Interactions Networks for Primary Heart Sarcomas
Abstract
Simple Summary
Abstract
1. Introduction
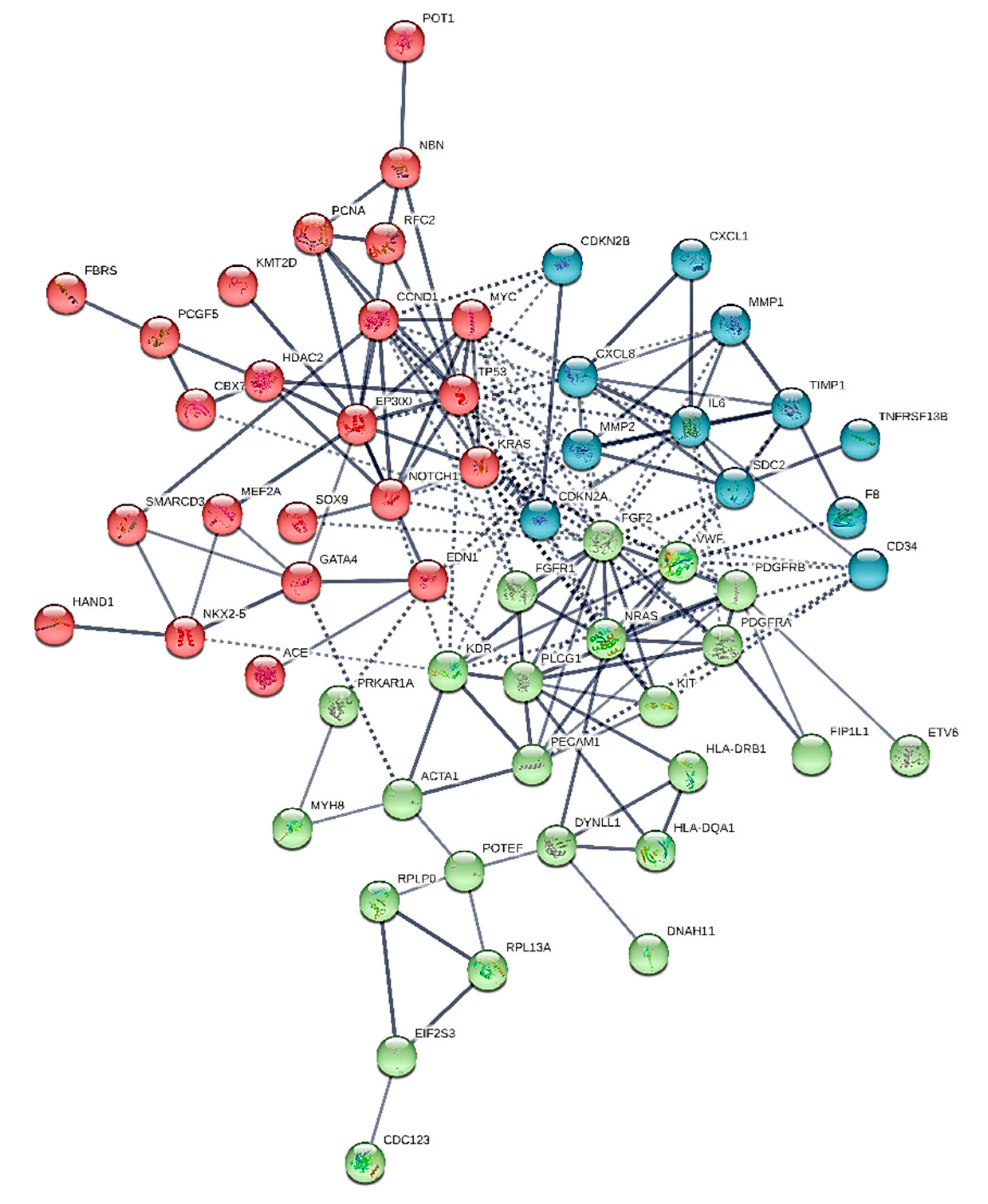
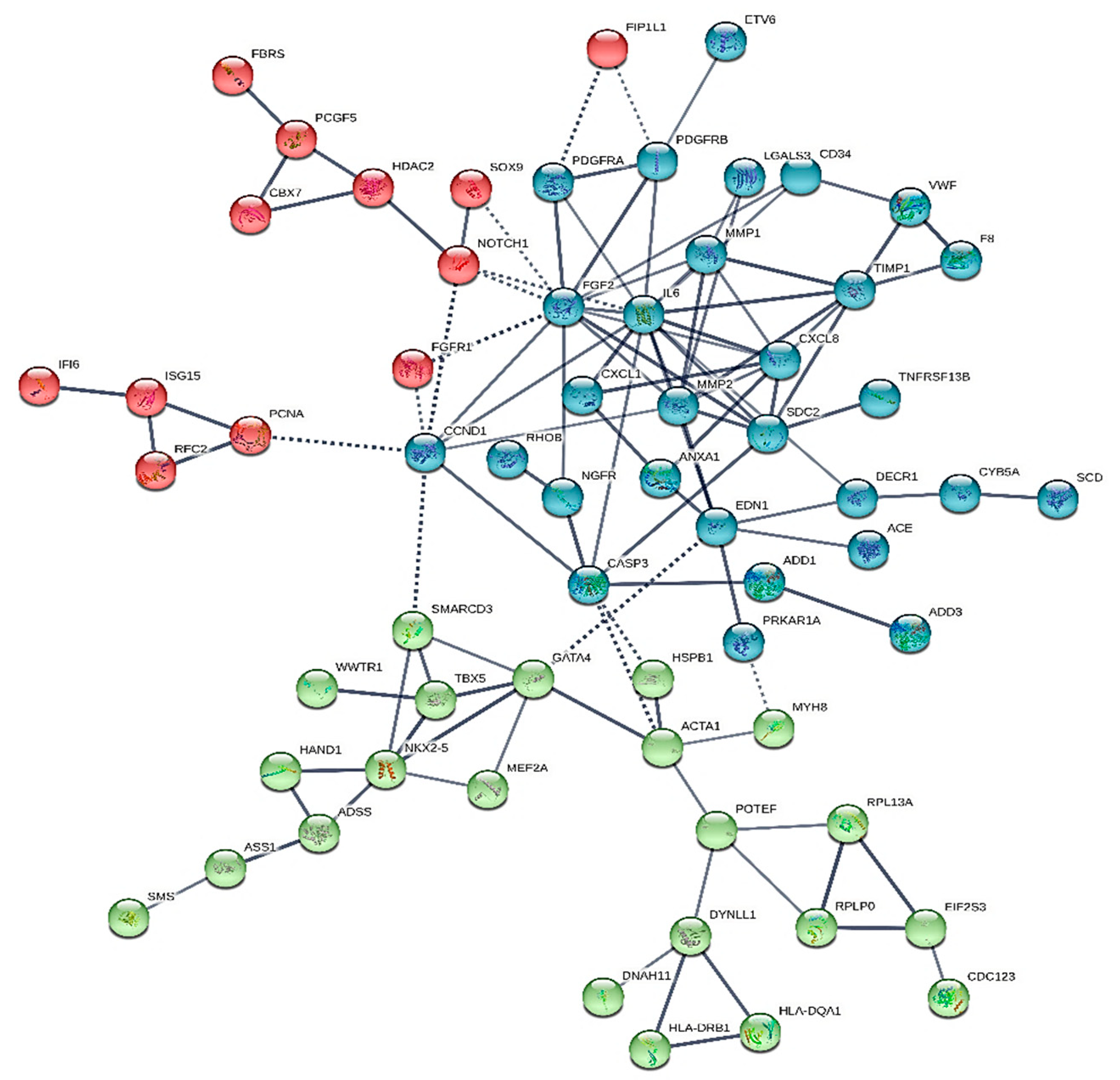
2. Results
2.1. Novelties
2.2. Major Hubs
3. Discussion
- (i)
- Angiosarcoma: (POT1, CDKN2A/B, PLCG1, KIT and KDR, MYC, TP53, KMT2D, NRAS or KRAS)
- (ii)
- Undiferrentiated pleomorphic sarcoma: (PDGFRB, FH and PIK3CA mutations KIT, PDGFRA/b, EGFR and MDM2 amplifications, CDKN2A deletion)
- (iii)
- Rhabdomyosarcoma: (KRAS)
- (iv)
- Leiomyosarcoma: (HRAS mutation)
- (v)
- Myxofibrosarcoma: (IFI6, LGALS3, ANXA1 and ASS1 downregulation CYB5A, SCD, ADD3, HSPB1, SMS, WWTR1 and RHOB upregulation)
- (vi)
- Synovial sarcoma: (SS18-SSX fusion)
- histone deacetylase 2-implicated in tumorigenesis (HDAC2), chronic obstructive pulmonary disease, lung diseases [38]
- fibroblast growth factor 2 (FGF2) implicated in cell survival and oncogenesis [38]
- polycomb group ring finger 5 that is involved in oncogenesis [39]
- syndecan 2 that has been associated to non-alcoholic fatty liver, hepatic fibrosis, post-traumatic stress disorder
- POTE ankyrin domain family member F, that has been related to dilated cardiomyopathy [41].
- adducin 1 implicated in the cell volume homeostasis, cell morphogenesis and cellular response to calcium ions [42]
- adenylosuccinate synthase 2 that is implicated in the de novo pathway and in the salvage pathway of purine nucleotide biosynthesis, it catalyzes the first committed step in the biosynthesis of AMP from IMP
- caspase 3, a canonical pro-apoptotic protein [43]
- cyclin dependent kinase 4—a cell division protein encoded by the CDK4 gene—which is associated to tumorigenesis of variant cancers [44]
- 2,4-dienoyl-CoA reductase 1 protein encoded by DECR1 gene participating in the beta-oxidation and metabolism of unsaturated fatty enoyl-CoA esters [45]
- E1A binding protein p300, associated to various syndromes and epithelial cancers [46]
- high mobility group AT-hook 2 which is a transcription factor related to malignancy and poor prognosis
- ISG15 ubiquitin like modifier—a small ribosomal subunit is a multi-modal unit implicated to immune response and more interestingly to cancer stem cells in a tumor [47]
- nerve growth factor receptor—a neurotrophic factor involved in the regulation and survival of certain neurons and pancreatic beta cells [50]
- platelet and endothelial cell adhesion molecule 1—a protein that removes aged neutrophils from the body, involving in leucocyte transmigration and angiogenesis [51].
- forty-four common nodes
- two common hubs: IL-6 and FGF2, that may be assumed as typical of the overall entity (heart sarcomas)
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wulff, H.R. The concept of disease: From Newton back to Aristotle. Lancet 1999, 54, 354. [Google Scholar] [CrossRef]
- Leopold, J.A.; Loscalzo, J. Emerging Role of Precision Medicine in Cardiovascular Disease. Circ. Res. 2018, 122, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The growing role of precision and personalized medicine for cancer treatment. Technology 2018, 6, 79–100. [Google Scholar] [CrossRef]
- Adams, K.F. Systems biology and heart failure: Concepts, methods, and potential research applications. Heart Fail. Rev. 2010, 15, 371–398. [Google Scholar] [CrossRef]
- Ren, D.Y.; Fuller, N.D.; Gilbert, S.A.B.; Zhang, Y. Cardiac Tumors: Clinical Perspective and Therapeutic Considerations. Curr. Drug Targets 2017, 18, 1805–1809. [Google Scholar] [CrossRef] [PubMed]
- Silverman, N.A. Primary cardiac tumors. Ann. Surg. 1980, 191, 127–138. [Google Scholar] [CrossRef]
- Burke, A.; Virmani, R. Classification and Incidence of Cardiac Tumors. Tumors of the Heart and Great Vessels; Armed Forces Institute of Pathology: Washington, DC, USA, 1996; pp. 1–11. [Google Scholar]
- Taguchi, S. Comprehensive review of the epidemiology and treatments for malignant adult cardiac tumors. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 257–262. [Google Scholar] [CrossRef]
- Urbini, M.; Astolfi, A.; Indio, V.; Nannini, M.; Pizzi, C.; Paolisso, P.; Tarantino, G.; Pantaleo, M.A.; Saponara, M. Genetic aberrations and molecular biology of cardiac sarcoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920918492. [Google Scholar] [CrossRef] [PubMed]
- Donsbeck, A.V.; Ranchere, D.; Coindre, J.M.; Le Gall, F.; Cordier, J.F.; Loire, R. Primary cardiac sarcomas: An immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology 1999, 34, 295–304. [Google Scholar] [CrossRef]
- Sarjeant, J.M.; Butany, J.; Cusimano, R.J. Cancer of the heart: Epidemiology and management of primary tumors and metastases. Am. J. Cardiovasc. Drugs 2003, 3, 407–421. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Geronikolou, S.; Pavlopoulou, A.; Chrousos, G.P.; Cokkinos, D.V. Heart Failure Due to Cardiac Cancer (HFCC1) Signalisome Guides Personalized Cardiotherapy; ESC: Munich, Germany, 2018. [Google Scholar]
- Geronikolou, S.; Pavlopoulou, A.; Chrousos, G.P.; Cokkinos, D.V. P3502 A novel interactome to guide therapy and avoid heart failure in cardiac cancer patients–figshare. Eur. Heart J. 2018, 39. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhang, L.; Qin, W.; Yao, X.H.; Zheng, L.Z.; Liu, X.; Li, J.; Guo, W.J. Oncogenic role of the chromobox protein CBX7 in gastric cancer. J. Exp. Clin. Cancer Res. 2010, 29, 114. [Google Scholar] [CrossRef]
- Fischer, M.; Schwieger, M.; Horn, S.; Niebuhr, B.; Ford, A.; Roscher, S.; Bergholz, U.; Greaves, M.; Löhler, J.; Stocking, C. Defining the oncogenic function of the TEL/AML1 (ETV6/RUNX1) fusion protein in a mouse model. Oncogene 2005, 24, 7579–7591. [Google Scholar] [CrossRef]
- Navid, S.; Fan, C.; O Flores-Villanueva, P.; Generali, D.; Li, Y. The Fibroblast Growth Factor Receptors in Breast Cancer: From Oncogenesis to Better Treatments. Int. J. Mol. Sci. 2020, 21, 2011. [Google Scholar]
- Jiang, X.H.; Liu, Y.Y. LINC00467 promotes proliferation and invasion in glioma via interacting with miRNA-485-5p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 766–772. [Google Scholar] [PubMed]
- Foley, C.J.; Fanjul-Fernández, M.; Bohm, A.; Nguyen, N.; Agarwal, A.; Austin, K.; Koukos, G.; Covic, L.; López-Otín, C.; Kuliopulos, A. Matrix metalloprotease 1a deficiency suppresses tumor growth and angiogenesis. Oncogene 2014, 33, 2264–2272. [Google Scholar] [CrossRef]
- Khanna, P.; Chua, P.J.; Wong, B.; Yin, C.; Thike, A.A.; Wan, W.K.; Tan, P.H. GRAM domain-containing protein 1B (GRAMD1B), a novel component of the JAK/STAT signaling pathway, functions in gastric carcinogenesis. Oncotarget 2017, 8, 115370–115383. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tong, X.; Liu, Y.; Liu, S.; Xiong, H.; Fan, H. ACE gene polymorphism is associated with COPD and COPD with pulmonary hypertension: A meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2435–2446. [Google Scholar] [CrossRef]
- Xie, X.; Shi, X.; Xun, X.; Rao, L. Endothelial nitric oxide synthase gene single nucleotide polymorphisms and the risk of hypertension: A meta-analysis involving 63,258 subjects. Clin. Exp. Hypertens. 2017, 39, 175–182. [Google Scholar] [CrossRef]
- Skrzynia, C.; Berg, J.S.; Willis, M.S.; Jensen, B.C. Genetics and heart failure: A concise guide for the clinician. Curr. Cardiol. Rev. 2015, 11, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bruno, C.M.; Neri, S.; Di Prima, P.; Sciacca, C. Pathophysiology of endothelin and medical emergencies. Panminerva Med. 2003, 45, 151–154. [Google Scholar] [PubMed]
- Posch, M.G.; Boldt, L.H.; Polotzki, M.; Richter, S.; Rolf, S.; Perrot, A.; Dietz, R.; Ozcelik, C.; Haverkamp, W. Mutations in the cardiac transcription factor GATA4 in patients with lone atrial fibrillation. Eur. J. Med. Genet. 2010, 53, 201–203. [Google Scholar] [CrossRef]
- Goette, A.; Jentsch-Ullrich, K.; Lendeckel, U.; Röcken, C.; Agbaria, M.; Auricchio, A.; Mohren, M.; Franke, A.; Klein, H.U. Effect of atrial fibrillation on hematopoietic progenitor cells: A novel pathophysiological role of the atrial natriuretic peptide? Circulation 2003, 108, 2446–2449. [Google Scholar] [CrossRef]
- Vieceli Dalla Sega, F.; Fortini, F.; Aquila, G.; Campo, G.; Vaccarezza, M.; Rizzo, P. Notch Signaling Regulates Immune Responses in Atherosclerosis. Front. Immunol. 2019, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Schulz, R. Activation of MMP-2 as a key event in oxidative stress injury to the heart. Front. Biosci. 2009, 14, 699–716. [Google Scholar]
- De Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Eiselleova, L.; Matulka, K.; Kriz, V.; Kunova, M.; Schmidtova, Z.; Neradil, J.; Tichy, B.; Dvorakova, D.; Pospisilova, S.; Hampl, A.; et al. A complex role for FGF-2 in self-renewal, survival, and adhesion of human embryonic stem cells. Stem Cells 2009, 27, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, F.; Garagiola, I.; Baronciani, L. Role of von Willebrand factor in the haemostasis. Blood Transfus. 2011, 9, s3–s8. [Google Scholar]
- Engelhardt, M.; Lübbert, M.; Guo, Y. CD34(+) or CD34(-): Which is the more primitive? Leukemia 2002, 16, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Nunemaker, C.S.; Chung, H.G.; Verrilli, G.M.; Corbin, K.L.; Upadhye, A.; Sharma, P.R. Increased serum CXCL1 and CXCL5 are linked to obesity, hyperglycemia, and impaired islet function. J. Endocrinol. 2014, 222, 267–276. [Google Scholar] [CrossRef]
- Höglinger, D.; Burgoyne, T.; Sanchez-Heras, E.; Hartwig, P.; Colaco, A.; Newton, J.; Futter, C.E.; Spiegel, S.; Platt, F.M.; Eden, E.R. NPC1 regulates ER contacts with endocytic organelles to mediate cholesterol egress. Nat. Commun. 2019, 10, 4276. [Google Scholar] [CrossRef] [PubMed]
- Neuville, A.; Collin, F.; Bruneval, P.; Parrens, M.; Thivolet, F.; Gomez-Brouchet, A.; Terrier, P.; de Montpreville, V.T.; Le Gall, F.; Hostein, I.; et al. Intimal sarcoma is the most frequent primary cardiac sarcoma: Clinicopathologic and molecular retrospective analysis of 100 primary cardiac sarcomas. Am. J. Surg. Pathol. 2014, 38, 461–469. [Google Scholar] [CrossRef]
- Parissis, J.; Arvanitis, D.; Sourvinos, G.; Spandidos, D. A primary cardiac leiomyosarcoma with mutation at H-ras codon 12. Oncol. Rep. 1997, 4, 807–808. [Google Scholar] [CrossRef]
- Liao, W.; Lim, A.; Tan, W.; Abisheganaden, J.; Wong, W. Restoration of HDAC2 and Nrf2 by andrographolide overcomes corticosteroid resistance in chronic obstructive pulmonary disease. Br. J. Pharmacol. 2020, 177, 3662–3673. [Google Scholar] [CrossRef]
- Jiang, S.; Tan, B.; Zhang, X. Identification of key lncRNAs in the carcinogenesis and progression of colon adenocarcinoma by co-expression network analysis. J. Cell. Biochem. 2019, 120, 6490–6501. [Google Scholar] [CrossRef]
- Contreras, H.R.; Ledezma, R.A.; Vergara, J.; Cifuentes, F.; Barra, C.; Cabello, P.; Gallegos, I.; Morales, B.; Huidobro, C.; Castellón, E.A. The expression of syndecan-1 and -2 is associated with Gleason score and epithelial-mesenchymal transition markers, E-cadherin and beta-catenin, in prostate cancer. Urol. Oncol. 2010, 28, 534–540. [Google Scholar] [CrossRef]
- Bergenfelz, C.; Larsson, A.M.; von Stedingk, K.; Gruvberger-Saal, S.; Aaltonen, K.; Jansson, S.; Jernstrom, H.; Janols, H.; Wullt, M.; Bredberg, A.; et al. Systemic Monocytic-MDSCs Are Generated from Monocytes and Correlate with Disease Progression in Breast Cancer Patients. PLoS ONE 2015, 10, e0127028. [Google Scholar] [CrossRef] [PubMed]
- Kugelmann, D.; Waschke, J.; Radeva, M.Y. Adducin is involved in endothelial barrier stabilization. PLoS ONE 2015, 10, e0126213. [Google Scholar] [CrossRef]
- Boudreau, M.W.; Peh, J.; Hergenrother, P.J. Procaspase-3 Overexpression in Cancer: A Paradoxical Observation with Therapeutic Potential. ACS Chem. Biol. 2019, 14, 2335–2348. [Google Scholar] [CrossRef]
- Sobhani, N.; D’Angelo, A.; Pittacolo, M.; Roviello, G.; Miccoli, A.; Corona, S.P.; Bernocchi, O.; Generali, D.; Otto, T. Updates on the CDK4/6 Inhibitory Strategy and Combinations in Breast Cancer. Cells 2019, 8, 321. [Google Scholar] [CrossRef]
- Cuebas, D.; Schulz, H. Evidence for a modified pathway of linoleate degradation. Metabolism of 2,4-decadienoyl coenzyme A. J. Biol. Chem. 1982, 257, 14140–14144. [Google Scholar] [CrossRef]
- Samuels, Y.; Wang, Z.; Bardelli, A.; Silliman, N.; Ptak, J.; Szabo, S.; Yan, H.; Gazdar, A.; Powell, S.M.; Riggins, G.J.; et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004, 304, 554. [Google Scholar] [CrossRef] [PubMed]
- Sainz, B., Jr.; Martín, B.; Tatari, M.; Heeschen, C.; Guerra, S. ISG15 is a critical microenvironmental factor for pancreatic cancer stem cells. Cancer Res. 2014, 74, 7309–7320. [Google Scholar] [CrossRef]
- Sharma, S.; Javadekar, S.M.; Pandey, M.; Srivastava, M.; Kumari, R.; Raghavan, S.C. Homology and enzymatic requirements of microhomology-dependent alternative end joining. Cell Death Dis. 2015, 6, e1697. [Google Scholar] [CrossRef] [PubMed]
- Uzunoglu, H.; Korak, T.; Ergul, E.; Uren, N.; Sazci, A.; Utkan, N.Z.; Kargi, E.; Triyaki, Ç.; Yirmibesoglu, O. Association of the nibrin gene (NBN) variants with breast cancer. Biomed. Rep. 2016, 4, 369–373. [Google Scholar] [CrossRef]
- Pierucci, D.; Cicconi, S.; Bonini, P.; Ferrelli, F.; Pastore, D.; Matteucci, C.; Marselli, L.; Marchetti, P.; Ris, F.; Halban, P.; et al. NGF-withdrawal induces apoptosis in pancreatic beta cells in vitro. Diabetologia 2001, 44, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Ganjei-Azar, P. Color. Atlas of Immunocytochemistry in Diagnostic Cytology; Springer Science+Business Media, LLC.: New York, NY, USA, 2007. [Google Scholar]
- Consortium, T.C.D. Coronary artery disease risk loci identified in over 190,000 individuals implicate lipid metabolism and inflammation as key causal pathways. Nat. Genet. 2013, 25–33. [Google Scholar]
- Mestroni, L.J.; Begay, R.; Graw, S.L.; Taylor, M.R.G. Pharmacogenetics of Heart Failure. Curr. Opin. Cardiol. 2014, 29, 227–234. [Google Scholar] [CrossRef][Green Version]
- Bristow, M.R. Pharmacogenetic targeting of drugs for heart failure. Pharmacol. Ther. 2012, 134, 107–115. [Google Scholar] [CrossRef]
- Mestroni, L.J.; Gilbert, E.M.; Lowes, B.L. Dilated Cardiomyopathies in Hurst’s the Heart, 13th ed.; Fuster, V., Walsh, R.A., Harrington, R.A., Eds.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2011; pp. 821–836. [Google Scholar]
- Burke, A.; Tavora, F. The 2015 WHO Classification of Tumors of the Heart and Pericardium. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 441–452. [Google Scholar] [CrossRef]
- Neragi-Miandoab, S.; Kim, J.; Vlahakes, G.J. Malignant tumours of the heart: A review of tumour type, diagnosis and therapy. Clin. Oncol. 2007, 19, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Chiosea, S.I.; Williams, L.; Griffith, C.C.; Thompson, L.D.; Weinreb, I.; Bauman, J.E.; Luvison, A.; Roy, S.; Seethala, R.R.; Nikiforova, M.N. Molecular characterization of apocrine salivary duct carcinoma. Am. J. Surg. Pathol. 2015, 39, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Chiosea, S.I.; Miller, M.; Seethala, R.R. HRAS mutations in epithelial-myoepithelial carcinoma. Head Neck Pathol. 2014, 8, 146–150. [Google Scholar] [CrossRef]
- Kovács, I.A.; Luck, K.; Spirohn, K.; Wang, Y.; Pollis, C.; Schlabach, S.; Bian, W.; Kim, D.K.; Kishore, N.; Hao, T.; et al. Network-based prediction of protein interactions. Nat. Commun. 2019, 10, 1240. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.; Cusick, M.E.; Barabási, A.L. Interactome networks and human disease. Cell 2011, 144, 986–998. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]






| Gene Symbol | Description | Accession Number 1 | Interactome 2 |
|---|---|---|---|
| ACE | angiotensin I converting enzyme | NM_000789 | CS 1–6 |
| ACTA1 | actin alpha 1, skeletal muscle | NM_001100 | CS 1–6 |
| ADD1 | adducin 1 | NM_014189 | CS 5 |
| ADD3 | adducin 3 | NM_019903 | CS 5 |
| ADSS | adenylosuccinate synthase 2 | NM_001126 | CS 5 |
| ANXA1 | annexin A1 | NM_000700 | CS 5 |
| ASS1 | argininosuccinate synthase 1 | NM_000050 | CS 5 |
| CASP3 | caspase 3 | NM_004346 | CS 5 |
| CBX7 | chromobox 7 | NM_175709.5 | CS 1–6 |
| CCND1 | cyclin D1 | NM_053056.3 | CS 1–6 |
| CD34 | CD34 molecule | NM_001773 | CS 1–6 |
| CDC123 | cell division cycle 123 | NM_006023 | CS 1–6 |
| CDK4 | cyclin dependent kinase 4 | NM_000075 | CS 2 |
| CDKN2A | Cyclin Dependent Kinase Inhibitor 2A | NM_001025109 | CS 1, 2 |
| CDKN2B | Cyclin Dependent Kinase Inhibitor 2B | NM_006023 | CS 1 |
| CXCL1 | C-X-C motif chemokine ligand 1 | NM_001511 | CS 1–6 |
| CXCL8 | C-X-C motif chemokine ligand 8 | NM_000584 | CS 1–6 |
| CYB5A | cytochrome b5 type A | NM_001914 | CS 5 |
| DECR1 | 2,4-dienoyl-CoA reductase 1 | NM_001330575 | CS 5 |
| DNAH11 | dynein axonemal heavy chain 11 | NM_001277115 | CS 1–6 |
| DYNLL1 | dynein light chain LC8-type 1 | NM_001037494 | CS 1–6 |
| EDN1 | endothelin 1 | NM_001955 | CS 1–6 |
| EIF2S3 | eukaryotic translation initiation factor 2 subunit gamma | NM_001415 | CS 1–6 |
| EGFR | epidermal growth factor receptor | NM_005228 | CS 2 |
| EP300 | E1A binding protein p300 | NM_001429 | CS 1 |
| ETV6 | ETS variant 6 | NM_001987 | CS 1–6 |
| F8 | coagulation factor VIII | NM_000132 | CS 1–6 |
| FBRS | Fibrosin | NM_001105079 | CS 1–6 |
| FGF2 | fibroblast growth factor 2 | NM_001361665 | CS1–6 |
| FGFR1 | fibroblast growth factor receptor 1 | NM_023110 | CS1–6 |
| FIP1L1 | factor interacting with PAPOLA and CPSF1 | NM_030917 | CS1–6 |
| GATA4 | GATA binding protein 4 | NM_001308093 | CS 1–6 |
| HAND1 | heart and neural crest derivatives expressed 1 | NM_004821 | CS 1–6 |
| HDAC2 | histone deacetylase 2 | NM_001527 | CS 1–6 |
| HLA-DQA1 | major histocompatibility complex, class II, DQ alpha 1 | NM_002122 | CS 1–6 |
| HLA-DRB1 | major histocompatibility complex, class II, DR beta 1 | NM_002124.4 | CS 1–6 |
| HMGA2 | high mobility group AT-hook 2 | NM_003483 | CS 2 |
| HRAS | HRas proto-oncogene, GTPase | NM_176795 | CS 4 |
| HSPB1 | heat shock protein family B (small) member 1 | NM_001540 | CS 5 |
| IFI6 | interferon alpha inducible protein 6 | NM_022873 | CS 5 |
| IL6 | interleukin 6 | NM_000600 | CS 1–6 |
| ISG15 | ISG15 ubiquitin like modifier | NM_005101 | CS 5 |
| KDR | kinase insert domain receptor | NM_002253 | CS 1 |
| KIT | KIT proto-oncogene, receptor tyrosine kinase | NM_000222 | CS 1 |
| KMT2D | lysine methyltransferase 2D | NM_003482 | CS 1 |
| KRAS | KRAS proto-oncogene, GTPase | NM_033360 | CS 1, CS 3 |
| LGALS3 | galectin 3 | NM_002306 | CS 5 |
| MDM2 | MDM2 proto-oncogene | NM_002392.6 | CS 2 |
| MEF2A | myocyte enhancer factor 2A | NM_001130926 | CS 1–6 |
| MMP1 | matrix metallopeptidase 1 | NM_002421 | CS 1–6 |
| MMP2 | matrix metallopeptidase 2 | NM_001127891 | CS 1–6 |
| MYC | MYC proto-oncogene, bHLH transcription factor | NM_001354870 | CS 1 |
| MYH8 | myosin heavy chain 8 | NM_002472 | CS 1–6 |
| NBN | nibrin | NM_001024688 | CS 1 |
| NGFR | nerve growth factor receptor | NM_002507 | CS 5 |
| NKX2-5 | NK2 homeobox 5 | NM_001166175 | CS 1–6 |
| NOTCH1 | notch 1 | NM_017617 | CS 1–6 |
| NRAS | NRAS Proto-Oncogene, GTPase | NM_002524.5 | CS 1 |
| PCGF5 | polycomb group ring finger 5 | NM_032373 | CS 1–6 |
| PCNA | proliferating cell nuclear antigen | NM_002592 | CS 1–6 |
| PDGFRA | platelet derived growth factor receptor alpha | NM_006206 | CS 1–6 |
| PDGFRB | platelet derived growth factor receptor beta | NM_002609 | CS 1–6 |
| PECAM1 | platelet and endothelial cell adhesion molecule 1 | NM_000442 | CS 1 |
| PIK3CA | phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha | NM_006218 | CS 2 |
| PLCG1 | phospholipase C gamma 1 | NM_182811 | CS 1 |
| POT1 | protection of telomeres 1 | NM_001042594 | CS 1 |
| POTEF | POTE ankyrin domain family member F | NM_001099771 | CS 1–6 |
| PRKAR1A | protein kinase cAMP-dependent type I regulatory subunit alpha | NM_001276289 | CS 1–6 |
| RHOB | ras homolog family member B | NM_004040 | CS 5 |
| RFC2 | replication factor C subunit 2 | NM_181471 | CS 1–6 |
| RPL13A | ribosomal protein L13a | NM_001270491 | CS 1–6 |
| RPLP0 | ribosomal protein lateral stalk subunit P0 | NM_053275 | CS 1–6 |
| SCD | stearoyl-CoA desaturase | NM_005063 | CS 5 |
| SDC2 | syndecan 2 | NM_002998 | CS 1–6 |
| SMARCD3 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily d, member 3 | NM_001003801 | CS 1–6 |
| SMS | spermine synthase | NM_004595 | CS 5 |
| SOX9 | SRY-box 9 | NM_000346 | CS 1–6 |
| SS18 | SS18 subunit of BAF chromatin remodeling complex | NM_001007559 | CS 6 |
| SSX2 | SSX family member 2 | NM_003147 | CS 6 |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | NM_003254 | CS 1–6 |
| TNFRSF13B | TNF receptor superfamily member 13B | NM_012452 | CS 1–6 |
| TP53 | tumor protein p53 | NM_000546 | CS 1 |
| VWF | von Willebrand factor | NM_000552 | CS 1–6 |
| WWTR1 | WW domain containing transcription regulator 1 | NM_015472 | CS 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geronikolou, S.A.; Pavlopoulou, A.; Chrousos, G.P.; Cokkinos, D.V. Interactions Networks for Primary Heart Sarcomas. Cancers 2021, 13, 3882. https://doi.org/10.3390/cancers13153882
Geronikolou SA, Pavlopoulou A, Chrousos GP, Cokkinos DV. Interactions Networks for Primary Heart Sarcomas. Cancers. 2021; 13(15):3882. https://doi.org/10.3390/cancers13153882
Chicago/Turabian StyleGeronikolou, Styliani A., Athanasia Pavlopoulou, George P. Chrousos, and Dennis V. Cokkinos. 2021. "Interactions Networks for Primary Heart Sarcomas" Cancers 13, no. 15: 3882. https://doi.org/10.3390/cancers13153882
APA StyleGeronikolou, S. A., Pavlopoulou, A., Chrousos, G. P., & Cokkinos, D. V. (2021). Interactions Networks for Primary Heart Sarcomas. Cancers, 13(15), 3882. https://doi.org/10.3390/cancers13153882







