Immunohistochemical Evaluation of FGD3 Expression: A New Strong Prognostic Factor in Invasive Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Descriptive Characteristics
2.2. Association between Traditional Prognostic Factors and Outcome
2.3. Association between FGD3 Expression and Outcome
2.4. Multivariate Analysis
2.5. Association between FGD3 Expression and Outcome Stratified by Age at Diagnosis
2.6. Association between FGD3 Expression and Outcome Stratified by AJCC Stage
2.7. Association between FGD3 Expression and Outcome Stratified by Factors Associated with Uncertain Need for Chemotherapy (Node-Negative vs. Node-Positive; Luminal A; Luminal B)
2.8. Association between FGD3 Expression and Lymph Node Involvement
3. Discussion
4. Materials and Methods
4.1. Patients Selection and Data Collection
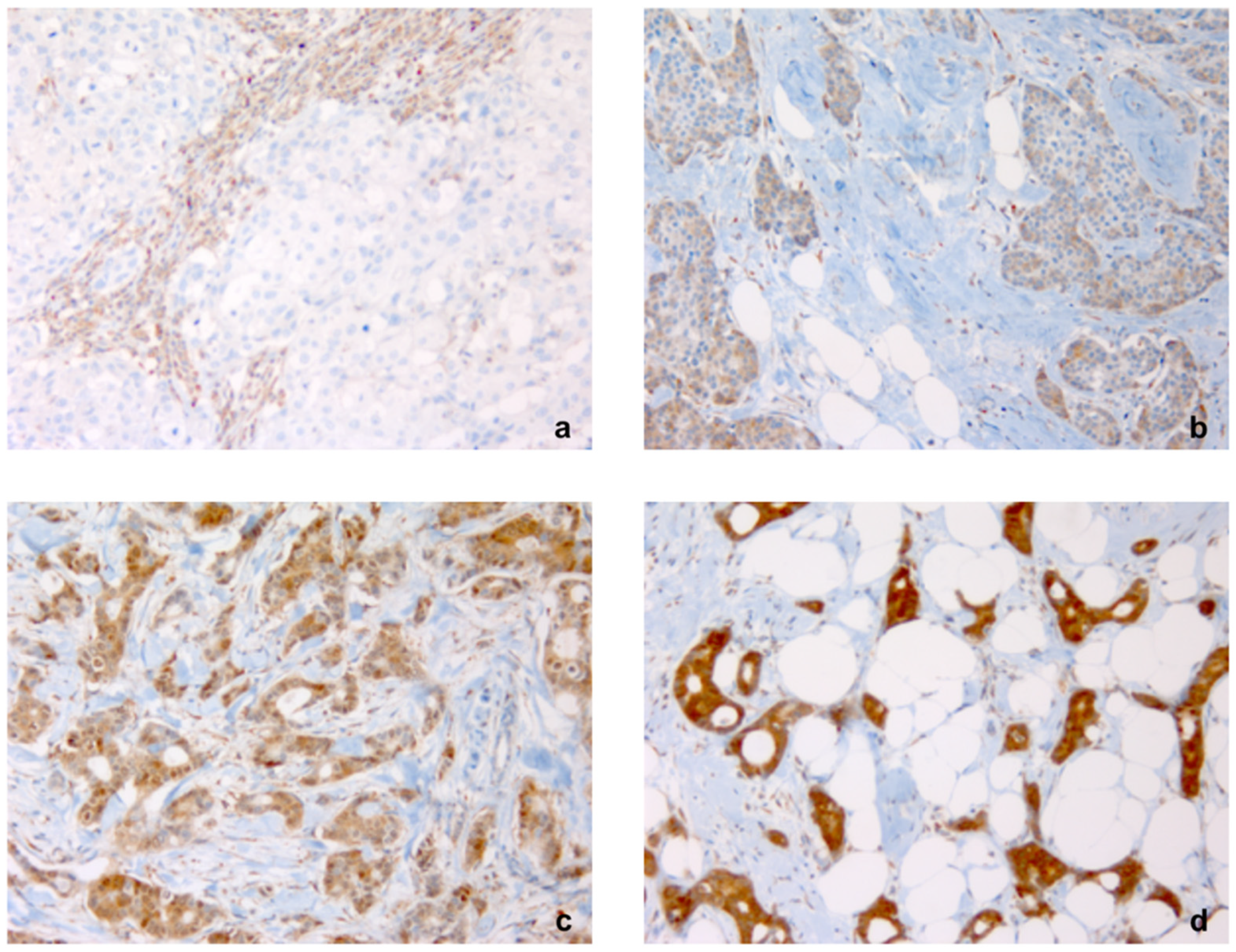
4.2. FGD3 Expression
4.3. Cellular Reactivity Cut-Off Point
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- I Numeri del Cancro in Italia 2019. (AIOM-AIRTUM). Available online: https://www.aiom.it/wp-content/uploads/2019/09/2019_Numeri_Cancro-operatori-web.pdf (accessed on 7 April 2021).
- Hayakawa, M.; Matsushima, M.; Hagiwara, H.; Oshima, T.; Fujino, T.; Ando, K.; Kikugawa, K.; Tanaka, H.; Miyazawa, K.; Kitagawa, M. Novel insights into FGD3, a putative GEF for Cdc42, that undergoes SCFFWD1/β-TrCP-mediated proteasomal degradation analogous to that of its homologue FGD1 but regulates cell morphology and motility differently from FGD1. Genes Cells 2008, 13, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.Y.; Ou Yang, T.H.; Anastassiou, D. Development of a prognostic model for breast cancer survival in an open challenge environment. Sci. Transl. Med. 2013, 5, 181ra50. [Google Scholar] [CrossRef]
- Margolin, A.A.; Bilal, E.; Huang, E.; Norman, T.C.; Ottestad, L.; Mecham, B.H.; Sauerwine, B.; Kellen, M.R.; Mangravite, L.M.; Furia, M.D.; et al. Systematic Analysis of Challenge-Driven Improvements in Molecular Prognostic Models for Breast Cancer. Sci. Transl. Med. 2013, 5, 181re1. [Google Scholar] [CrossRef] [PubMed]
- Ou Yang, T.H.; Cheng, W.Y.; Zheng, T.; Maurer, M.A.; Anastassiou, D. Breast cancer prognostic biomarker using attractor metagenes and the FGD3-SUSD3 metagene. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2850–2856. [Google Scholar] [CrossRef]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, L.N.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Lv, Q.; Zhang, J.; Yi, Y.; Huang, Y.; Wang, Y.; Wang, Y.; Zhang, W. Proliferating Cell Nuclear Antigen Has an Association with Prognosis and Risks Factors of Cancer Patients: A Systematic Review. Mol. Neurobiol. 2016, 53, 6209–6217. [Google Scholar] [CrossRef]
- Falchook, G.S.; Bastida, C.C.; Kurzrock, R. Aurora kinase inhibitors in oncology clinical trials: Current state of the progress. Semin. Oncol. 2015, 42, 832–848. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.; Sun, Y.; Abramovitz, M.; Young, B.; Lin, X.; Ni, M.; Achua, J.; Regan, M.M.; Gray, K.P.; Wang, V.; et al. High expression of FGD3, a putative regulator of cell morphology and motility, is prognostic of favorable outcome in multiple cancers. JCO Precis Oncol. 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Renda, I.; Bianchi, S.; Vezzosi, V.; Nori, J.; Vanzi, E.; Tavella, K.; Susini, T. Expression of FGD3 gene as prognostic factor in young breast cancer patients. Sci. Rep. 2019, 9, 15204. [Google Scholar] [CrossRef]
- Bryan, S.; Masoud, H.; Weir, H.K.; Woods, R.; Lockwood, G.; Smith, L.; Brierley, J.; Gospodarowicz, M.; Badets, N. Cancer in Canada: Stage at diagnosis. Health Rep. 2018, 29, 21–25. [Google Scholar]
- Agarwal, S.; Pappas, L.; Neumayer, L.; Kokeny, K.; Agarwal, J. Effect of Breast Conservation Therapy vs Mastectomy on Disease-Specific Survival for Early-Stage Breast Cancer. JAMA Surg. 2014, 149, 267–274. [Google Scholar] [CrossRef]
- Gherghe, M.; Bordea, C.; Blidaru, A. Sentinel lymph node biopsy (SLNB) vs. axillary lymph node dissection (ALND) in the current surgical treatment of early stage breast cancer. J. Med. Life. 2015, 8, 176–180. [Google Scholar]
- Fitzgibbons, P.L.; Page, D.L.; Weaver, D.; Thor, A.D.; Craig Allred, D.; Clark, G.M.; Ruby, S.G.; O’Malley, F.; Simpson, J.F.; Connolly, J.L.; et al. Prognostic Factors in Breast Cancer of College of American Pathologists Consensus Statement. Arch. Pathol. Lab. Med. 2000, 124, 966–978. [Google Scholar] [CrossRef]
- Bloom, H.J.G.; Richardson, W.W. Histological Grading And Prognosis In Breast Cancer. A Study Of 1409 Cases Of Which 359 Have Been Followed For 15 Years. Br. J. Cancer. 1957, 11, 359–377. [Google Scholar] [CrossRef]
- Sandi, S.; Guihua, W.; Gaofang, X.; Richang, D.; Ningdong, H.; Xu, X.; Haibo, Z. Prediction model of lymphovascular invasion based on clinicopathological factors in Chinese patients with invasive breast cancer. Medicine 2018, 97, e12973. [Google Scholar] [CrossRef]
- Rahka, E.A.; Reis-Filho, J.S.; Baehner, F.; Dabbs, D.J.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Breast cancer prognostic classification in the molecular era: The role of histological grade. Breast Cancer Res. 2010, 12, 207. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Davies, C.; Godwin, J.; Gray, R.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Pan, H.C.; Taylor, C.; et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [CrossRef]
- Osborne, C.K. Tamoxifen in the Treatment of Breast Cancer. N. Engl. J. Med. 1998, 339, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Madani, S.H.; Payandeh, M.; Sadeghi, M.; Motamed, H.; Sadeghi, E. The correlation between Ki-67 with other prognostic factors in breast cancer: A study in Iranian patients. Indian J. Med. Paediatr. Oncol. 2016, 37, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Wang, S.; Shi, Y.; Liu, D.; Teng, X.; Qin, T.; Zeng, Y.; Yuan, Z. Patients 35 years old or younger with operable breast cancer are more at risk for relapse and survival: A retrospective matched caseecontrol study. Breast 2011, 20, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Passhak, M.; Shachar, S.S.; Bar-Sela, G.; Fried, G. Breast cancer in young women aged 35 and under: Patterns of care and outcome. Breast J. 2017, 24, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, A.; Iwamoto, T.; Tokunaga, E.; Tomotaki, A.; Kumamaru, H.; Miyata, H.; Niikura, N.; Kawai, M.; Anan, K.; Hayashi, N.; et al. Young adult breast cancer patients have a poor prognosis independent of prognostic clinicopathological factors: A study from the Japanese Breast Cancer Registry. Breast Cancer Res. Treat. 2016, 160, 163–172. [Google Scholar] [CrossRef]
- Cardoso, F.; Loibl, S.; Pagani, O.; Graziottin, A.; Panizza, P.; Martincich, L.; Gentilini, O.; Peccatori, F.; Fourquet, A.; Delaloge, S.; et al. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur. J. Cancer. 2012, 48, 3355–3377. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- The Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: Collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996, 347, 1713–1727. [Google Scholar] [CrossRef]
- The Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 1997, 350, 1047–1059. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Taube, S.E.; McShane, L.M.; Cavenagh, M.M.; Altman, D.G. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): An Abridged Explanation and Elaboration. J. Natl. Cancer Inst. 2018, 110, 803–811. [Google Scholar] [CrossRef]







| Characteristic | All | % | FGD3− | % | FGD3+ | % | p Value |
|---|---|---|---|---|---|---|---|
| Age, median (range) | 57 (22–89) | ___ | 58.5 (23–87) | ___ | 55.0 (22–89) | ___ | ___ |
| Histological grade | |||||||
| G1 | 87 | 21.7 | 18 | 4.5 | 69 | 17.2 | <0.001 |
| G2 | 166 | 41.4 | 39 | 9.7 | 127 | 31.7 | |
| G3 | 148 | 36.9 | 83 | 20.7 | 65 | 16.2 | |
| LVI | |||||||
| No | 270 | 67.3 | 83 | 20.6 | 187 | 46.7 | 0.012 |
| Yes | 131 | 32.7 | 57 | 14.2 | 74 | 18.5 | |
| Molecular Subtype | |||||||
| Luminal A | 137 | 34.2 | 27 | 6.8 | 110 | 27.4 | <0.001 |
| Luminal B | 145 | 36.2 | 45 | 11.2 | 100 | 25.0 | |
| HER2+ | 87 | 21.7 | 48 | 12.0 | 39 | 9.7 | |
| Triple Negative | 32 | 7.9 | 20 | 4.9 | 12 | 3.0 | |
| Ki67 expression | |||||||
| Ki67 ≤ 20% | 185 | 46.1 | 40 | 10.0 | 145 | 36.1 | <0.001 § |
| Ki67 > 20% | 208 | 51.9 | 96 | 24.0 | 112 | 27.9 | |
| unknown | 8 | 2.0 | 4 | 1.0 | 4 | 1.0 | |
| AJCC stage | |||||||
| I | 227 | 56.6 | 56 | 14.0 | 171 | 42.6 | <0.001 |
| II | 103 | 25.7 | 47 | 11.7 | 56 | 14.0 | |
| III | 66 | 16.5 | 34 | 8.5 | 32 | 8.0 | |
| IV | 5 | 1.2 | 3 | 0.7 | 2 | 0.5 | |
| Primary Tumor Surgery | |||||||
| BCS | 281 | 70.1 | 89 | 22.2 | 192 | 47.9 | 0.037 |
| Mastectomy | 120 | 29.9 | 51 | 12.7 | 69 | 17.2 | |
| Axillary Lymph Node Surgery | |||||||
| SLB | 268 | 66.8 | 72 | 18.0 | 196 | 48.8 | <0.001 |
| AD | 133 | 33.2 | 68 | 17.0 | 65 | 16.2 | |
| Neoadjuvant Chemotherapy | |||||||
| No | 352 | 87.8 | 122 | 30.4 | 230 | 57.4 | 0.775 |
| Yes | 49 | 12.2 | 18 | 4.5 | 31 | 7.7 | |
| Adjuvant Chemotherapy | |||||||
| No | 250 | 62.3 | 70 | 17.5 | 170 | 44.8 | <0.001 |
| Yes | 151 | 37.7 | 70 | 17.5 | 81 | 20.2 | |
| Hormonotherapy | |||||||
| No | 115 | 28.7 | 52 | 13.0 | 63 | 15.7 | 0.006 |
| Yes | 286 | 71.3 | 88 | 21.9 | 198 | 49.4 | |
| Trastuzumab | |||||||
| No | 345 | 86.0 | 109 | 27.2 | 236 | 58.8 | 0.001 |
| Yes | 56 | 14.0 | 31 | 7.7 | 25 | 6.3 | |
| Adjuvant Radiotherapy | |||||||
| No | 99 | 24.7 | 37 | 9.2 | 62 | 15.5 | 0.554 |
| Yes | 302 | 75.3 | 103 | 25.7 | 199 | 49.6 | |
| Parameters | Disease-Free Survival | |||
| HR | 95% CI | p | ||
| Age at diagnosis | Age > 40 ys | Ref. | <0.001 | |
| Age ≤ 40 ys | 2.747 | 1.60–4.72 | ||
| FGD3 expression | High | Ref. | 0.003 | |
| Low | 2.252 | 1.31–3.87 | ||
| AJCC stage | I, II | Ref. | 0.012 | |
| III, IV | 2.018 | 1.16–3.50 | ||
| Hormonal receptor status | ER/PgR− | Ref. | 0.033 | |
| ER/PgR+ | 0.535 | 0.30–0.94 | ||
| Parameters | Overall Survival | |||
| HR | 95% CI | p | ||
| AJCC stage | I, II | Ref. | <0.001 | |
| III, IV | 4.802 | 2.28–10.09 | ||
| FGD3 expression | High | Ref. | 0.007 | |
| Low | 3.021 | 1.35–6.74 | ||
| FGD3 Expression | pN+ | Total | % | p |
| Low (− or ≤30% +) | 64 | 140 | 45.7 | <0.001 |
| High (>30% +, ++, +++) | 72 | 261 | 27.6 | |
| Total | 136 | 401 | 33.9 | |
| FGD3 Expression | pN+ ≥ 10 | Total | % | p |
| Low (− or ≤30% +) | 14 | 64 | 21.9 | 0.882 |
| High (>30% +, ++, +++) | 15 | 72 | 20.8 | |
| Total | 29 | 136 | 21.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susini, T.; Saccardin, G.; Renda, I.; Giani, M.; Tartarotti, E.; Nori, J.; Vanzi, E.; Pasqualini, E.; Bianchi, S. Immunohistochemical Evaluation of FGD3 Expression: A New Strong Prognostic Factor in Invasive Breast Cancer. Cancers 2021, 13, 3824. https://doi.org/10.3390/cancers13153824
Susini T, Saccardin G, Renda I, Giani M, Tartarotti E, Nori J, Vanzi E, Pasqualini E, Bianchi S. Immunohistochemical Evaluation of FGD3 Expression: A New Strong Prognostic Factor in Invasive Breast Cancer. Cancers. 2021; 13(15):3824. https://doi.org/10.3390/cancers13153824
Chicago/Turabian StyleSusini, Tommaso, Giulia Saccardin, Irene Renda, Milo Giani, Enrico Tartarotti, Jacopo Nori, Ermanno Vanzi, Elisa Pasqualini, and Simonetta Bianchi. 2021. "Immunohistochemical Evaluation of FGD3 Expression: A New Strong Prognostic Factor in Invasive Breast Cancer" Cancers 13, no. 15: 3824. https://doi.org/10.3390/cancers13153824
APA StyleSusini, T., Saccardin, G., Renda, I., Giani, M., Tartarotti, E., Nori, J., Vanzi, E., Pasqualini, E., & Bianchi, S. (2021). Immunohistochemical Evaluation of FGD3 Expression: A New Strong Prognostic Factor in Invasive Breast Cancer. Cancers, 13(15), 3824. https://doi.org/10.3390/cancers13153824





