Geospatial Cellular Distribution of Cancer-Associated Fibroblasts Significantly Impacts Clinical Outcomes in Metastatic Clear Cell Renal Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
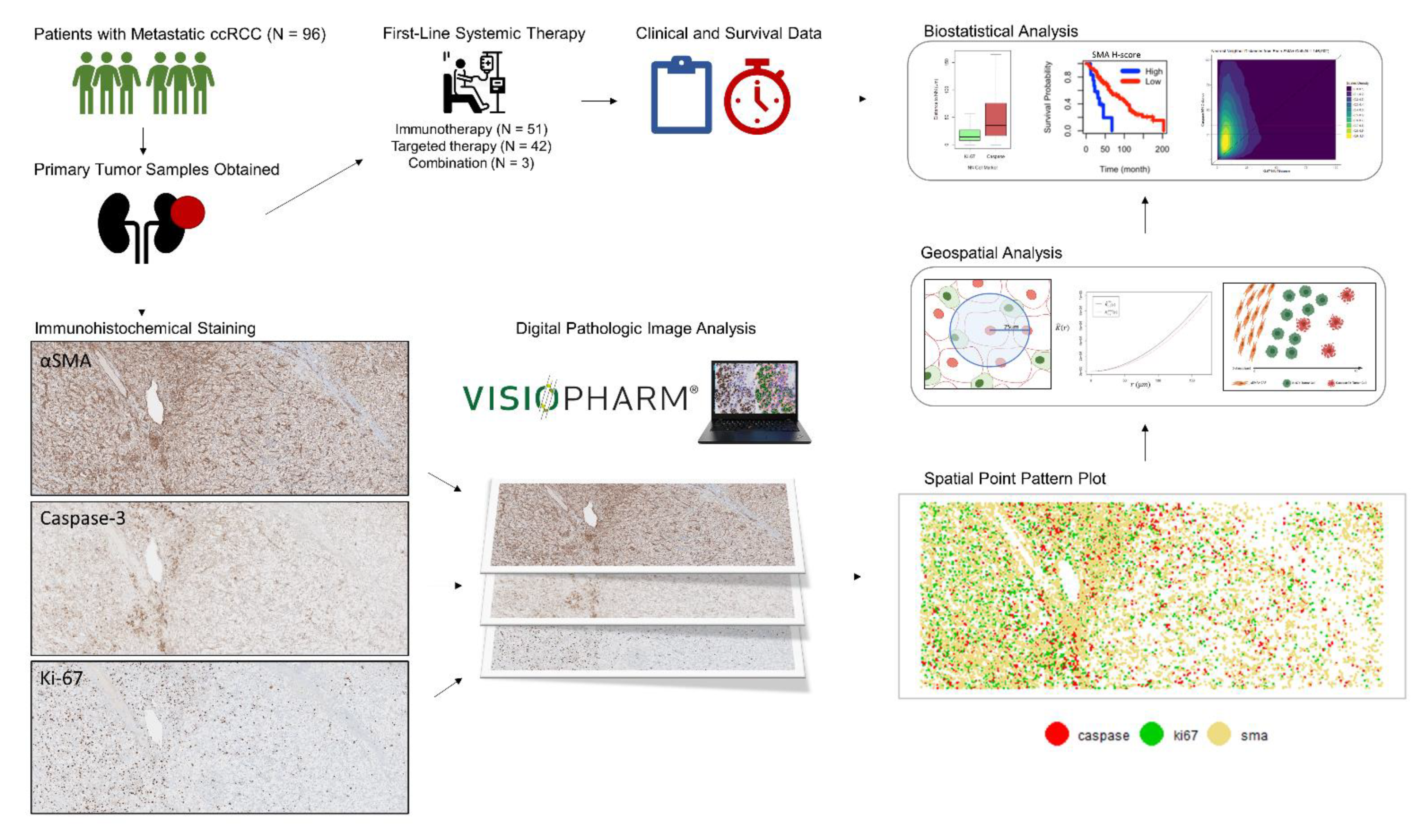
2. Materials and Methods
2.1. Patient and Sample Selection
2.2. Immunohistochemical Specimen Preparation
2.3. Quantitative Digital Image Analysis
2.4. Cellular Distribution and Spatial Analysis
2.5. Variable Definitions
2.6. Statistical Analysis
2.7. Statistical Software
3. Results
3.1. Study Population
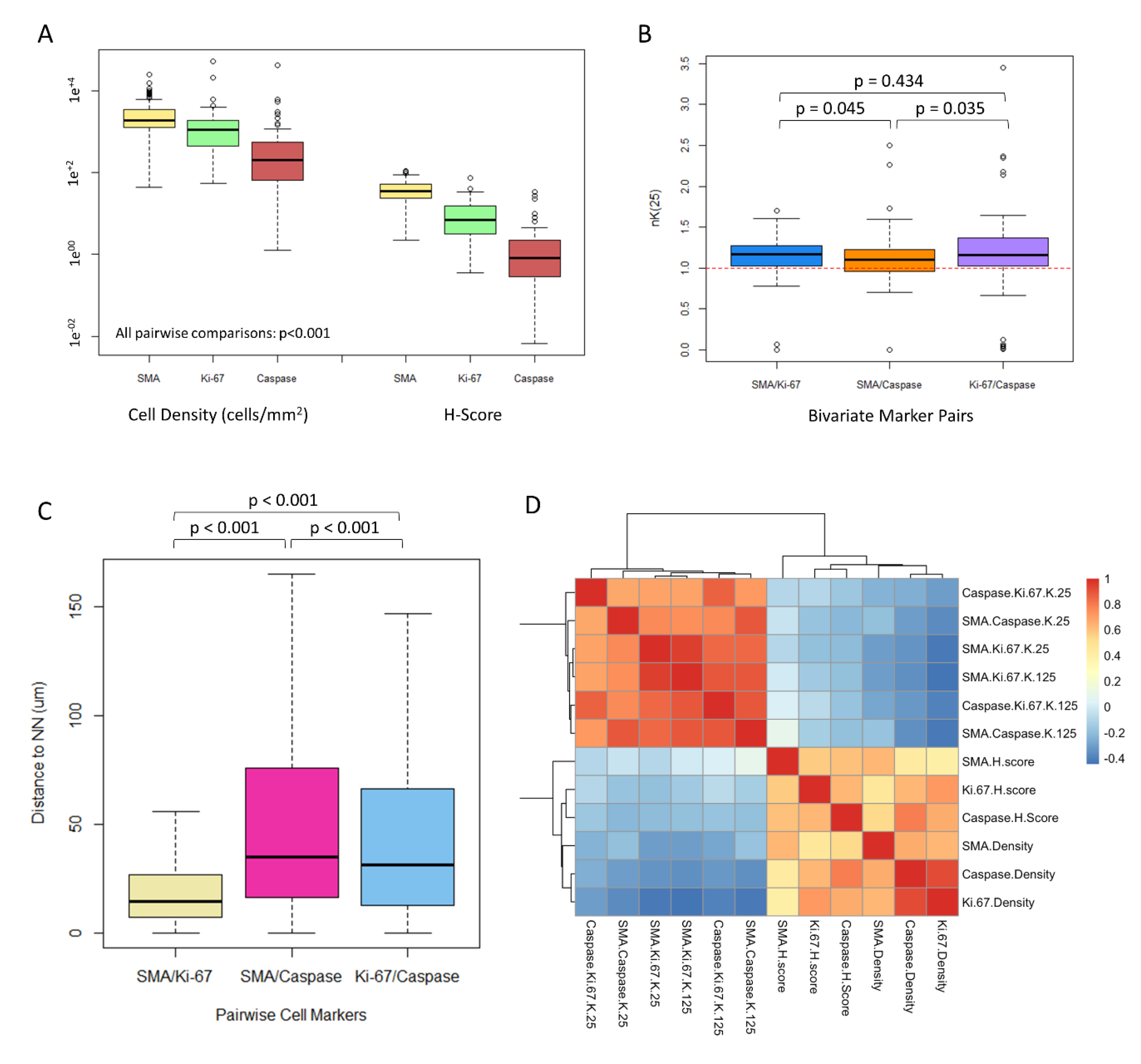
3.2. CAFs Are Highly Prevalent in the ccRCC Tumor Microenvironment
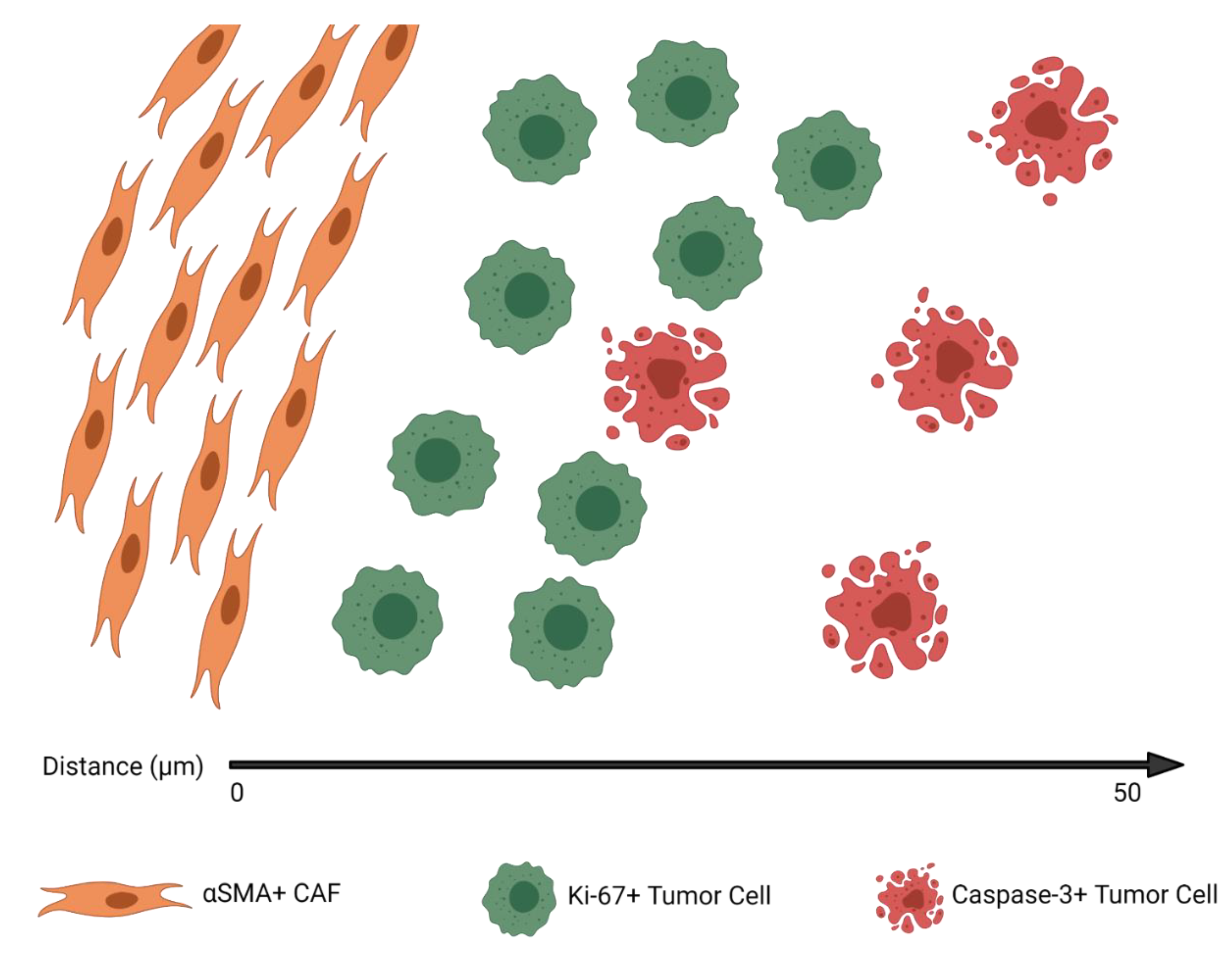
3.3. CAFs Are Spatially Clustered with Proliferating Tumor Cells
3.4. CAF Density and αSMA H-Score Are Positively Correlated with Proliferating and Apoptotic Tumor Cells
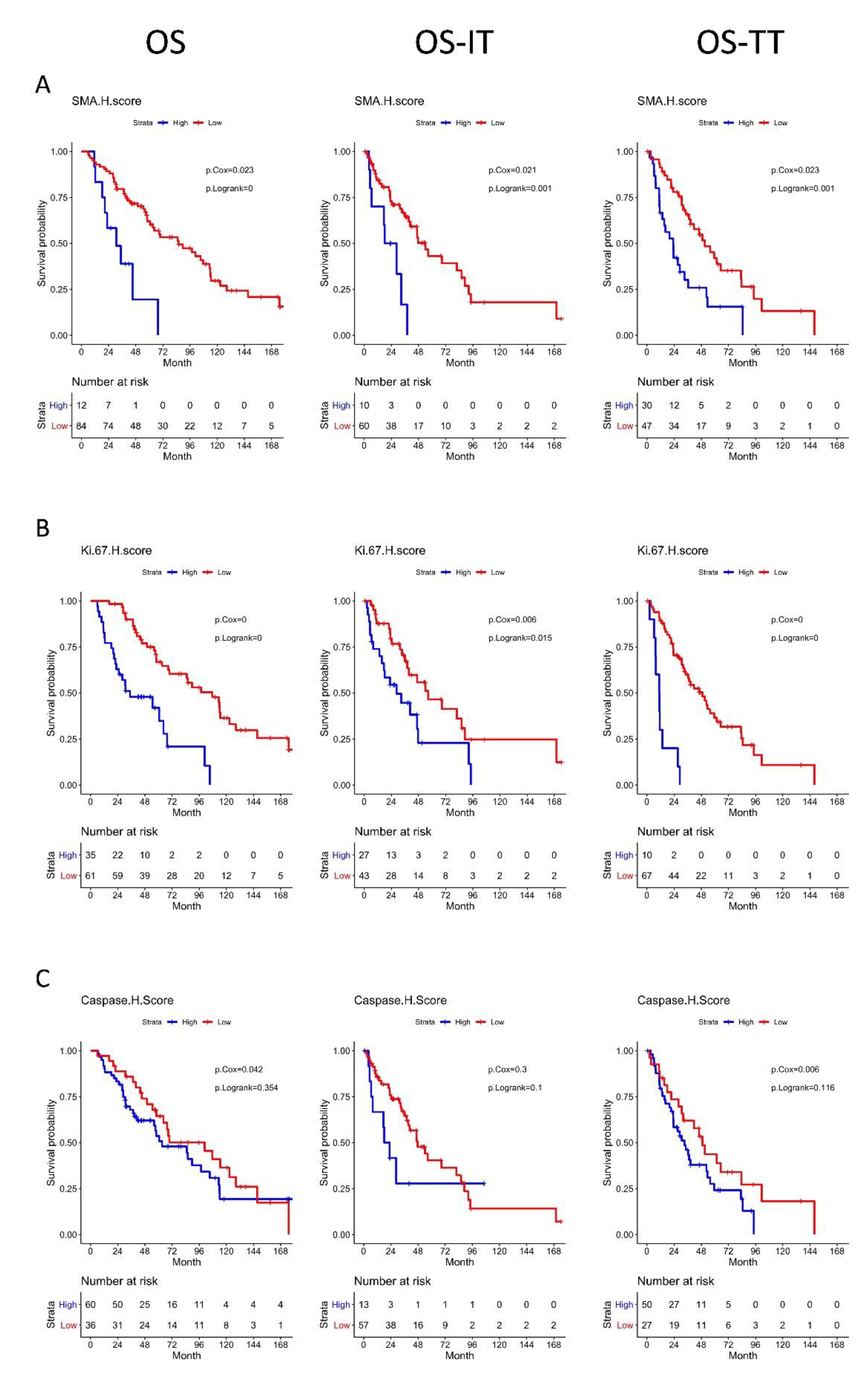
3.5. High αSMA, Ki-67, and Caspase-3 H-Scores Are Associated with Significantly Worse OS
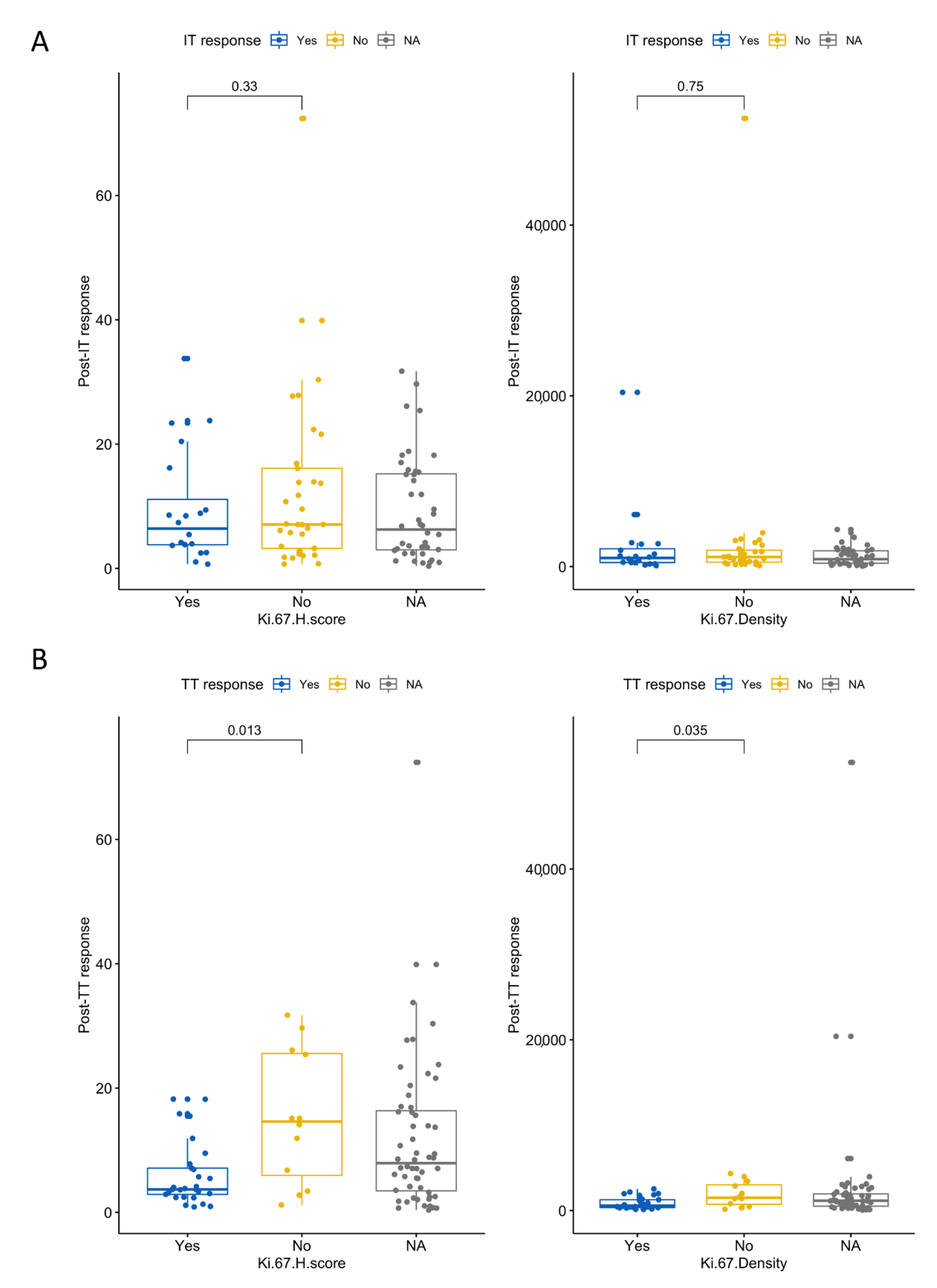
3.6. High Ki-67 Density and H-Score Are Associated with Resistance to First-Line TT
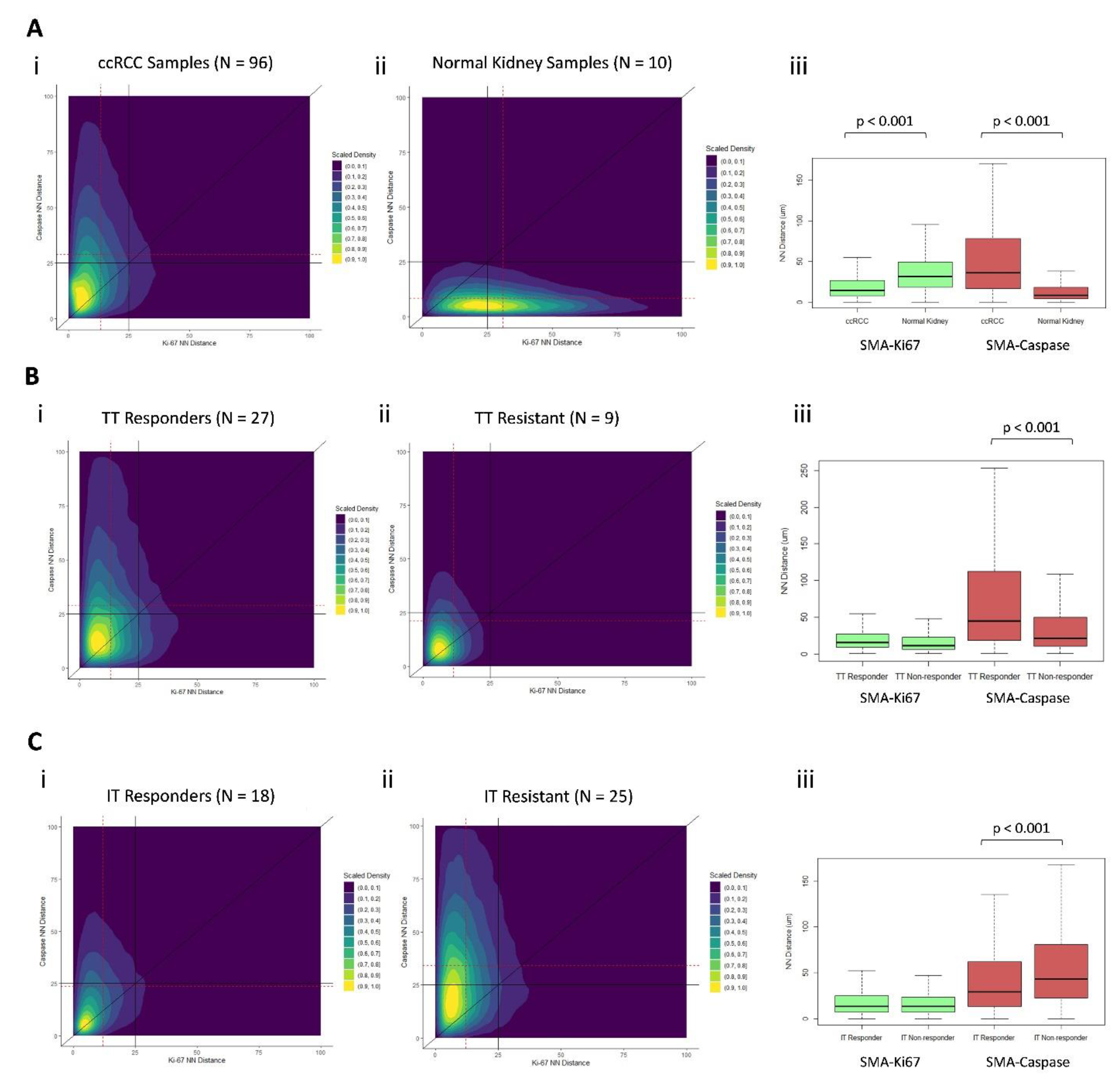
3.7. Distinct Geospatial Distributions of CAFs Are Associated with Treatment Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Tao, L.; Huang, G.; Song, H.; Chen, Y.; Chen, L. Cancer associated fibroblasts: An essential role in the tumor microenvironment. Oncol. Lett. 2017, 14, 2611–2620. [Google Scholar] [CrossRef]
- Harper, J.; Sainson, R.C. Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Semin. Cancer Biol. 2014, 25, 69–77. [Google Scholar] [CrossRef]
- Errarte, P.; Larrinaga, G.; Lopez, J.I. The role of cancer-associated fibroblasts in renal cell carcinoma. An example of tumor modulation through tumor/non-tumor cell interactions. J. Adv. Res. 2020, 21, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Bakhtyar, N.; Wong, N.; Kapoor, A.; Cutz, J.C.; Hill, B.; Ghert, M.; Tang, D. Clear cell renal cell carcinoma induces fibroblast-mediated production of stromal periostin. Eur. J. Cancer 2013, 49, 3537–3546. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.I.; Errarte, P.; Erramuzpe, A.; Guarch, R.; Cortes, J.M.; Angulo, J.C.; Pulido, R.; Irazusta, J.; Llarena, R.; Larrinaga, G. Fibroblast activation protein predicts prognosis in clear cell renal cell carcinoma. Hum. Pathol. 2016, 54, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi, T.; Marini, A.; Giannoni, E.; Taddei, M.L.; Gandellini, P.; De Donatis, A.; Lanciotti, M.; Serni, S.; Cirri, P.; Chiarugi, P. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012, 72, 5130–5140. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Small, E.J. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J. Clin. Oncol. 2005, 23, 1028–1043. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef]
- Nishishita, R.; Morohashi, S.; Seino, H.; Wu, Y.; Yoshizawa, T.; Haga, T.; Saito, K.; Hakamada, K.; Fukuda, S.; Kijima, H. Expression of cancer-associated fibroblast markers in advanced colorectal cancer. Oncol. Lett. 2018, 15, 6195–6202. [Google Scholar] [CrossRef]
- Soliman, N.A.; Yussif, S.M. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol. Med. 2016, 13, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, L.; Ma, X.; Li, H.; Gu, L.; Gao, Y.; Fan, Y.; Zhang, Y.; Zhang, X. Prognostic and clinicopathological role of high Ki-67 expression in patients with renal cell carcinoma: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Gayed, B.A.; Youssef, R.F.; Bagrodia, A.; Darwish, O.M.; Kapur, P.; Sagalowsky, A.; Lotan, Y.; Margulis, V. Ki67 is an independent predictor of oncological outcomes in patients with localized clear-cell renal cell carcinoma. BJU Int. 2014, 113, 668–673. [Google Scholar] [CrossRef]
- Bui, M.H.; Visapaa, H.; Seligson, D.; Kim, H.; Han, K.R.; Huang, Y.; Horvath, S.; Stanbridge, E.J.; Palotie, A.; Figlin, R.A.; et al. Prognostic value of carbonic anhydrase IX and KI67 as predictors of survival for renal clear cell carcinoma. J. Urol. 2004, 171, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Jonasch, E.; Michaelson, M.D.; Nandagopal, L.; Gore, J.L.; George, S.; Alva, A.; Haas, N.; Harrison, M.R.; Plimack, E.R.; et al. NCCN Guidelines Insights: Kidney Cancer, Version 2.2020. J. Natl. Compr. Cancer Netw. 2019, 17, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Rizzardi, A.E.; Johnson, A.T.; Vogel, R.I.; Pambuccian, S.E.; Henriksen, J.; Skubitz, A.P.; Metzger, G.J.; Schmechel, S.C. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn. Pathol. 2012, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McClelland, R.A.; Finlay, P.; Walker, K.J.; Nicholson, D.; Robertson, J.F.; Blamey, R.W.; Nicholson, R.I. Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res. 1990, 50, 3545–3550. [Google Scholar] [PubMed]
- Marcon, E.; Puech, F. A typology of distance-based measures of spatial concentration. Reg. Sci. Urban. Economics 2017, 62, 56–67. [Google Scholar] [CrossRef]
- Chakiryan, N.H.; Kimmel, G.J.; Kim, Y.; Hajiran, A.; Aydin, A.M.; Zemp, L.; Katende, E.; Nguyen, J.; Lopez-Blanco, N.; Chahoud, J.; et al. Spatial clustering of CD68+ tumor associated macrophages with tumor cells is associated with worse overall survival in metastatic clear cell renal cell carcinoma. PLoS ONE 2021, 16, e0245415. [Google Scholar] [CrossRef]
- Warren, A.Y.; Harrison, D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: Standards and controversies. World J. Urol. 2018, 36, 1913–1926. [Google Scholar] [CrossRef]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef]
- Faraggi, D.; Simon, R. A simulation study of cross-validation for selecting an optimal cutpoint in univariate survival analysis. Stat. Med. 1996, 15, 2203–2213. [Google Scholar] [CrossRef]
- Gnarra, J.R.; Tory, K.; Weng, Y.; Schmidt, L.; Wei, M.H.; Li, H.; Latif, F.; Liu, S.; Chen, F.; Duh, F.M.; et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat. Genet. 1994, 7, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef]
- Mehdi, M.Z.; Nagi, A.H.; Naseem, N. MCM-2 and Ki-67 as proliferation markers in renal cell carcinoma: A quantitative and semi-quantitative analysis. Int. braz j urol 2016, 42, 1121–1128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morgan, T.M.; Mehra, R.; Tiemeny, P.; Wolf, J.S.; Wu, S.; Sangale, Z.; Brawer, M.; Stone, S.; Wu, C.L.; Feldman, A.S. A Multigene Signature Based on Cell Cycle Proliferation Improves Prediction of Mortality Within 5 Yr of Radical Nephrectomy for Renal Cell Carcinoma. Eur. Urol. 2018, 73, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Tosoian, J.J.; Feldman, A.S.; Abbott, M.R.; Mehra, R.; Tiemeny, P.; Wolf, J.S., Jr.; Stone, S.; Wu, S.; Daignault-Newton, S.; Taylor, J.M.G.; et al. Biopsy Cell Cycle Proliferation Score Predicts Adverse Surgical Pathology in Localized Renal Cell Carcinoma. Eur. Urol. 2020, 78, 657–660. [Google Scholar] [CrossRef]
| Characteristic. | No. (%) |
|---|---|
| Median age, range | 59 (55–67) |
| Gender | |
| Female | 28 (29) |
| Male | 68 (71) |
| Race | |
| White | 90 (94) |
| Black | 2 (2.1) |
| Asian | 1 (1.0) |
| Other | 3 (3.1) |
| IMDC Risk Category | |
| Favorable | 20 (21) |
| Intermediate | 56 (58) |
| Poor | 9 (9) |
| Indeterminate | 11 (12) |
| Primary tumor size (cm) | 8.2 (6.0, 11.0) |
| ISUP Grade | |
| 2 | 11 (11) |
| 3 | 60 (62) |
| 4 | 25 (26) |
| Sarcomatoid Variant | |
| No | 87 (91) |
| Yes | 9 (9.4) |
| Rhabdoid Variant | |
| No | 88 (92) |
| Yes | 8 (8.3) |
| First Line Therapy | |
| IT | 51 (53) |
| TT | 42 (44) |
| Both | 3 (3.1) |
| Response Category | |
| IT Resistance | 25 (32) |
| IT Response | 18 (23) |
| IT Indeterminate | 8 (8.3) |
| TT Resistance | 9 (9.4) |
| TT Response | 27 (28) |
| TT Indeterminate | 6 (6.3) |
| A. | ||||||||
| Metric | Cutoff | N High | N Low | Survival High (mo.) | Survival Low (mo.) | Cox HR | 95% CI | p |
| SMA H-score | 37.028 | 12 | 84 | 63.2 | 97.6 | 1.45 | 1.05–2.01 | 0.02 |
| Ki 67 H-score | 7.088 | 35 | 61 | 54.7 | 97.6 | 1.79 | 1.40–2.29 | <0.001 |
| Caspase H-Score | 0.834 | 60 | 36 | 85.8 | 68.6 | 1.33 | 1.01–1.75 | 0.04 |
| SMA Density | 1892.363 | 48 | 48 | 85.1 | 63.2 | 1.17 | 0.86–1.60 | 0.32 |
| Ki 67 Density | 1097.034 | 48 | 48 | 64.4 | 85.1 | 1.30 | 1.06–1.61 | 0.01 |
| Caspase Density | 205.198 | 47 | 49 | 85.8 | 68.6 | 1.26 | 1.02–1.56 | 0.03 |
| SMA-Ki67 nK(25) | 1.167 | 46 | 48 | 69.6 | 68.6 | 0.98 | 0.71–1.34 | 0.90 |
| SMA-Caspase nK(25) | 1.106 | 38 | 44 | 85.1 | 67.6 | 0.92 | 0.65–1.30 | 0.64 |
| Caspase-Ki67 nK(25) | 1.163 | 42 | 42 | 67.6 | 89.7 | 0.82 | 0.58–1.16 | 0.26 |
| B. | ||||||||
| Metric | Cutoff | N High | N Low | Survival High (mo.) | Survival Low (mo.) | Cox HR | 95% CI | p |
| SMA H-score | 36.346 | 10 | 60 | 32.6 | 47.6 | 1.51 | 1.05–2.17 | 0.03 |
| Ki 67 H-score | 7.06 | 27 | 43 | 33.3 | 56.5 | 1.47 | 1.13–1.91 | <0.001 |
| Caspase H-Score | 1.137 | 13 | 57 | 46.7 | 46.7 | 1.19 | 0.89–1.58 | 0.24 |
| SMA Density | 1911.912 | 35 | 35 | 46.7 | 35.9 | 1.24 | 0.91–1.68 | 0.17 |
| Ki 67 Density | 1097.034 | 36 | 34 | 40.5 | 46.7 | 1.21 | 0.98–1.50 | 0.08 |
| Caspase Density | 189.285 | 35 | 35 | 86.1 | 40.5 | 1.18 | 0.95–1.46 | 0.13 |
| SMA-Ki67 nK(25) | 1.139 | 35 | 33 | 40.5 | 46.7 | 0.94 | 0.67–1.31 | 0.70 |
| SMA-Caspase nK(25) | 1.085 | 28 | 32 | 47.6 | 46.7 | 0.83 | 0.56–1.26 | 0.39 |
| Caspase-Ki67 nK(25) | 1.131 | 31 | 31 | 54.1 | 40.5 | 0.72 | 0.47–1.09 | 0.12 |
| C. | ||||||||
| Metric | Cutoff | N High | N Low | Survival High (mo.) | Survival Low (mo.) | Cox HR | 95% CI | p |
| SMA H-score | 37.2 | 30 | 47 | 27.2 | 48.9 | 1.46 | 1.06–2.02 | 0.02 |
| Ki 67 H-score | 7.06 | 10 | 67 | 23.1 | 55.8 | 1.71 | 1.35–2.17 | <0.001 |
| Caspase H-Score | 0.742 | 50 | 27 | 37.2 | 33.1 | 1.46 | 1.14–1.88 | <0.001 |
| SMA Density | 1861.339 | 38 | 39 | 37.2 | 41.5 | 1.19 | 0.89–1.60 | 0.24 |
| Ki 67 Density | 1107.625 | 38 | 39 | 27.2 | 41.5 | 1.27 | 1.04–1.57 | 0.02 |
| Caspase Density | 174.07 | 37 | 40 | 36.4 | 37.2 | 1.25 | 1.02–1.53 | 0.03 |
| SMA-Ki67 nK(25) | 1.156 | 37 | 38 | 36.4 | 37.2 | 1.05 | 0.76–1.45 | 0.76 |
| SMA-Caspase nK(25) | 1.1 | 31 | 35 | 34.2 | 37.2 | 1.03 | 0.76–1.42 | 0.84 |
| Caspase-Ki67 nK(25) | 1.164 | 35 | 33 | 33.1 | 38.4 | 0.92 | 0.65–1.31 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakiryan, N.H.; Kimmel, G.J.; Kim, Y.; Johnson, J.O.; Clark, N.; Hajiran, A.; Chang, A.; Aydin, A.M.; Zemp, L.; Katende, E.; et al. Geospatial Cellular Distribution of Cancer-Associated Fibroblasts Significantly Impacts Clinical Outcomes in Metastatic Clear Cell Renal Cell Carcinoma. Cancers 2021, 13, 3743. https://doi.org/10.3390/cancers13153743
Chakiryan NH, Kimmel GJ, Kim Y, Johnson JO, Clark N, Hajiran A, Chang A, Aydin AM, Zemp L, Katende E, et al. Geospatial Cellular Distribution of Cancer-Associated Fibroblasts Significantly Impacts Clinical Outcomes in Metastatic Clear Cell Renal Cell Carcinoma. Cancers. 2021; 13(15):3743. https://doi.org/10.3390/cancers13153743
Chicago/Turabian StyleChakiryan, Nicholas H., Gregory J. Kimmel, Youngchul Kim, Joseph O. Johnson, Noel Clark, Ali Hajiran, Andrew Chang, Ahmet M. Aydin, Logan Zemp, Esther Katende, and et al. 2021. "Geospatial Cellular Distribution of Cancer-Associated Fibroblasts Significantly Impacts Clinical Outcomes in Metastatic Clear Cell Renal Cell Carcinoma" Cancers 13, no. 15: 3743. https://doi.org/10.3390/cancers13153743
APA StyleChakiryan, N. H., Kimmel, G. J., Kim, Y., Johnson, J. O., Clark, N., Hajiran, A., Chang, A., Aydin, A. M., Zemp, L., Katende, E., Chahoud, J., Ferrall-Fairbanks, M. C., Spiess, P. E., Francis, N., Fournier, M., Dhillon, J., Park, J. Y., Wang, L., Mulé, J. J., ... Manley, B. J. (2021). Geospatial Cellular Distribution of Cancer-Associated Fibroblasts Significantly Impacts Clinical Outcomes in Metastatic Clear Cell Renal Cell Carcinoma. Cancers, 13(15), 3743. https://doi.org/10.3390/cancers13153743









