Survival of Patients with Epidermal Growth Factor Receptor-Mutated Metastatic Non-Small Cell Lung Cancer Treated beyond the Second Line in the Tyrosine Kinase Inhibitor Era
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
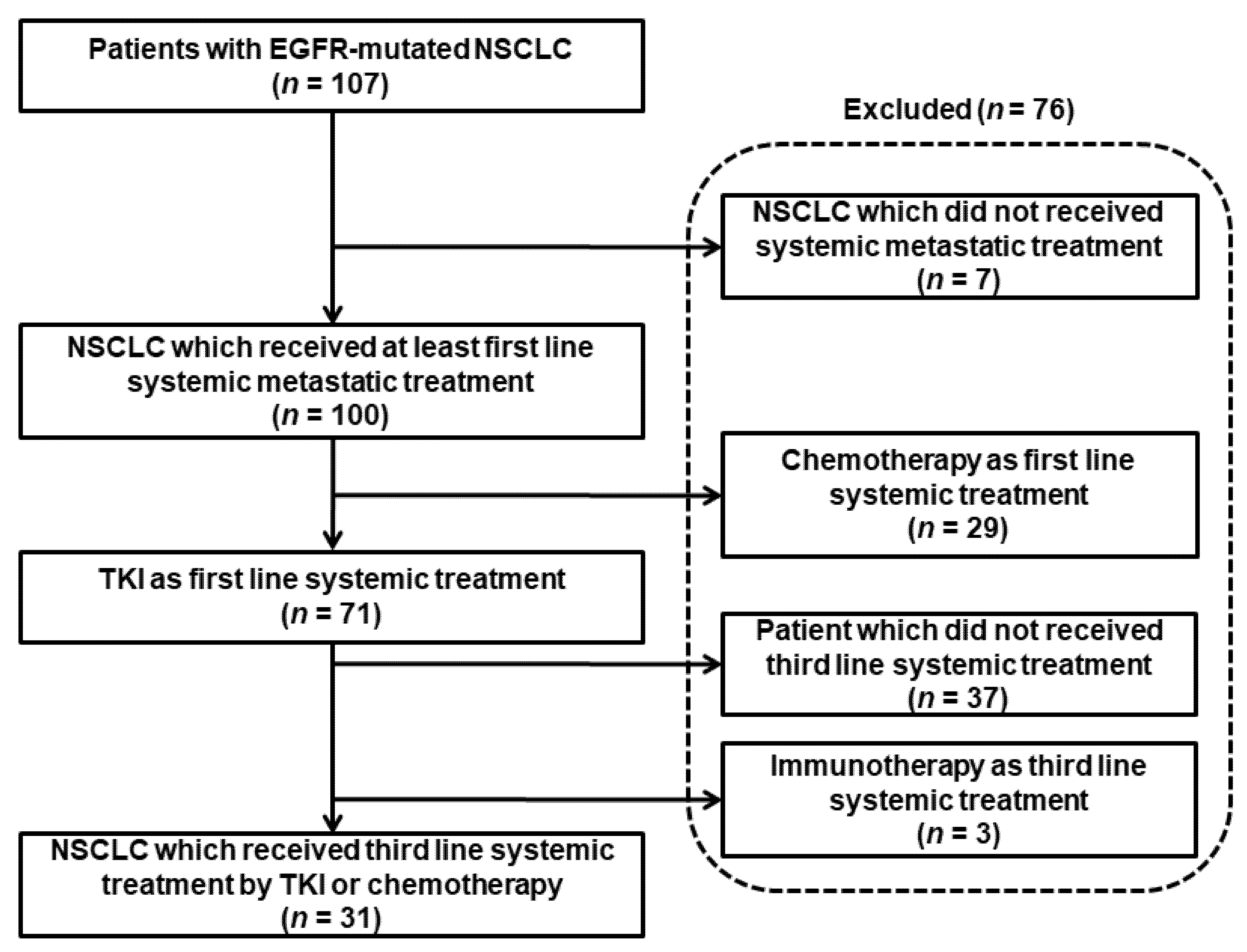
3.1. Description of the Study Population
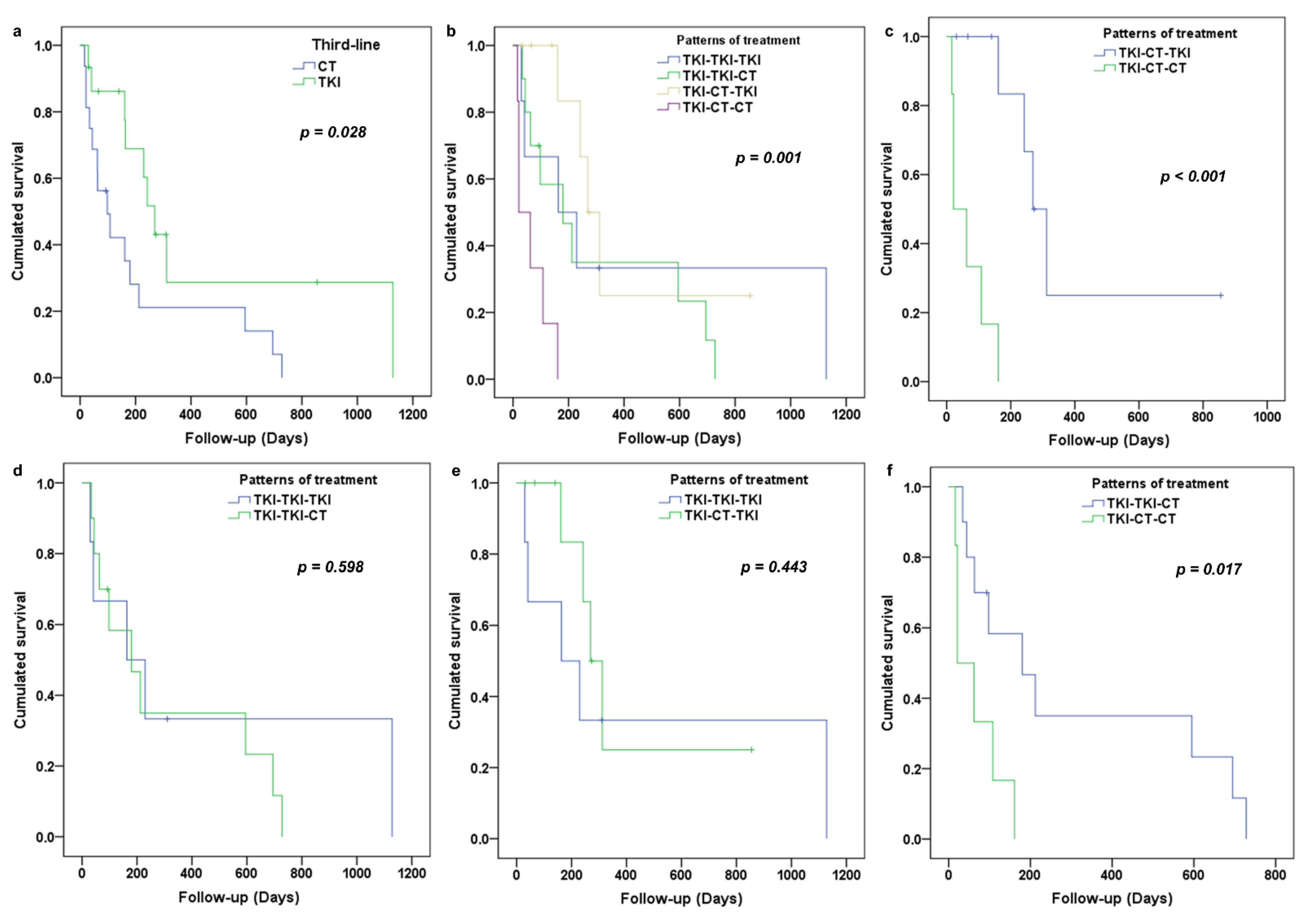
3.2. Analysis of PFS in Third-Line
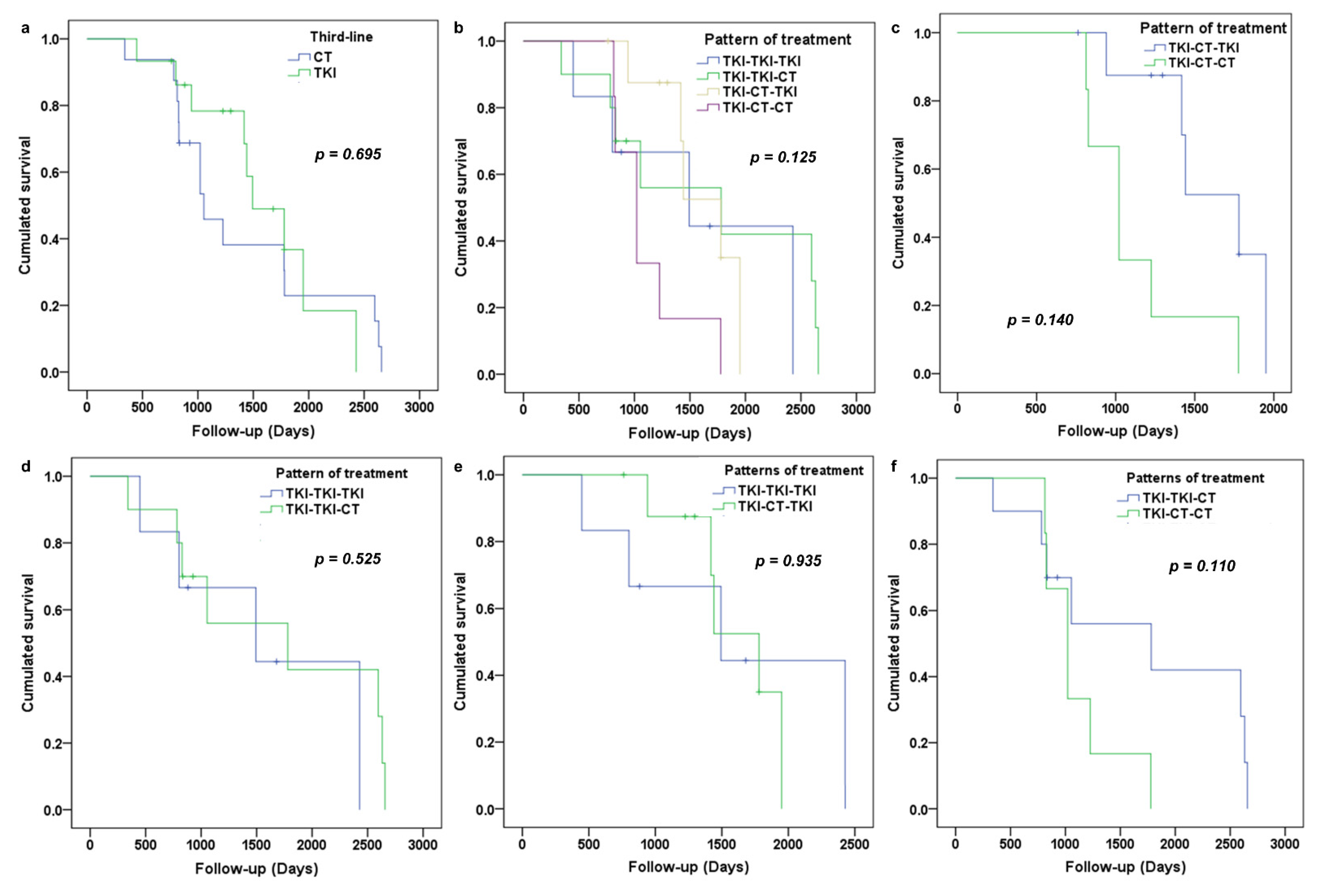
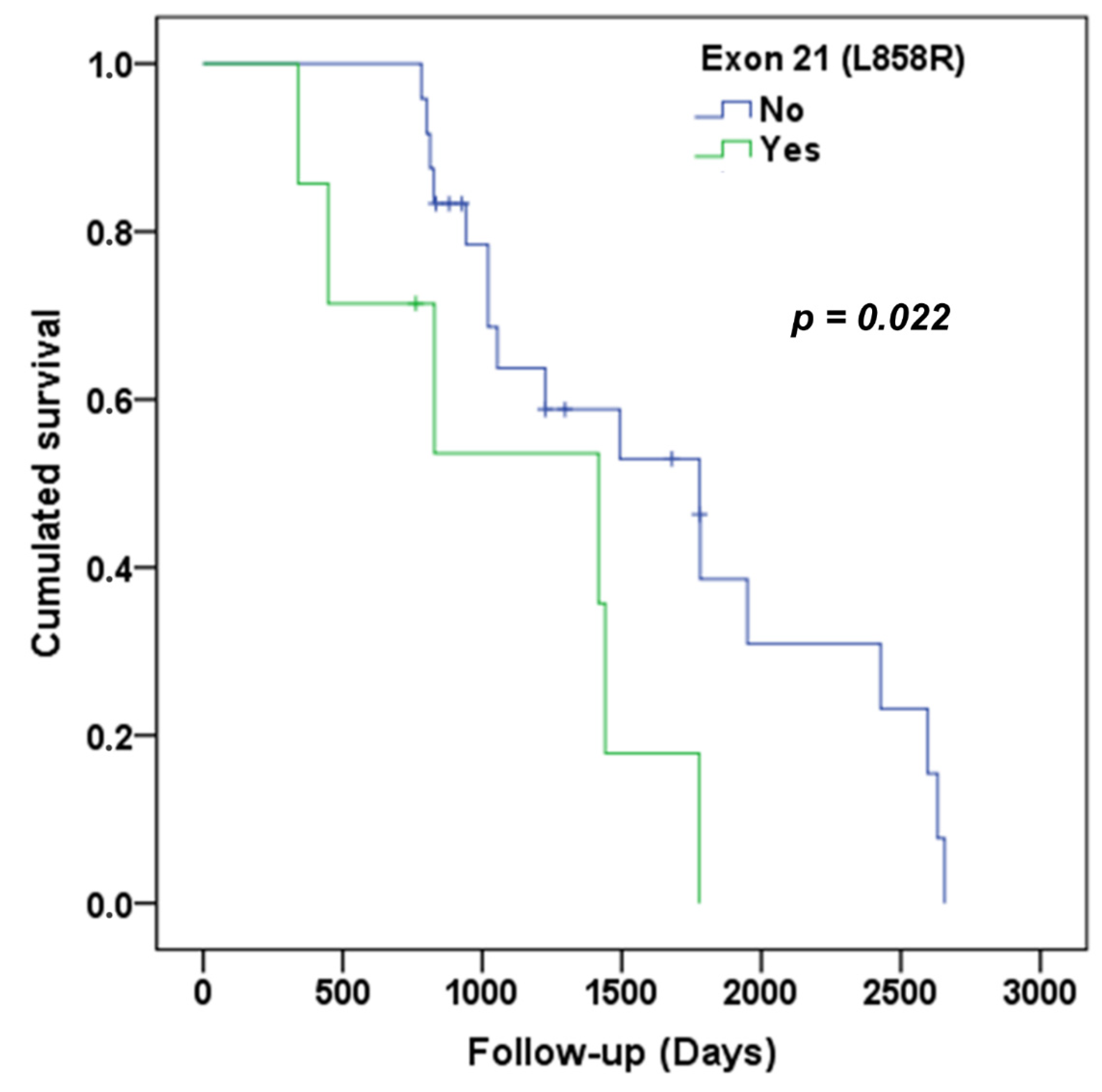
3.3. Analysis of Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calibasi-Kocal, G.; Amirfallah, A.; Sever, T.; Unal, O.U.; Gurel, D.; Oztop, I.; Ellidokuz, H.; Basbinar, Y. EGFR mutation status in a series of Turkish non-small cell lung cancer patients. Biomed. Rep. 2020, 13, 1. [Google Scholar] [CrossRef]
- Grigoriu, B.; Berghmans, T.; Meert, A.-P. Management of EGFR mutated nonsmall cell lung carcinoma patients. Eur. Respir. J. 2015, 45, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.H.; Schneider, B.J.; Temin, S.; Baker, S.; Brahmer, J.; Ellis, P.M.; Gaspar, L.E.; Haddad, R.Y.; Hesketh, P.J.; Jain, D.; et al. Therapy for Stage IV Non–Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2020, 38, 1608–1632. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Liu, T.; Dang, J.; Li, G. First-line treatments in EGFR-mutated advanced non-small cell lung cancer: A network meta-analysis. PLoS ONE 2019, 14, e0223530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in UntreatedEGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Imai, H.; Minemura, H.; Sugiyama, T.; Yamada, Y.; Kaira, K.; Kanazawa, K. Efficacy and safety of cytotoxic drug chemo-therapy after first-line EGFR-TKI treatment in elderly patients with non-small-cell lung cancer harboring sensitive EGFR mu-tations. Cancer Chemother. Pharmacol. 2018, 82, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, Y.-L.; Cao, L.-J.; Chen, J.-H.; Ma, Z.-Y.; Cui, J.-W.; Wang, J.; Liu, H.-B.; Ding, J.-Y.; Hu, M. Efficacy and Safety of Gefitinib as Third-line Treatment in NSCLC Patients With Activating EGFR Mutations Treated With First-line Gefitinib Followed by Second-line Chemotherapy: A Single-Arm, Prospective, Multicenter Phase II Study (RE-CHALLENGE, CTONG1304). Am. J. Clin. Oncol. 2019, 42, 432–439. [Google Scholar]
- Oxnard, G.R.; Arcila, M.E.; Chmielecki, J.; Ladanyi, M.; Miller, V.A.; Pao, W. New Strategies in Overcoming Acquired Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Lung Cancer. Clin. Cancer Res. 2011, 17, 5530–5537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westover, D.; Zugazagoitia, J.; Cho, B.C.; Lovly, C.; Paz-Ares, L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann. Oncol. 2018, 29, i10–i19. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Ahn, M.-J.; Kim, D.-W.; Ramalingam, S.S.; Sequist, L.V.; Su, W.-C.; Kim, S.W.; Kim, J.H.; Planchard, D.; Felip, E.; et al. Osimertinib in Pretreated T790M-Positive Ad-vanced Non–Small-Cell Lung Cancer: AURA Study Phase II Extension Component. J. Clin. Oncol. 2017, 35, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Auliac, J.B.; Pérol, M.; Planchard, D.; Monnet, I.; Wislez, M.; Doubre, H.; Guisier, F.; Pichon, E.; Greillier, L.; Mastroianni, B.; et al. Real-life efficacy of osimertinib in pretreated patients with advanced non-small cell lung cancer harboring EGFR T790M mutation. Lung Cancer 2019, 127, 96–102. [Google Scholar] [CrossRef]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bernards, R. Taking advantage of drug resistance, a new approach in the war on cancer. Front. Med. 2018, 12, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Amirouchene-Angelozzi, N.; Swanton, C.; Bardelli, A. Tumor Evolution as a Therapeutic Target. Cancer Discov. 2017, 7, 805–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, R.L.; Wang, L.; Bernards, R. With great power comes great vulnerability. Mol. Cell. Oncol. 2018, 5, e1509488. [Google Scholar] [CrossRef] [Green Version]
- Morgillo, F.; Fasano, M.; Della Corte, C.; Sasso, F.; Papaccio, F.; Viscardi, G. Results of the safety run-in-part of the METAL (METformin in Advanced lung cancer) study: A multicenter, open-label phase I-II study of metformin with erlotinib in sec-ond-line therapy of patients with stage IV non-small lung cancer. ESMO Open 2017, 2, e000132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Xu, Y.; Zhou, Y.; Yang, A.; Cui, J.; Chen, P.; Zhao, H.; Zhou, X.; Shen, C.; Yu, J.; et al. The effect of TKI therapy and chemotherapy treatment delivery sequence on total progression-free survival in patients with advanced EGFR-mutated NSCLC. Oncol. Lett. 2020, 20, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Eriguchi, D.; Okano, T.; Kawaguchi, Y.; Maeda, J.; Hagiwara, M.; Kakihana, M. P3.01–24 The Importance to Switch from EGFR-TKI to Cytotoxic Chemotherapy for EGFR Mutation-Positive Adenocarcinoma (Abstract). J. Thorac. Oncol. 2018, 13, S876. [Google Scholar] [CrossRef] [Green Version]
- Tamiya, M.; Tamiya, A.; Suzuki, H.; Moriizumi, K.; Nakahama, K.; Taniguchi, Y.; Kunimasa, K.; Kimura, M.; Inoue, T.; Kuhara, H.; et al. Which Is Better EGFR-TKI Followed by Osimertinib: Afatinib or Gefitinib/Erlotinib? Anticancer Res. 2019, 39, 3923–3929. [Google Scholar] [CrossRef]
- Park, K.; Bennouna, J.; Boyer, M.; Hida, T.; Hirsh, V.; Kato, T.; Lu, S.; Mok, T.; Nakagawa, K.; O’Byrne, K.; et al. Sequencing of therapy following first-line afatinib in patients with EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2019, 132, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Hochmair, M.J.; Morabito, A.; Hao, D.; Yang, C.-T.; Soo, R.A.; Yang, J.C.-H.; Gucalp, R.; Halmos, B.; Märten, A.; Cufer, T. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: Final analysis of the GioTag study. Futur. Oncol. 2020, 16, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Sutiman, N.; Tan, S.W.; Tan, E.H.; Lim, W.T.; Kanesvaran, R.; Ng, Q.S.; Jain, A.; Ang, M.K.; Tan, W.L.; Toh, C.-K.; et al. EGFR Mutation Subtypes Influence Survival Outcomes following First-Line Gefitinib Therapy in Advanced Asian NSCLC Patients. J. Thorac. Oncol. 2017, 12, 529–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Zhu, M.; Li, Y.; Li, Q. Association between EGFR exon 19 or exon 21 mutations and survival rates after first-line EGFR-TKI treatment in patients with non-small cell lung cancer. Mol. Clin. Oncol. 2019, 11, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Cardarella, S.; Lydon, C.A.; Dahlberg, S.; Jackman, D.M.; Jänne, P.A.; Johnson, B.E. Five-Year Survival in EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs. J. Thorac. Oncol. 2016, 11, 556–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Patients Treated by Chemotherapy in Third-Line (n = 16) | Patients Treated by TKI in Third-Line (n = 15) | |
|---|---|---|
| Meanage (year) | 55.5 ± 11.89 | 64.93 ± 10.20 |
| Sex-ratio (male/female) | 0.23 | 0.25 |
| Non-smokers [n (%)] | 9 (56.25) | 14 (93.33) |
| Comorbidities [n (%)) | 12 (75.00) | 11 (73.33) |
| Frequency of EGFR-mutations | ||
| Exon 21(L858R) [n (%)] | 3 (18.75) | 4 (26.67) |
| Exon 20 (T790M) [n (%)) | 4 (25.00) | 3 (20.00) |
| Exon 19 (délétion) [n (%)] | 11 (68.75) | 10 (66.67) |
| Exon 18 [n (%)] | 4 (25.00) | 1 (6.67) |
| Median number of received line of treatment | 3.50 (range 3–7) | 3.00 (range 3–7) |
| Median duration of third-line (months) | 3.18 (range 0.53–24.27) | 7.63 (range 1–37.60) |
| Median duration of follow-up (months) | 34.03 (range 0.53–24.26) | 47.23 (range 1–37.6) |
| Breakdown by state at the latest news | ||
| Deceased [n (%)] | 14 (87.50) | 9 (60.00) |
| Alive [n (%)] | 1 (6.25) | 5 (33.33) |
| Lost to follow-up [n (%)] | 1 (6.25) | 1 (6.67) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Refeno, V.; Lamuraglia, M.; Terrisse, S.; Bonnet, C.; Dumont, C.; Doucet, L.; Pouessel, D.; Culine, S. Survival of Patients with Epidermal Growth Factor Receptor-Mutated Metastatic Non-Small Cell Lung Cancer Treated beyond the Second Line in the Tyrosine Kinase Inhibitor Era. Cancers 2021, 13, 3887. https://doi.org/10.3390/cancers13153887
Refeno V, Lamuraglia M, Terrisse S, Bonnet C, Dumont C, Doucet L, Pouessel D, Culine S. Survival of Patients with Epidermal Growth Factor Receptor-Mutated Metastatic Non-Small Cell Lung Cancer Treated beyond the Second Line in the Tyrosine Kinase Inhibitor Era. Cancers. 2021; 13(15):3887. https://doi.org/10.3390/cancers13153887
Chicago/Turabian StyleRefeno, Valéry, Michele Lamuraglia, Safae Terrisse, Clément Bonnet, Clément Dumont, Ludovic Doucet, Damien Pouessel, and Stephane Culine. 2021. "Survival of Patients with Epidermal Growth Factor Receptor-Mutated Metastatic Non-Small Cell Lung Cancer Treated beyond the Second Line in the Tyrosine Kinase Inhibitor Era" Cancers 13, no. 15: 3887. https://doi.org/10.3390/cancers13153887
APA StyleRefeno, V., Lamuraglia, M., Terrisse, S., Bonnet, C., Dumont, C., Doucet, L., Pouessel, D., & Culine, S. (2021). Survival of Patients with Epidermal Growth Factor Receptor-Mutated Metastatic Non-Small Cell Lung Cancer Treated beyond the Second Line in the Tyrosine Kinase Inhibitor Era. Cancers, 13(15), 3887. https://doi.org/10.3390/cancers13153887






