Review of Treatment Options for the Management of Advanced Stage Hodgkin Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. ABVD versus BEACOPP
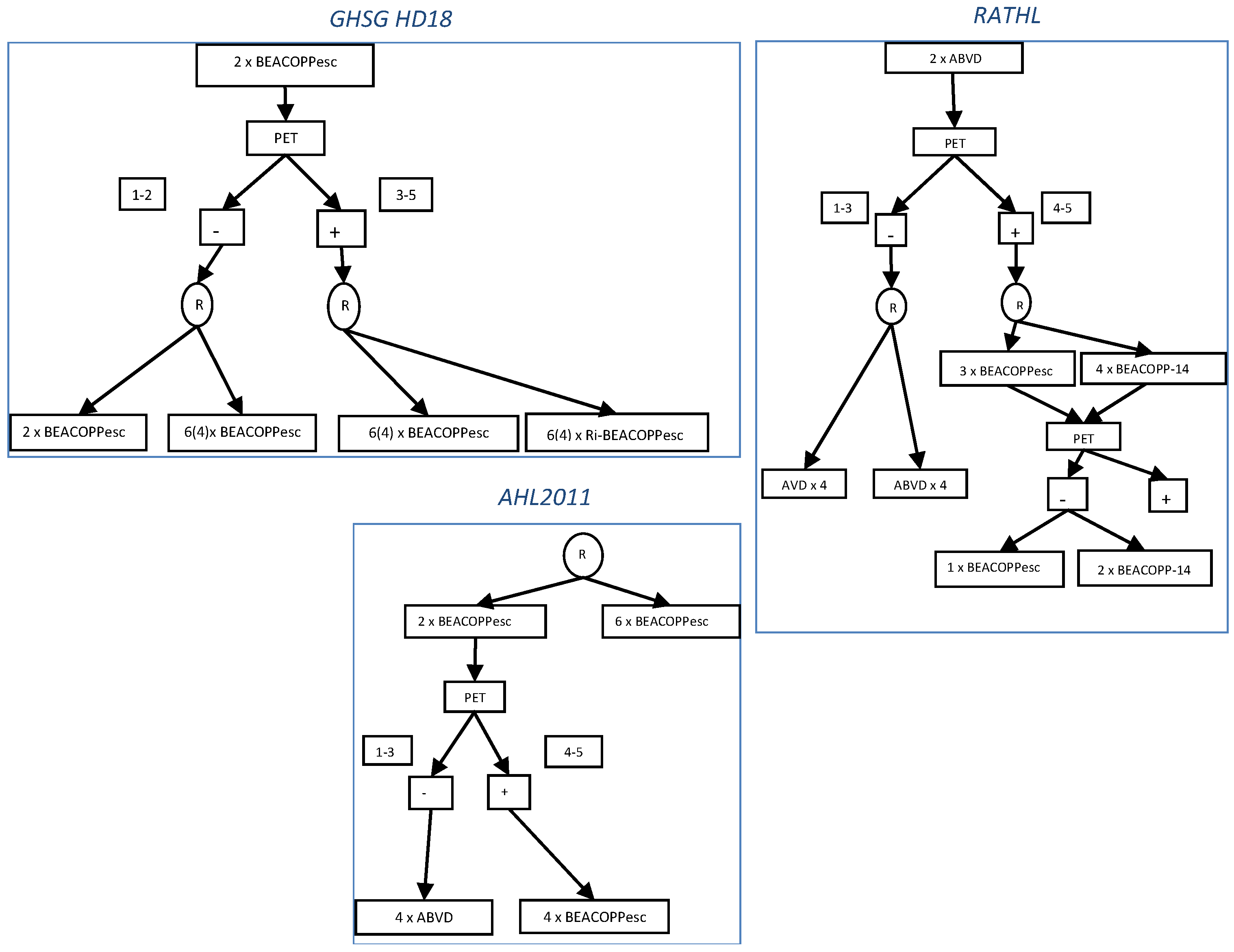
3. PET-Adapted Treatment
4. New Drugs
4.1. Brentuximab Vedotin
4.2. Checkpoint Inhibitors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shanbhag, S.; Ambinder, R.F. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J. Clin. 2018, 68, 116–132. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Eichenauer, D.A.; André, M.; Johnson, P.; Fossa, A.; Casasnovas, O.; Engert, A. Controversies in the treatment of classical Hodgkin lymphoma. Hemasphere 2018, 2, e149. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Johnson, P.W.M. Optimizing therapy in advanced stage Hodgkin lymphoma. Blood 2018, 131, 1679–1688. [Google Scholar] [CrossRef] [Green Version]
- Eichenauer, D.A.; Aleman, B.M.P.; André, M.; Federico, M.; Hutchings, M.; Illidge, T.; Engert, A.; Ladetto, M.; ESMO Guidelines Committee. Hodgkin lymphoma. ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv19–iv29. [Google Scholar] [CrossRef] [PubMed]
- Schaapveld, M.; Aleman, B.M.; van Eggermond, A.M.; Janus, C.P.; Krol, A.D.; van der Maazen, R.W.; Roesink, J.; Raemaekers, J.M.; de Boer, J.P.; Zijlstra, J.M.; et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef]
- Carde, P.; Karrasch, M.; Fortpied, C.; Brice, P.; Khaled, H.; Casasnovas, O.; Caillot, D.; Gaillard, I.; Bologna, S.; Ferme, C.; et al. Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, international prognostic score 3, high-risk Hodgkin lymphoma: First results of the phase III EORTC 20012 Intergroup Trial. J. Clin. Oncol. 2016, 34, 2028–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federico, M.; Luminari, S.; Iannitto, E.; Polimeno, G.; Marcheselli, L.; Montanini, A.; La Sala, A.; Merli, F.; Stelitano, C.; Pozzi, S.; et al. HD2000 Gruppo Italiano per lo Studio dei Limfomi Trial. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin’s lymphoma: Results from the HD2000 Gruppo Italiano per lo Studio dei Limfomi Trial. J. Clin. Oncol. 2009, 27, 805–811. [Google Scholar] [CrossRef]
- Merli, F.; Luminari, S.; Gobbi, P.G.; Cascavilla, N.; Mammi, C.; Ilariucci, F.; Stelitano, C.; Musso, M.; Baldini, L.; Galimberti, S.; et al. Long-term results of the HD2000 Trial comparing ABVD versus BEACOPP versus COPP-EBV-CAD in untreated patients with advanced Hodgkin lymphoma: A study by Fondazione Italiana Linfomi. J. Clin. Oncol. 2016, 34, 1175–1181. [Google Scholar] [CrossRef] [Green Version]
- Mounier, N.; Brice, P.; Bologna, S.; Briere, J.; Gaillard, I.; Heczko, M.; Gabarre, J.; Casasnovas, O.; Jaubert, J.; Colin, P.; et al. Lymphoma Study Association (LYSA). ABVD (8 cycles) versus BEACOPP (4 escalated cycles ≥ 4 baseline): Final results in stage III-IV low-risk Hodgkin lymphoma (IPS 0-2) of the LYSA H34 randomized trial. Ann. Oncol. 2014, 25, 1622–1628. [Google Scholar] [CrossRef]
- Viviani, S.; Zinzani, P.L.; Rambaldi, A.; Brusamolino, E.; Levis, A.; Bonfante, V.; Vitolo, U.; Pulsoni, A.; Liberati, A.M.; Specchia, G.; et al. Michelangelo Foundation; Gruppo Italiano di Terapie Innovative nei Linfomi; Intergruppo Italiano Linfomi. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N. Engl. J. Med. 2011, 365, 203–212. [Google Scholar] [CrossRef] [Green Version]
- André, M.P.E.; Carde, P.; Viviani, S.; Bellei, M.; Fortpied, C.; Hutchings, M.; Gianni, A.M.; Brice, P.; Casasnovas, O.; Gobbi, P.G.; et al. Long-term overall survival and toxicities of ABVD vs BEACOPP in advanced Hodgkin lymphoma: A pooled analysis of four randomized trials. Cancer Med. 2020, 9, 6565–6575. [Google Scholar] [CrossRef] [PubMed]
- Skoetz, N.; Trelle, S.; Rancea, M.; Haverkamp, H.; Diehl, V.; Engert, A.; Borchmann, P. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: A systematic review and network meta-analysis. Lancet Oncol. 2013, 14, 943–952. [Google Scholar] [CrossRef]
- Amin, M.S.A.; Brunckhorst, O.; Scott, C.; Wrench, D.; Gleeson, M.; Kazmi, M.; Ahmed, K. ABVD and BEACOPP regimens’ effects on fertility in young males with Hodgkin lymphoma. Clin. Transl. Oncol. 2021, 23, 1067–1077. [Google Scholar] [CrossRef]
- Behringer, K.; Mueller, H.; Goergen, H.; Thielen, I.; Eibl, A.D.; Stumpf, V.; Wessels, C.; Wiehlputz, M.; Rosenbrock, J.; Halbsguth, T.; et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J. Clin. Oncol. 2012, 31, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.A.; Remedios, R.; Kinkwood, A.A.; Patrick, P.; Stevens, L.; Clifton-Hadley, L.; Roberts, T.; Hatton, C.; Kalakonda, N.; Miligan, D.W.; et al. Determinants of ovarian function after response-adapted therapy in patients with advanced Hodgkin’s lymphoma (RATHL): A secondary analysis of a randomised phase 3 trial. Lancet Oncol. 2018, 19, 1328–1337. [Google Scholar] [CrossRef] [Green Version]
- Cheson, B.D. Role of functional imaging in the management of lymphoma. J. Clin. Oncol. 2011, 29, 1844–1854. [Google Scholar] [CrossRef]
- Hutchings, M.; Loft, A.; Hansen, M.; Pedersen, L.M.; Buhl, T.; Jurlander, J.; Buus, S.; Keiding, S.; D’Amore, F.; Boesen, A.M.; et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 2006, 107, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallamini, A.; Rigacci, L.; Merli, F.; Nassi, L.; Bosi, A.; Capodanno, I.; Luminari, S.; Vitolo, U.; Sancetta, R.; Iannitto, E.; et al. The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin’s disease. Haematologica 2006, 91, 475–481. [Google Scholar]
- Meignan, M.; Gallamini, A.; Meignan, M.; Gallamini, A.; Haioun, C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk. Lymphoma 2009, 50, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano Classification. J. Clin. Oncol. 2014, 32, 3059–3067. [Google Scholar] [CrossRef]
- Gallamini, A.; Barrington, S.F.; Biggi, A.; Chauvie, S.; Kostakoglu, L.; Gregianin, M.; Meignan, M.; Mikhaeel, G.N.; Loft, A.; Zaucha, J.M.; et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica 2014, 99, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Kanoun, S.; Rossi, C.; Casanovas, O. [18F]FDG-PET/CT in Hodgkin lymphoma: Current usefulness and perspectives. Cancers 2018, 10, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borchmann, P.; Goergen, H.; Kobe, C.; Lohri, A.; Greil, R.; Eichenauer, D.A.; Zijlstra, J.M.; Markova, J.; Meissner, J.; Feuring-Buske, M.; et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): Final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 2017, 390, 2790–2802. [Google Scholar] [CrossRef]
- Casasnovas, R.O.; Bouabdallah, R.; Brice, P.; Lazarovici, J.; Ghesquieres, H.; Stamatoullas, A.; Dupuis, J.; Gac, A.C.; Gastinne, T.; Joly, B.; et al. PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): A randomised, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2019, 20, 202–215. [Google Scholar] [CrossRef]
- Johnson, P.; Federico, M.; Kirkwood, A.; Fosså, A.; Berkahn, L.; Carella, A.; d’Amore, F.; Enblad, G.; Franceschetto, A.; Fulham, M.; et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N. Engl. J. Med. 2016, 374, 2419–2429. [Google Scholar] [CrossRef]
- Press, O.W.; Li, H.; Schöder, H.; Straus, D.J.; Moskowitz, C.H.; LeBlanc, M.; Rimsza, L.M.; Bartlett, N.L.; Evens, A.M.; Mittra, E.S.; et al. US Intergroup Trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: Southwest Oncology Group S0816. J. Clin. Oncol. 2016, 34, 2020–2027. [Google Scholar] [CrossRef] [Green Version]
- Gallamini, A.; Tarella, C.; Viviani, S.; Rossi, A.; Patti, C.; Mulé, A.; Picardi, M.; Romano, A.; Cantonetti, M.; La Nasa, G.; et al. Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: Long-term results of the GITIL/FIL HD 0607 Trial. J. Clin. Oncol. 2018, 36, 454–462. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Broccoli, A.; Gioia, D.M.; Castagnoli, A.; Ciccone, G.; Evangelista, A.; Santoro, A.; Ricardi, U.; Bonfichi, M.; Brusamolino, E.; et al. Interim positron emission tomography response-adapted therapy in advanced-stage Hodgkin lymphoma: Final results of the phase II part of the HD0801 study. J. Clin. Oncol. 2016, 34, 1376–1385. [Google Scholar] [CrossRef]
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illés, Á.; Picardi, M.; et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N. Engl. J. Med. 2018, 378, 331–344. [Google Scholar] [CrossRef]
- Straus, D.J.; Długosz-Danecka, M.; Alekseev, S.; Illés, Á.; Picardi, M.; Lech-Maranda, E.; Feldman, T.; Smolewski, P.; Savage, K.J.; Bartlett, N.L.; et al. Brentuximab vedotin with chemotherapy for stage III/IV classical Hodgkin lymphoma: 3-year update of the ECHELON-1 study. Blood 2020, 135, 735–741. [Google Scholar] [CrossRef]
- Eichenauer, D.A.; Plütschow, A.; Kreissl, S.; Sökler, M.; Hellmuth, J.C.; Meissner, J.; Mathas, S.; Topp, M.S.; Behringer, K.; Klapper, W.; et al. Incorporation of brentuximab vedotin into first-line treatment of advanced classical Hodgkin’s lymphoma: Final analysis of a phase 2 randomised trial by the German Hodgkin Study Group. Lancet Oncol. 2017, 18, 1680–1687. [Google Scholar] [CrossRef]
- Ramchandren, R.; Domingo-Domènech, E.; Rueda, A.; Trněný, M.; Feldman, T.A.; Lee, H.J.; Provencio, M.; Sillaber, C.; Cohen, J.B.; Savage, K.J.; et al. Nivolumab for newly diagnosed advanced-stage classic Hodgkin lymphoma: Safety and efficacy in the phase II CheckMate 205 Study. J. Clin. Oncol. 2019, 37, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. KEYNOTE-087. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.B.; Savas, H.; Evens, A.M.; Advani, R.H.; Palmer, B.; Pro, B.; Karmali, R.; Mou, E.; Bearden, J.; Dillehay, G.; et al. Pembrolizumab followed by AVD in untreated early unfavorable and advanced-stage classical Hodgkin lymphoma. Blood 2021, 137, 1318–1326. [Google Scholar] [CrossRef]
| EORTC/LYSA | GHSG | |
|---|---|---|
| Risk factors | Mediastinum-to-thorax ratio ≥ 0.35 | Mediastinal mass larger than 1/3 of the maximum thoracic width (A) |
| Age ≥ 50 years | Extranodal disease (B) | |
| ESR > 50 mm/h without B symptoms or >30 mm/h with B symptoms | ESR > 50 mm/h without B symptoms or >30 mm/h with B symptoms (C) | |
| Involvement of ≥4 out of 5 supradiaphragmatic nodal areas | Involvement of ≥3 out of 11 nodal areas on both sides of the diaphragm (D) | |
| Treatment group | ||
| Earlystage | I–II without risk factors | I–II without risk factors |
| Intermediate stage | I–II ≥ 1 risk factors | I–IIA with ≥1 risk factors. IIB with risk factors C and/or D, but not A/B |
| Advanced stage | III–IV | IIB with risk factors A and/or B, III/IV |
| Trial | Regimen | No. of Pts | PFS (%) | OS (%) | No. of Second Cancers |
|---|---|---|---|---|---|
| EORTC 20012 Intergroup Trial [7] | ABVD × 8 | 275 | 73 (4y) | 87 (4y) | 14 (5.1%) |
| BEACOPPesc × 4, then BEACOPPstd × 4 | 274 | 83 (4y) | 90 (4y) | 25 (9.3%) | |
| HD2000 Trial [8,9] | ABVD × 6 | 99 | 69 (10y) | 85 (10y) | 1 (1.0%) |
| BEACOPPesc × 4, then BEACOPPstd × 2 | 98 | 75 (10y) | 84 (10y) | 6 (6.7%) | |
| CEC × 6 | 98 | 76 (10y) | 86 (10y) | 6 (6.7%) | |
| LYSA H34 Trial [10] | ABVD × 8 | 80 | 75 (5y) | 92 (5y) | 7 (9.1%) |
| BEACOPPesc × 4, then ≥ BEACOPP × 4 | 70 | 93 (5y) | 99 (5y) | 2 (2.9%) | |
| Viviani et al. [11] | ABVD × 6–8, then reinduction and HDT/ASCT if less than CR or PD | 168 | 73 (7y) | 84 (7y) | 4 (2.4%) |
| BEACOPPesc × 4, then BEACOPP × 4, then reinduction and HDT/ASCT if less than CR or PD | 163 | 85 (7y) | 89 (7y) | 3 (1.8%) |
| Trial | Regimen | No. of Pts | Stage III/IV (%) | PFS (%) | OS (%) |
|---|---|---|---|---|---|
| GHSG HD18 Trial [24] | BEACOPPesc × 2, if PET-2+, randomization to BEACOPPesc × 4–6 | 219 | 78 | 91 (3y) | 97 (3y) |
| BEACOPPesc ×2, if PET-2+ randomization to BEACOPPesc + rituximab × 4–6 | 220 | 75 | 93 (3y) | 94 (3y) | |
| LYSA AHL2011 Trial [25] | BEACOPPesc × 2, if PET-2–, ABVD × 4 | 319 | 88 | 88 (2y) | 96 (5y) |
| BEACOPPesc × 2, if PET+, BEACOPPesc × 4 | 49 | ||||
| BEACOPPesc × 6 (no PET adaptation) | 401 | 92 (2y) | 95 (5y) | ||
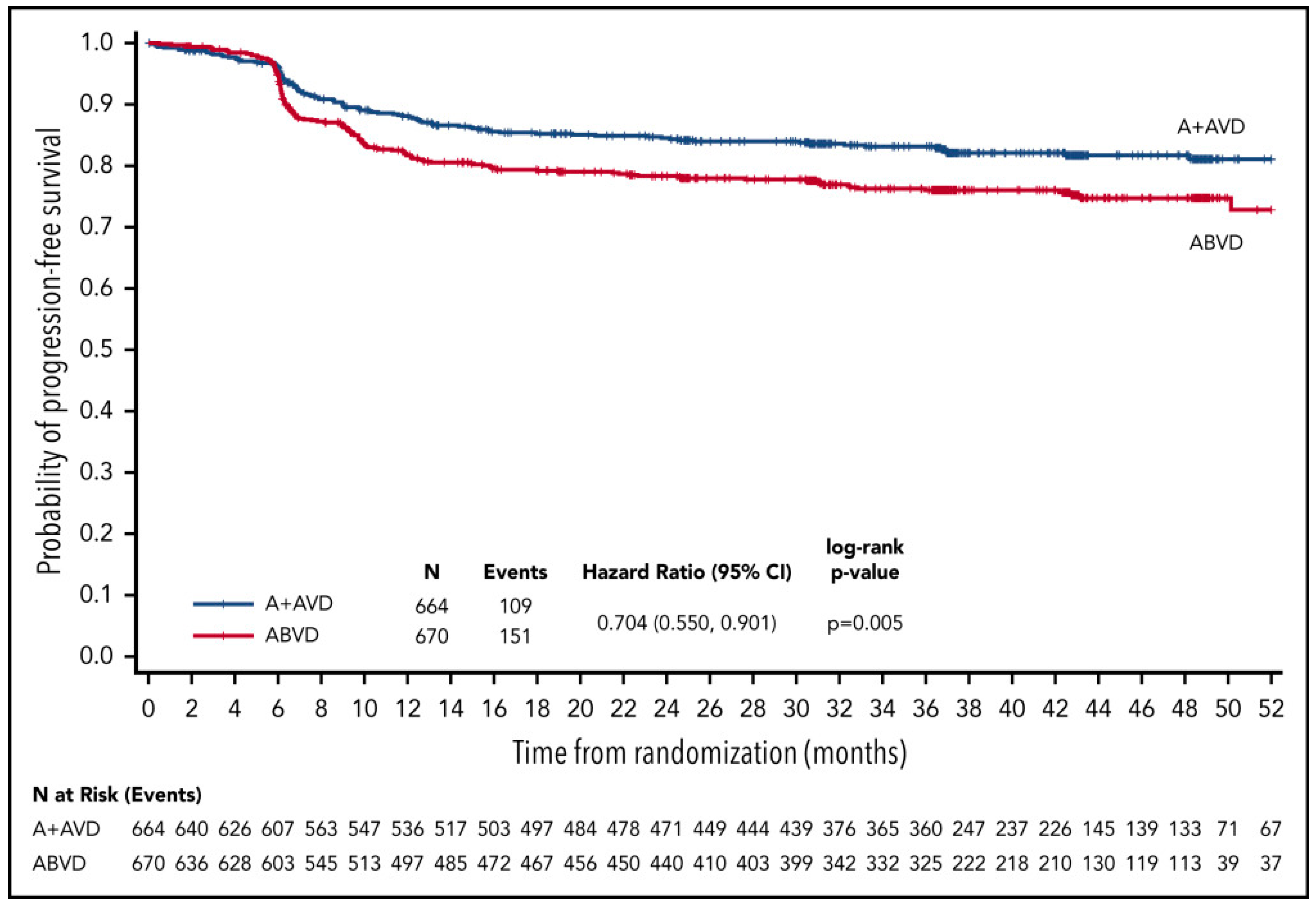
| RATHL Trial [26] | ABVD × 2, if PET-2–, randomization to AVD × 4 | 470 | 59 | 86 (3y) | 97 (3y) |
| ABVD × 2 , if PET-2–, randomization to ABVD × 4 | 465 | 58 | 84 (3y) | 98 (3y) | |
| ABVD × 2, if PET-2+, BEACOPPesc × 4 or BEACOPP-14 × 6 | 172 | 58 | 66 (3y) | 88 (3y) | |
| US Intergroup SWOG Trial S0816 [27] | ABVD × 2, if PET-2–, for ABVD × 4 | 370 | 100 | 82 (2y) | NA |
| ABVD × 2, if PET-2+, for BEACOPPesc × 6 | 55 | 64 (2y) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellemans, H.; André, M.P.E. Review of Treatment Options for the Management of Advanced Stage Hodgkin Lymphoma. Cancers 2021, 13, 3745. https://doi.org/10.3390/cancers13153745
Vellemans H, André MPE. Review of Treatment Options for the Management of Advanced Stage Hodgkin Lymphoma. Cancers. 2021; 13(15):3745. https://doi.org/10.3390/cancers13153745
Chicago/Turabian StyleVellemans, Hélène, and Marc P. E. André. 2021. "Review of Treatment Options for the Management of Advanced Stage Hodgkin Lymphoma" Cancers 13, no. 15: 3745. https://doi.org/10.3390/cancers13153745
APA StyleVellemans, H., & André, M. P. E. (2021). Review of Treatment Options for the Management of Advanced Stage Hodgkin Lymphoma. Cancers, 13(15), 3745. https://doi.org/10.3390/cancers13153745





