Why Do Children with Acute Lymphoblastic Leukemia Fare Better Than Adults?
Abstract
Simple Summary
Abstract
1. Introduction
2. Socio-Economic Factors
3. Host Factors
3.1. Age
3.2. Sex and Ethnicity
3.3. Disease Biology
3.4. Treatment Related Differences
3.5. Treatment Related Toxicities
3.6. Drug Tolerance and Drug Resistance
3.7. Time to Complete Remission
4. MRD Evaluation
5. HSCT in First Complete Remission
6. Future Directions in Treating ALL
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frei, E., 3rd; Sallan, S.E. Acute lymphoblastic leukemia: Treatment. Cancer 1978, 42, 828–838. [Google Scholar]
- Ledford, H. The perfect blend. Nature 2016, 532, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Kansagra, A.; Litzow, M. Treatment of Young Adults with Acute Lymphoblastic Leukemia. Curr. Hematol. Malign. Rep. 2017, 12, 187–196. [Google Scholar] [CrossRef]
- Hochberg, J.; Khaled, S.; Forman, S.J.; Cairo, M.S. Criteria for and outcomes of allogeneic haematopoietic stem cell transplant in children, adolescents and young adults with acute lymphoblastic leukaemia in first complete remission. Br. J. Haematol. 2013, 161, 27–42. [Google Scholar] [CrossRef]
- Hallbook, H.; Gustafsson, G.; Smedmyr, B.; Soderhall, S.; Heyman, M.; Swedish Adult Acute Lymphocytic Leukemia Group. Treatment outcome in young adults and children >10 years of age with acute lymphoblastic leukemia in Sweden: A comparison between a pediatric protocol and an adult protocol. Cancer 2006, 107, 1551–1561. [Google Scholar] [CrossRef]
- Hocking, J.; Schwarer, A.P.; Gasiorowski, R.; Patil, S.; Avery, S.; Gibson, J.; Iland, H.; Ho, P.J.; Joshua, D.; Muirhead, J.; et al. Excellent outcomes for adolescents and adults with acute lymphoblastic leukemia and lymphoma without allogeneic stem cell transplant: The FRALLE-93 pediatric protocol. Leuk. Lymphoma 2014, 55, 2801–2807. [Google Scholar] [CrossRef] [PubMed]
- Sallan, S.E. Myths and Lessons from the Adult/Pediatric Interface un Acute Lymphoblastic Leukemia. ASH Educ. Program Book 2006, 2006, 128–132. [Google Scholar]
- Mattison, R.; Stock, W. Approaches to treatment for acute lymphoblastic leukemia in adolescents and young adults. Curr. Hematol. Malign. Rep. 2008, 3, 144–151. [Google Scholar] [CrossRef]
- Ram, R.; Wolach, O.; Vidal, L.; Gafter-Gvili, A.; Shpilberg, O.; Raanani, P. Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric-inspired regimens: Systematic review and meta-analysis. Am. J. Hematol. 2012, 87, 472–478. [Google Scholar] [CrossRef]
- Pulte, D.; Gondos, A.; Brenner, H. Trends in 5-and 10-year Survival After Diagnosis with Childhood Hematologic Malignancies in the United States, 1990–2004. JNCI J. Natl. Cancer Inst. 2008, 100, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Dores, G.M.; Devesa, S.S.; Curtis, R.E.; Linet, M.S.; Morton, L.M. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood J. Am. Soc. Hematol. 2012, 119, 34–43. [Google Scholar] [CrossRef]
- Litzow, M.; Kenderian, S. Acute lymphoblastic leukemia in adolescents and young adults—from genomics to the clinics. Clin. Oncol. Adolesc. Young Adults 2013, 3, 49–62. [Google Scholar] [CrossRef]
- Stock, W.; La, M.; Sanford, B.; Bloomfield, C.D.; Vardiman, J.W.; Gaynon, P. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood 2008, 112, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Curran, E.; Stock, W. How I treat acute lymphoblastic leukemia in older adolescents and young adults. Blood J. Am. Soc. Hematol. 2015, 125, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, C.A. Differences in outcome in adolescents with acute lymphoblastic leukemia: A consequence of better regimens? Better doctors? Both? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Rytting, M.E.; Jabbour, E.J.; O’Brien, S.M.; Kantarjian, H.M. Acute lymphoblastic leukemia in adolescents and young adults. Cancer 2017, 123, 2398–2403. [Google Scholar] [CrossRef] [PubMed]
- Advani, A.S.; Hunger, S.P.; Burnett, A.K. Acute Leukemia in Adolescents and Young Adults. Semin. Oncol. 2009, 36, 213–226. [Google Scholar] [CrossRef]
- DeAngelo, D.J.; Stevenson, K.E.; Dahlberg, S.; Silverman, L.B.; Couban, S.; Supko, J.G.; Amrein, P.C.; Ballen, K.K.; Seftel, M.D.; Turner, A.R.; et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia 2014, 29, 526–534. [Google Scholar] [CrossRef] [PubMed]
- DeAngelo, D.J. The Treatment of Adolescents and Young Adults with Acute Lymphoblastic Leukemia. DeAngelo D J. The treatment of adolescents and young adults with acute lymphoblastic leukemia. ASH Educ. Program Book 2005, 2005, 123–130. [Google Scholar] [CrossRef][Green Version]
- Kahn, J.; Keegan, T.H.; Tao, L.; Abrahão, R.; Bleyer, A.; Viny, A.D. Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer 2016, 122, 2723–2730. [Google Scholar] [CrossRef]
- Thomas, D.M.; Seymour, J.F.; O’Brien, T.; Sawyer, S.M.; Ashley, D.M. Adolescent and young adult cancer: A revolution in evolution? Intern. Med. J. 2006, 36, 302–307. [Google Scholar] [CrossRef]
- Ramanujachar, R.; Richards, S.; Hann, I.; Goldstone, A.; Mitchell, C.; Vora, A.; Rowe, J.; Webb, D. Adolescents with acute lymphoblastic leukaemia: Outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr. Blood Cancer 2007, 48, 254–261. [Google Scholar] [CrossRef]
- Utuama, O.; Mukhtar, F.; Pham, Y.T.-H.; Dabo, B.; Manani, P.; Moser, J.; Michael-Asalu, A.; Tran, C.T.; Le, L.C.; Le, T.V.; et al. Racial/ethnic, age and sex disparities in leukemia survival among adults in the United States during 1973–2014 period. PLoS ONE 2019, 14, e0220864. [Google Scholar] [CrossRef] [PubMed]
- Stock, W. Adolescents and Young Adults with Acute Lymphoblastic Leukemia. Hematol. Am. Soc. Hematol. Educ. Program Book 2010, 2010, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Dombret, H.; Cluzeau, T.; Huguet, F.; Boissel, N. Pediatric-Like Therapy for Adults with ALL. Curr. Hematol. Malign. Rep. 2014, 9, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Boissel, N.; Auclerc, M.-F.; Lhéritier, V.; Perel, Y.; Thomas, X.; Leblanc, T.; Rousselot, P.; Cayuela, J.-M.; Gabert, J.; Fegueux, N.; et al. Should Adolescents with Acute Lymphoblastic Leukemia Be Treated as Old Children or Young Adults? Comparison of the French FRALLE-93 and LALA-94 Trials. J. Clin. Oncol. 2003, 21, 774–780. [Google Scholar] [CrossRef]
- Chiaretti, S.; Gianfelici, V.; O’Brien, S.M.; Mullighan, C.G. Advances in the Genetics and Therapy of Acute Lymphoblastic Leukemia. Am. Soc. Clin. Oncol. Educ. Book 2016, e314–e322. [Google Scholar] [CrossRef] [PubMed]
- Balbach, S.T.; Makarova, O.; Bonn, B.R.; Zimmermann, M.; Rohde, M.; Oschlies, I.; Klapper, W.; Rossig, C.; Burkhardt, B. Proposal of a genetic classifier for risk group stratification in pediatric T-cell lymphoblastic lymphoma reveals differences from adult T-cell lymphoblastic leukemia. Leukemia 2015, 30, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; O’Brien, S.; Huang, X.; Thomas, D.; Rytting, M.; Sasaki, K.; Cortes, J.; Garcia-Manero, G.; Kadia, T.; Ravandi, F.; et al. Prognostic factors for outcome in patients with refractory and relapsed acute lymphocytic leukemia treated with inotuzumab ozogamicin, a CD22 monoclonal antibody. Am. J. Hematol. 2014, 90, 193–196. [Google Scholar] [CrossRef]
- Boissel, N.; Baruchel, A. Acute lymphoblastic leukemia in adolescent and young adults: Treat as adults or as children? Blood 2018, 132, 351–361. [Google Scholar] [CrossRef]
- Roberts, K.G.; Li, Y.; Payne-Turner, D.; Harvey, R.; Yang, Y.-L.; Pei, D.; McCastlain, K.; Ding, L.; Lu, C.; Song, G.; et al. Targetable Kinase-Activating Lesions in Ph-like Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2014, 371, 1005–1015. [Google Scholar] [CrossRef]
- Moorman, A.V. New and emerging prognostic and predictive genetic biomarkers in B-cell precursor acute lymphoblastic leukemia. Haematologica 2016, 101, 407–416. [Google Scholar] [CrossRef]
- Fang, Q.; Song, Y.; Gong, X.; Wang, J.; Li, Q.; Liu, K.; Feng, Y.; Hao, Q.; Li, Y.; Wei, H.; et al. Gene Deletions and Prognostic Values in B-Linage Acute Lymphoblastic Leukemia. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Advani, A.S. Perspectives and Future Directions for Acute Lymphoblastic Leukemia. Clin. Lymphoma Myeloma Leuk. 2016, 16, S6–S9. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.; Enshaei, A.; Jones, L.; Erhorn, A.; Masic, D.; Bentley, H.; Laczko, K.S.; Fielding, A.K.; Goldstone, A.H.; Goulden, N.; et al. IGH@ Translocations Are Prevalent in Teenagers and Young Adults with Acute Lymphoblastic Leukemia and Are Associated with a Poor Outcome. J. Clin. Oncol. 2014, 32, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G. Molecular genetics of B-precursor acute lymphoblastic leukemia. J. Clin. Investig. 2012, 122, 3407–3415. [Google Scholar] [CrossRef] [PubMed]
- DeAngelo, D.J.; Jabbour, E.; Advani, A. Recent Advances in Managing Acute Lymphoblastic Leukemia. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 330–342. [Google Scholar] [CrossRef]
- Faderl, S.; Jeha, S.; Kantarjian, H.M. The biology and therapy of adult acute lymphoblastic leukemia. Cancer 2003, 98, 1337–1354. [Google Scholar] [CrossRef]
- Mullighan, C.; Su, X.; Zhang, J.; Radtke, I.; Phillips, L.A.; Miller, C.B.; Ma, J.; Liu, W.; Cheng, C.; Schulman, B.A.; et al. Deletion ofIKZF1and Prognosis in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2009, 360, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.; Zhang, J.; Harvey, R.; Collins-Underwood, J.R.; Schulman, B.A.; Phillips, L.A.; Tasian, S.; Loh, M.L.; Su, X.; Liu, W.; et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 2009, 106, 9414–9418. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.; Mullighan, C.G.; Chen, I.-M.; Wharton, W.; Mikhail, F.M.; Carroll, A.J.; Kang, H.; Liu, W.; Dobbin, K.K.; Smith, M.A.; et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood J. Am. Soc. Hematol. 2010, 115, 5312–5321. [Google Scholar] [CrossRef]
- Bond, J.; Graux, C.; Lhermitte, L.; Lara, D.; Cluzeau, T.; Leguay, T.; Cieslak, A.; Trinquand, A.; Pastoret, C.; Belhocine, M.; et al. Early Response–Based Therapy Stratification Improves Survival in Adult Early Thymic Precursor Acute Lymphoblastic Leukemia: A Group for Research on Adult Acute Lymphoblastic Leukemia Study. J. Clin. Oncol. 2017, 35, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Beldjord, K.; Chevret, S.; Asnafi, V.; Huguet, F.; Boulland, M.-L.; Leguay, T.; Thomas, X.; Cayuela, J.-M.; Grardel, N.; Chalandon, Y.; et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood 2014, 123, 3739–3749. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Wang, B.-Y.; Zhang, W.-N.; Huang, J.-Y.; Li, B.-S.; Zhang, M.; Jiang, L.; Li, J.-F.; Wang, M.-J.; Dai, Y.-J.; et al. Genomic Profiling of Adult and Pediatric B-cell Acute Lymphoblastic Leukemia. EBioMedicine 2016, 8, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Albitar, M.; Potts, S.J.; Giles, F.J.; O’Brien, S.; Keating, M.; Thomas, D.; Clarke, C.; Jilani, I.; Aguilar, C.; Estey, E.; et al. Proteomic-based prediction of clinical behavior in adult acute lymphoblastic leukemia. Cancer 2006, 106, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- de Bont, J.M.; Holt, B.; Dekker, A.W.; van der Does-van den Berg, A.; Sonneveld, P.; Pieters, R. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia 2004, 18, 2032–2035. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, U.; Mahadeo, K.M.; Kebriaei, P.; Shpall, E.J.; Saini, N.Y. Chimeric Antigen Receptor T-Cells in B-Acute Lymphoblastic Leukemia: State of the Art and Future Directions. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Inaba, H.; Mullighan, C.G. Pediatric acute lymphoblastic leukemia. Haematologica 2020, 105, 2524–2539. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y. Recent updates for antibody therapy for acute lymphoblastic leukemia. Exp. Hematol. Oncol. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Alcantara, M.; Simonin, M.; Lhermitte, L.; Touzart, A.; Dourthe, M.E.; Latiri, M.; Grardel, N.; Cayuela, J.M.; Chalandon, Y.; Graux, C.; et al. Clinical and biological features of PTPN2-deleted adult and pediatric T-cell acute lymphoblastic leukemia. Blood Adv. 2019, 3, 1981–1988. [Google Scholar] [CrossRef]
- Genesca, E.; Morgades, M.; Gonzalez-Gil, C.; Fuster-Tormo, F.; Haferlach, C.; Meggendorfer, M. Adverse prognostic impact of complex karyotype (>/=3 cytogenetic alterations) in adult T-cell acute lymphoblastic leukemia (T-ALL). Leuk. Res. 2021, 109, 106612. [Google Scholar] [CrossRef]
- Andrieu, G.P.; Kohn, M.; Simonin, M.; Smith, C.; Cieslak, A.; Dourthe, M.-E.; Charbonnier, G.; Graux, C.; Rigal-Huguet, F.; Lheritier, V.; et al. PRC2 loss of function confers a targetable vulnerability to BET proteins in T-ALL. Blood 2021. [Google Scholar] [CrossRef] [PubMed]
- Huguet, F.; Leguay, T.; Raffoux, E.; Thomas, X.; Beldjord, K.; Delabesse, E.; Chevallier, P.; Buzyn, A.; Delannoy, A.; Chalandon, Y.; et al. Pediatric-Inspired Therapy in Adults with Philadelphia Chromosome–Negative Acute Lymphoblastic Leukemia: The GRAALL-2003 Study. J. Clin. Oncol. 2009, 27, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Ribera, J.-M.; Oriol, A.; Sanz, M.-A.; Tormo, M.; Abellan, P.F.; Del Potro, E.; Abella, E.; Bueno, J.; Parody, R.; Bastida, P.; et al. Comparison of the Results of the Treatment of Adolescents and Young Adults with Standard-Risk Acute Lymphoblastic Leukemia with the Programa Español de Tratamiento en Hematología Pediatric-Based Protocol ALL-96. J. Clin. Oncol. 2008, 26, 1843–1849. [Google Scholar] [CrossRef]
- Storring, J.M.; Minden, M.D.; Kao, S.; Gupta, V.; Schuh, A.C.; Schimmer, A.; Yee, K.W.L.; Kamel-Reid, S.; Chang, H.; Lipton, J.H.; et al. Treatment of adults with BCR-ABL negative acute lymphoblastic leukaemia with a modified paediatric regimen. Br. J. Haematol. 2009, 146, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Stock, W.; Johnson, J.L.; Stone, R.M.; Kolitz, J.E.; Powell, B.L.; Wetzler, M. Dose intensification of daunorubicin and cytarabine during treatment of adult acute lymphoblastic leukemia: Results of Cancer and Leukemia Group B Study 19802. Cancer 2013, 119, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Thomas, X.; Le Jeune, C. Treating adults with acute lymphocytic leukemia: New pharmacotherapy options. Expert. Opin. Pharmacother. 2016, 17, 2319–2330. [Google Scholar] [CrossRef]
- Gavralidis, A.; Brunner, A.M. Novel Therapies in the Treatment of Adult Acute Lymphoblastic Leukemia. Curr. Hematol. Malign. Rep. 2020, 15, 294–304. [Google Scholar] [CrossRef]
- Contreras, C.F.; Higham, C.S.; Behnert, A.; Kim, K.; Stieglitz, E.; Tasian, S.K. Clinical utilization of blinatumomab and inotuzumab immunotherapy in children with relapsed or refractory B-acute lymphoblastic leukemia. Pediatr. Blood Cancer 2020, 68, e28718. [Google Scholar] [CrossRef]
- Styczyński, J.; Wysocki, M. In Vitro Drug Resistance Profiles of Adult Acute Lymphoblastic Leukemia: Possible Explanation for Difference in Outcome to Similar Therapeutic Regimens. Leuk. Lymphoma 2002, 43, 301–307. [Google Scholar] [CrossRef]
- Bassan, R.; Spinelli, O. Minimal Residual Disease Monitoring in Adult ALL to Determine Therapy. Curr. Hematol. Malign. Rep. 2015, 10, 86–95. [Google Scholar] [CrossRef]
- Contreras Yametti, G.P.; Ostrow, T.H.; Jasinski, S.; Raetz, E.A.; Carroll, W.L.; Evensen, N.A. Minimal Residual Disease in Acute Lymphoblastic Leukemia: Current Practice and Future Directions. Cancers 2021, 13, 1847. [Google Scholar] [CrossRef]
- Gökbuget, N.; Zugmaier, G.; Dombret, H.; Stein, A.; Bonifacio, M.; Graux, C.; Faul, C.; Brüggemann, M.; Taylor, K.; Mergen, N.; et al. Curative outcomes following blinatumomab in adults with minimal residual disease B-cell precursor acute lymphoblastic leukemia. Leuk. Lymphoma 2020, 61, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Campana, D. Role of Minimal Residual Disease Monitoring in Adult and Pediatric Acute Lymphoblastic Leukemia. Hematol. Clin. North. Am. 2009, 23, 1083–1098. [Google Scholar] [CrossRef] [PubMed]
- Lepretre, S.; Touzart, A.; Vermeulin, T.; Picquenot, J.-M.; Tanguy-Schmidt, A.; Salles, G.; Lamy, T.; Béné, M.-C.; Raffoux, E.; Huguet, F.; et al. Pediatric-Like Acute Lymphoblastic Leukemia Therapy in Adults with Lymphoblastic Lymphoma: The GRAALL-LYSA LL03 Study. J. Clin. Oncol. 2016, 34, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.; Abdel-Azim, N.; Kim, Y.-M.; Ruan, Y.; Phan, V.; Ogana, H.; Wang, W.; Lee, R.; Gang, E.J.; Khazal, S.; et al. Minimal Residual Disease Detection in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2020, 21, 1054. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.A.; Zhou, S.; Higley, H.; Mukundan, L.; Fu, S.; Reaman, G.H. Association of Minimal Residual Disease with Clinical Outcome in Pediatric and Adult Acute Lymphoblastic Leukemia: A Meta-analysis. JAMA Oncol. 2017, 3, e170580. [Google Scholar] [CrossRef]
- Liu, X.; Zou, Y.; Chen, X.; Wang, S.; Guo, Y.; Yang, W.; Zhang, L.; Chen, Y.; Zhang, Y.; Zhu, X. Minimal residual disease surveillance at day 90 predicts long-term survival in pediatric patients with T-cell acute lymphoblastic leukemia. Leuk. Lymphoma 2020, 61, 3460–3467. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.J.; Gossai, N.; Wagner, J.E.; Smith, A.R.; Bachanova, V.; Cao, Q.; MacMillan, M.; Stefanski, H.S.; Weisdorf, D.J.; Verneris, M.R. Survival Differences between Adolescents/Young Adults and Children with B Precursor Acute Lymphoblastic Leukemia after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2013, 19, 138–142. [Google Scholar] [CrossRef]
- Litzow, M.R.; Ferrando, A.A. How I treat T-cell acute lymphoblastic leukemia in adults. Blood 2015, 126, 833–841. [Google Scholar] [CrossRef]
- Seftel, M.D.; Neuberg, D.; Zhang, M.-J.; Wang, H.-L.; Ballen, K.K.; Bergeron, J.; Couban, S.; Freytes, C.O.; Hamadani, M.; Kharfan-Dabaja, M.A.; et al. Pediatric-inspired therapy compared to allografting for Philadelphia chromosome-negative adult ALL in first complete remission. Am. J. Hematol. 2015, 91, 322–329. [Google Scholar] [CrossRef]
- Topp, M.S.; Gökbuget, N.; Zugmaier, G.; Stein, A.S.; Dombret, H.; Chen, Y.; Ribera, J.; Bargou, R.C.; Horst, H.; Kantarjian, H.M. Long-term survival of patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab. Cancer 2020, 127, 554–559. [Google Scholar] [CrossRef]
- Stock, W.; Martinelli, G.; Stelljes, M.; Deangelo, D.J.; Gökbuget, N.; Advani, A.S.; O’Brien, S.; Liedtke, M.; Merchant, A.A.; Cassaday, R.D.; et al. Efficacy of inotuzumab ozogamicin in patients with Philadelphia chromosome–positive relapsed/refractory acute lymphoblastic leukemia. Cancer 2020, 127, 905–913. [Google Scholar] [CrossRef]
- Pui, C.-H. Precision medicine in acute lymphoblastic leukemia. Front. Med. 2020, 14, 689–700. [Google Scholar] [CrossRef]
- Ai, J.; Advani, A. Current status of antibody therapy in ALL. Br. J. Haematol. 2014, 168, 471–480. [Google Scholar] [CrossRef]
- Samra, B.; Jabbour, E.; Ravandi, F.; Kantarjian, H.; Short, N.J. Evolving therapy of adult acute lymphoblastic leukemia: State-of-the-art treatment and future directions. J. Hematol. Oncol. 2020, 13. [Google Scholar] [CrossRef]
- Carol, H.; Szymanska, B.; Evans, K.; Boehm, I.; Houghton, P.J.; Smith, M.; Lock, R.B. The Anti-CD19 Antibody–Drug Conjugate SAR3419 Prevents Hematolymphoid Relapse Postinduction Therapy in Preclinical Models of Pediatric Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2013, 19, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.; Raetz, E.; Cairo, M.S. The future role of monoclonal antibody therapy in childhood acute leukaemias. Br. J. Haematol. 2012, 159, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Pikman, Y.; Stieglitz, E. Targeting the Ras pathway in pediatric hematologic malignancies. Curr. Opin. Pediatr. 2020, 33, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S. Minimal residual disease in acute lymphoblastic leukemia: Technical aspects and implications for clinical interpretation. Blood Res. 2020, 55, S19–S26. [Google Scholar] [CrossRef]
- Sherali, N.; Hamadneh, T.; Aftab, S.; Alfonso, M.S.; Tsouklidis, N. Integration of Next-Generation Sequencing in Diagnosing and Minimal Residual Disease Detection in Patients with Philadelphia Chromosome-Like Acute Lymphoblastic Leukemia. Cureus 2020, 12, e10696. [Google Scholar] [CrossRef] [PubMed]
| Disease Characteristic | Adults | AYA | Children | References |
|---|---|---|---|---|
| High WBC count | More frequent | More frequent | Less frequent | [26,29] |
| T-cell | 25% | Intermediate | 15% | [30] |
| ETP-ALL | 7.4% | - | 10–15% | [12,31] |
| Hyperdiploidy | 5% | Less than 20% | 30–40% | [12] |
| Trisomies of chromosomes 4, 10, 17 | Rare | Rare | Frequent | [16,32] |
| Philadelphia chromosome | 53% | 14% | 3% | [17] |
| t (12;21)/ETV6/RUNX1 | 2% | 7% | 25% | [12] |
| IKZF1 gene deletions | 20.3% | - | 15% | [32,33] |
| IKZF1plus | 21.3% | - | 6% | [33] |
| Ph-like mutations | 27% | 25% | 3% | [3,34] |
| JAK mutations | 5% | 60% | 5.6% | [31] |
| CRLF2 gene alterations | 4% | 11% | 5–7% | [12] |
| iAMP21 | 12% | 5.8% | 1.5% | [30] |
| IGH translocations | More frequent | 11% | <3% | [32,35] |
| DUX4/ERG | ~2% | 15% | 5% | [30,36] |
| t (4;11)/MLL | 8–10% | 4.5–5.7 | 2–3% (85% in infants) | [32,37] |
| t (1;19)/TCF3-PBX1 | 6% | 3% | 3% | [12,32] |
| CNS involvement | Higher | 10% | 3% | [29] |
| Characteristic | Adults | Children | References |
|---|---|---|---|
| Chemotherapy | Myelotoxic agents (anthracyclines, cytarabine, cyclophosphamide, etoposide) | Non-myelotoxic agents (VCR, asparaginase, MTX, higher doses of Prednisone) | [25,26] |
| Longer delays between courses | Greater adherence to schedules | ||
| Early and more intensive CNS chemotherapy | [18] | ||
| More prolonged maintenance chemotherapy | |||
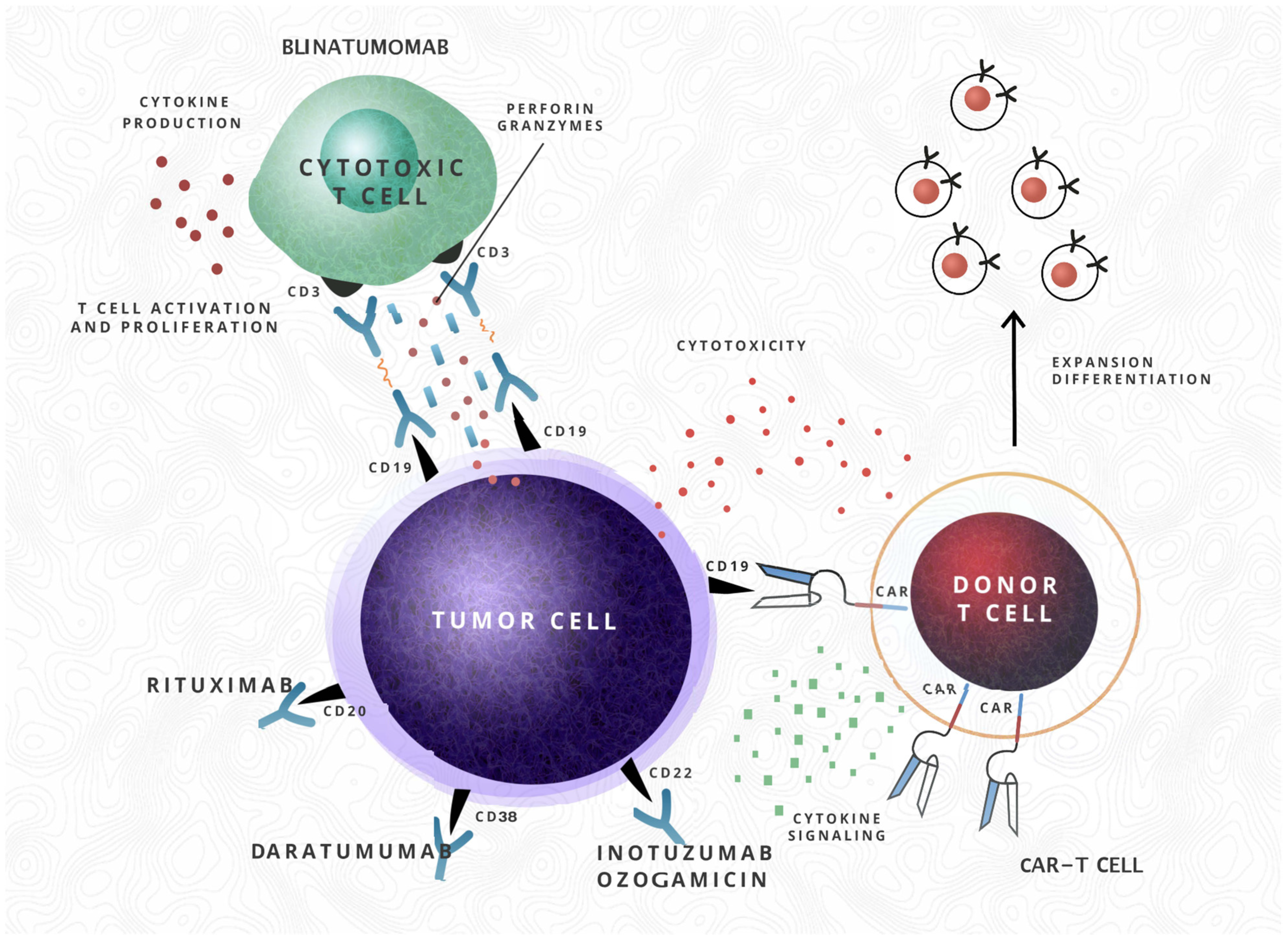
| Increased frequency of drug toxicity | Asparaginase hypersensitivity reactions, asparaginase, corticosteroids, cytarabine, daunorubicin, VCR toxicities Blinatumomab CRS CAR-T cells CRS, aplasia, hypogammaglobulinemia, immune effector cell-associated neurotoxicity syndrome | Inotuzumab ozogamicin (higher rates of veno-oclusive disease) Blinatumomab CRS CAR-T cells CRS, aplasia, hypogammaglobulinemia, immune effector cell-associated neurotoxicity syndrome) | [3,14,46,47,48,49] |
| Alterations in drug metabolism | |||
| Drug resistance | Resistance to prednisolone with increasing age | [50] | |
| Complex karyotype associated with steroid resistance in T-ALL | [51] | ||
| PRC2 loss-of-function alterations associated with PPR in T-ALL | [52] | ||
| Time to complete remission | Children achieve CR earlier than most adults | [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neaga, A.; Jimbu, L.; Mesaros, O.; Bota, M.; Lazar, D.; Cainap, S.; Blag, C.; Zdrenghea, M. Why Do Children with Acute Lymphoblastic Leukemia Fare Better Than Adults? Cancers 2021, 13, 3886. https://doi.org/10.3390/cancers13153886
Neaga A, Jimbu L, Mesaros O, Bota M, Lazar D, Cainap S, Blag C, Zdrenghea M. Why Do Children with Acute Lymphoblastic Leukemia Fare Better Than Adults? Cancers. 2021; 13(15):3886. https://doi.org/10.3390/cancers13153886
Chicago/Turabian StyleNeaga, Alexandra, Laura Jimbu, Oana Mesaros, Madalina Bota, Diana Lazar, Simona Cainap, Cristina Blag, and Mihnea Zdrenghea. 2021. "Why Do Children with Acute Lymphoblastic Leukemia Fare Better Than Adults?" Cancers 13, no. 15: 3886. https://doi.org/10.3390/cancers13153886
APA StyleNeaga, A., Jimbu, L., Mesaros, O., Bota, M., Lazar, D., Cainap, S., Blag, C., & Zdrenghea, M. (2021). Why Do Children with Acute Lymphoblastic Leukemia Fare Better Than Adults? Cancers, 13(15), 3886. https://doi.org/10.3390/cancers13153886





