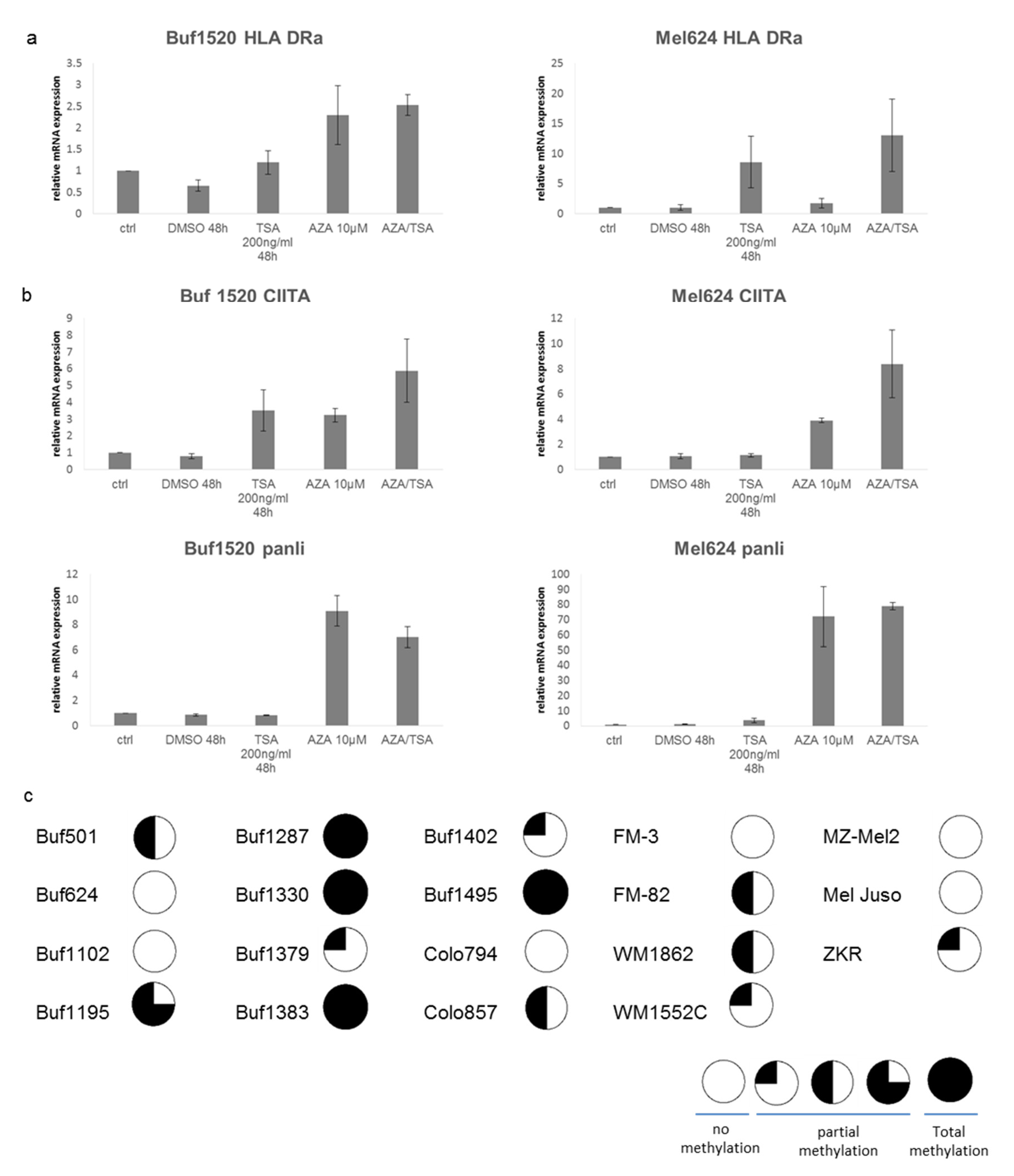
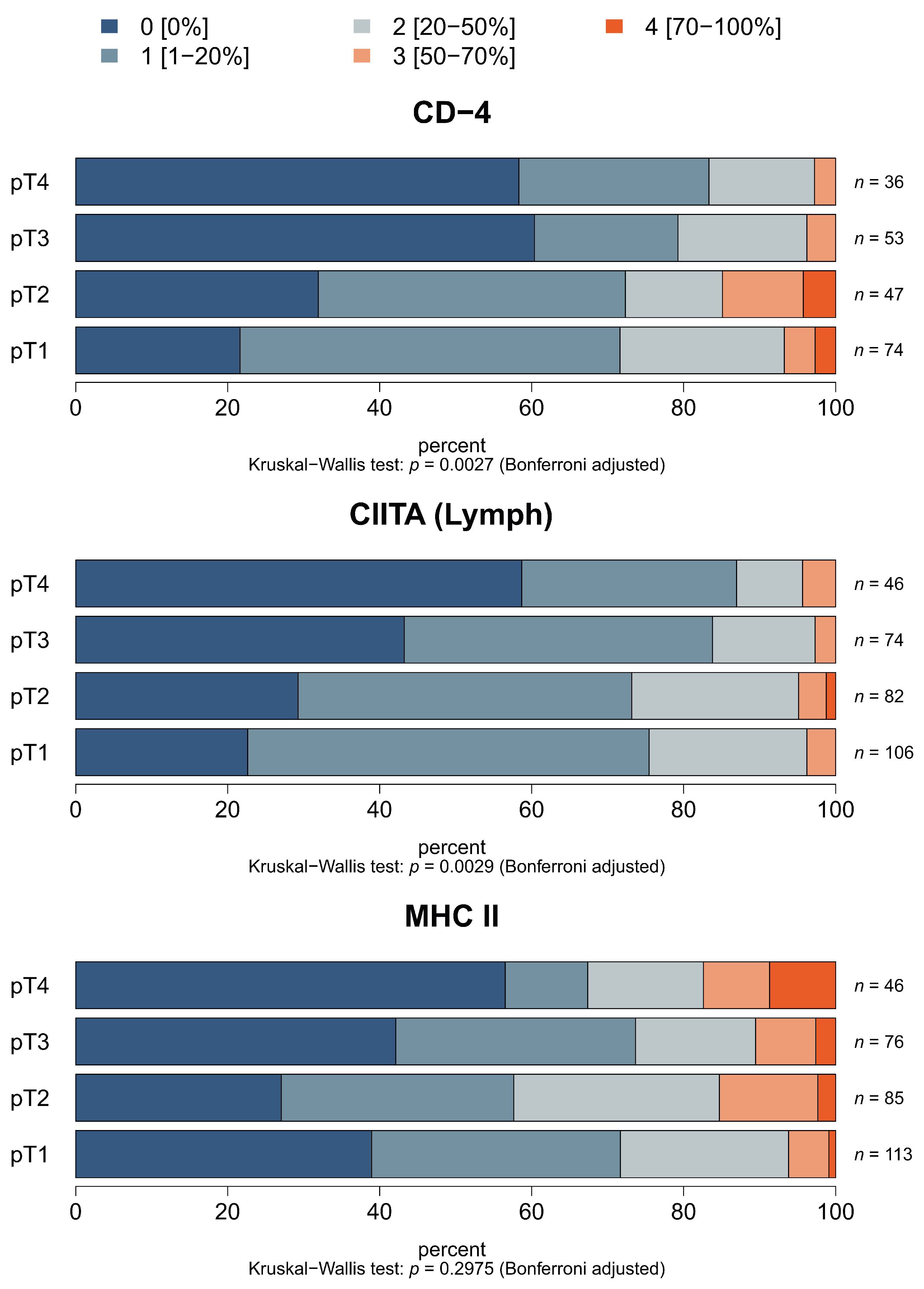
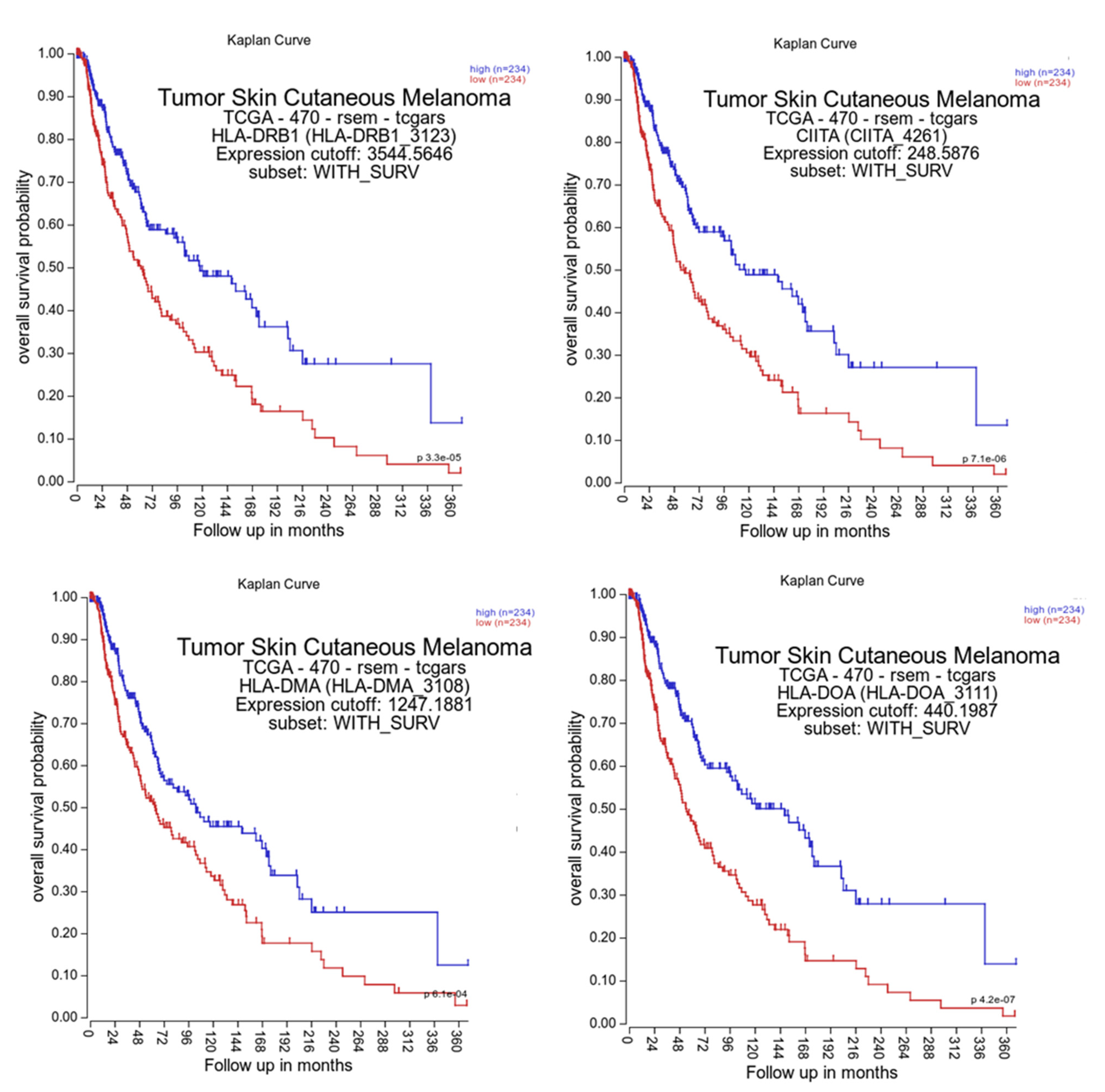
| Samples | HLA-II APM | n 1 | High | Medium | Low | Negative |
|---|---|---|---|---|---|---|
| Melanoma lesions | HLA class II | 326 | 37 (11.33%) | 68 (20.86%) | 95 (29.14%) | 126 (38.65%) |
| HLA-DO | 294 | 0 | 13 (4.42%) | 26 (8.84%) | 255 (86.73%) | |
| CIITA tumor | 314 | 0 | 1 (0.32%) | 23 (7.32%) | 284 (90.45%) | |
| CIITA lymph. | 314 | 12 (3.82%) | 55 (17.52%) | 139 (44.27%) | 108 (34.39%) | |
| Melanoma cell lines | HLA class II | 47 | 4 (8.51%) | 13 (27.66%) | 5 (10.64%) | 25 (53.19%) |
| Cell Line | qPCR 1 | Flow Cytometry 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CIITA | HLA-DRa | HLA pan li | Cathepsin | HLA-DMa | HLA-DMb | HLA-DOA | HLA-DOB | CLIP | Classification | % Positive | |
| melanocytes | 2 | 2 | 3 | 3 | 3 | 2 | 1 | 1 | 2 | neg. | |
| HaCAT | 2 | 2 | 3 | 4 | 3 | 2 | 1 | 2 | 2 | neg. | |
| BUF836 | 1 | 2 | 3 | 3 | 2 | 2 | 1 | 1 | 3 | neg. | |
| Mel 624 | 1 | 1 | 1 | 2 | 3 | 2 | 1 | 1 | 1 | neg. | |
| BUF1495 | 1 | 1 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | neg. | |
| BUF1286 | 1 | 1 | 2 | 3 | 3 | 3 | 2 | 1 | 1 | neg. | |
| BUF1182 | 1 | 1 | 2 | 1 | 3 | 2 | 1 | 1 | 1 | neg. | |
| BUF553b | 1 | 1 | 1 | 3 | 3 | 2 | 1 | 1 | 1 | neg. | |
| FM6 | 2 | 1 | 3 | 3 | 3 | 2 | 1 | 2 | 3 | neg. | |
| BUF1330 | 1 | 1 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | neg. | |
| BUF501 ATC | 1 | 1 | 2 | 3 | 3 | 2 | 1 | 1 | 1 | neg. | |
| BUF1011 | 1 | 1 | 2 | 2 | 3 | 2 | 1 | 1 | 2 | neg. | |
| BUF1195 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | neg. | |
| BUF1383 | 1 | 1 | 1 | 3 | 2 | 1 | 1 | 1 | 1 | neg. | |
| FM81 | 1 | 1 | 1 | 3 | 3 | 3 | 2 | 2 | 1 | neg. | |
| BUF1402 | 1 | 1 | 2 | 2 | 3 | 2 | 1 | 1 | 1 | neg. | |
| BUF1287 | 2 | 3 | 5 | 3 | 3 | 2 | 2 | 1 | 3 | neg. | |
| 1727D | 2 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | 2 | neg. | |
| SK Mel29 1 | 2 | 2 | 2 | 2 | 3 | 2 | 1 | 2 | 1 | neg. | |
| BUF1280 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | neg. | |
| Brooks 86 | 1 | 1 | 1 | 3 | 3 | 2 | 1 | 1 | 1 | neg. | |
| UKRV Mel14a | 2 | 1 | 2 | 2 | 3 | 2 | 1 | 3 | 1 | neg. | |
| BUF1379 | 1 | 1 | 2 | 2 | 3 | 2 | 1 | 1 | 1 | neg. | |
| COLO 857 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | neg. | |
| NA-8 | 1 | 1 | 2 | 2 | 3 | 1 | 1 | 1 | 1 | neg. | |
| BUF1520 | 1 | 1 | 3 | 2 | 2 | 2 | 1 | 1 | 3 | neg. | |
| BUF624 | 1 | 2 | 2 | 3 | 3 | 2 | 1 | 1 | 2 | neg. | |
| WM1862 | 1 | 2 | 3 | 3 | 3 | 2 | 1 | 2 | 2 | low | 11.9 |
| MZ Mel3 | 2 | 4 | 3 | 2 | 3 | 3 | 1 | 1 | 3 | low | 13.3 |
| M17 | 3 | 4 | 4 | 3 | 3 | 3 | 3 | 1 | 4 | low | 11.5 |
| FM82 | 2 | 3 | 3 | 2 | 2 | 1 | 1 | 1 | 4 | low | 3.5 |
| BUF1088 | 1 | 1 | 2 | 3 | 3 | 3 | 2 | 1 | 2 | low | 13.7 |
| FM79 | 2 | 3 | 3 | 1 | 4 | 3 | 2 | 2 | 3 | med. | 23.3 |
| BUF537 | 3 | 4 | 4 | 3 | 4 | 3 | 1 | 1 | 4 | med. | 27.4 |
| FM28 | 3 | 4 | 4 | 3 | 4 | 3 | 2 | 2 | 3 | med. | 24.9 |
| WM39 | 2 | 4 | 4 | 3 | 3 | 2 | 1 | 2 | 4 | med. | all |
| FM3 | 4 | 5 | 5 | 2 | 4 | 4 | 4 | 3 | 5 | med. | 35.8 |
| MKR | 3 | 4 | 4 | 2 | 4 | 2 | 2 | 1 | 4 | med. | 58.4 |
| WM1552c | 1 | 2 | 1 | 3 | 3 | 3 | 2 | 2 | 1 | med. | all |
| 2058 Brooks | 2 | 4 | 4 | 4 | 3 | 2 | 2 | 1 | 4 | med. | all |
| BUF526 | 2 | 3 | 4 | 2 | 3 | 2 | 1 | 2 | 5 | med. | all |
| BUF1317 | 2 | 3 | 4 | 2 | 3 | 2 | 1 | 2 | 4 | med. | all |
| GR-M | 3 | 4 | 4 | 3 | 3 | 2 | 1 | 2 | 4 | med. | all |
| Mel1359 | 3 | 4 | 4 | 3 | 4 | 4 | 3 | 2 | 4 | med. | all |
| Mel JUSO | 4 | 5 | 5 | 1 | 4 | 4 | 4 | 2 | 5 | med. | all |
| COLO794 | 3 | 4 | 5 | 3 | 4 | 4 | 2 | 2 | 4 | high | all |
| MZMel2 | 4 | 5 | 5 | 2 | 4 | 4 | 4 | 3 | 5 | high | all |
| ZKR | 3 | 4 | 5 | 2 | 4 | 4 | 4 | 2 | 5 | high | all |
| BUF1102 | 3 | 5 | 5 | 1 | 4 | 3 | 4 | 2 | 5 | high | all |
| HLA Component | Melanoma Cells | ||
|---|---|---|---|
| High | Medium | Negative | |
| CIITA | 3 | 21 | 23 |
| CLIP | 16 | 13 | 18 |
| pan-li | 16 | 23 | 8 |
| HLA-DRa | 15 | 11 | 21 |
| HLA-DOA | 5 | 12 | 30 |
| HLA-DOB | 0 | 19 | 28 |
| cathepsin S | 1 | 40 | 6 |
| HLA-DMa | 11 | 36 | 0 |
| HLA-DMb | 6 | 36 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, S.; Handke, D.; Mueller, A.; Biehl, K.; Kreuz, M.; Bukur, J.; Koehl, U.; Lazaridou, M.-F.; Berneburg, M.; Steven, A.; et al. Distinct Molecular Mechanisms of Altered HLA Class II Expression in Malignant Melanoma. Cancers 2021, 13, 3907. https://doi.org/10.3390/cancers13153907
Meyer S, Handke D, Mueller A, Biehl K, Kreuz M, Bukur J, Koehl U, Lazaridou M-F, Berneburg M, Steven A, et al. Distinct Molecular Mechanisms of Altered HLA Class II Expression in Malignant Melanoma. Cancers. 2021; 13(15):3907. https://doi.org/10.3390/cancers13153907
Chicago/Turabian StyleMeyer, Stefanie, Diana Handke, Anja Mueller, Katharina Biehl, Markus Kreuz, Jürgen Bukur, Ulrike Koehl, Maria-Filothei Lazaridou, Mark Berneburg, André Steven, and et al. 2021. "Distinct Molecular Mechanisms of Altered HLA Class II Expression in Malignant Melanoma" Cancers 13, no. 15: 3907. https://doi.org/10.3390/cancers13153907
APA StyleMeyer, S., Handke, D., Mueller, A., Biehl, K., Kreuz, M., Bukur, J., Koehl, U., Lazaridou, M.-F., Berneburg, M., Steven, A., Massa, C., & Seliger, B. (2021). Distinct Molecular Mechanisms of Altered HLA Class II Expression in Malignant Melanoma. Cancers, 13(15), 3907. https://doi.org/10.3390/cancers13153907






