Effectiveness of a Novel Covered Stent without External Thread Fixation for Anastomotic Leakage after Total or Proximal Gastrectomy for Gastric Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Gastrectomy Protocol
2.3. Diagnosis and Initial Management of Anastomotic Leakage
2.4. Stent Placement and Follow-Up
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Characteristics and Initial Management for Anastomotic Leakage
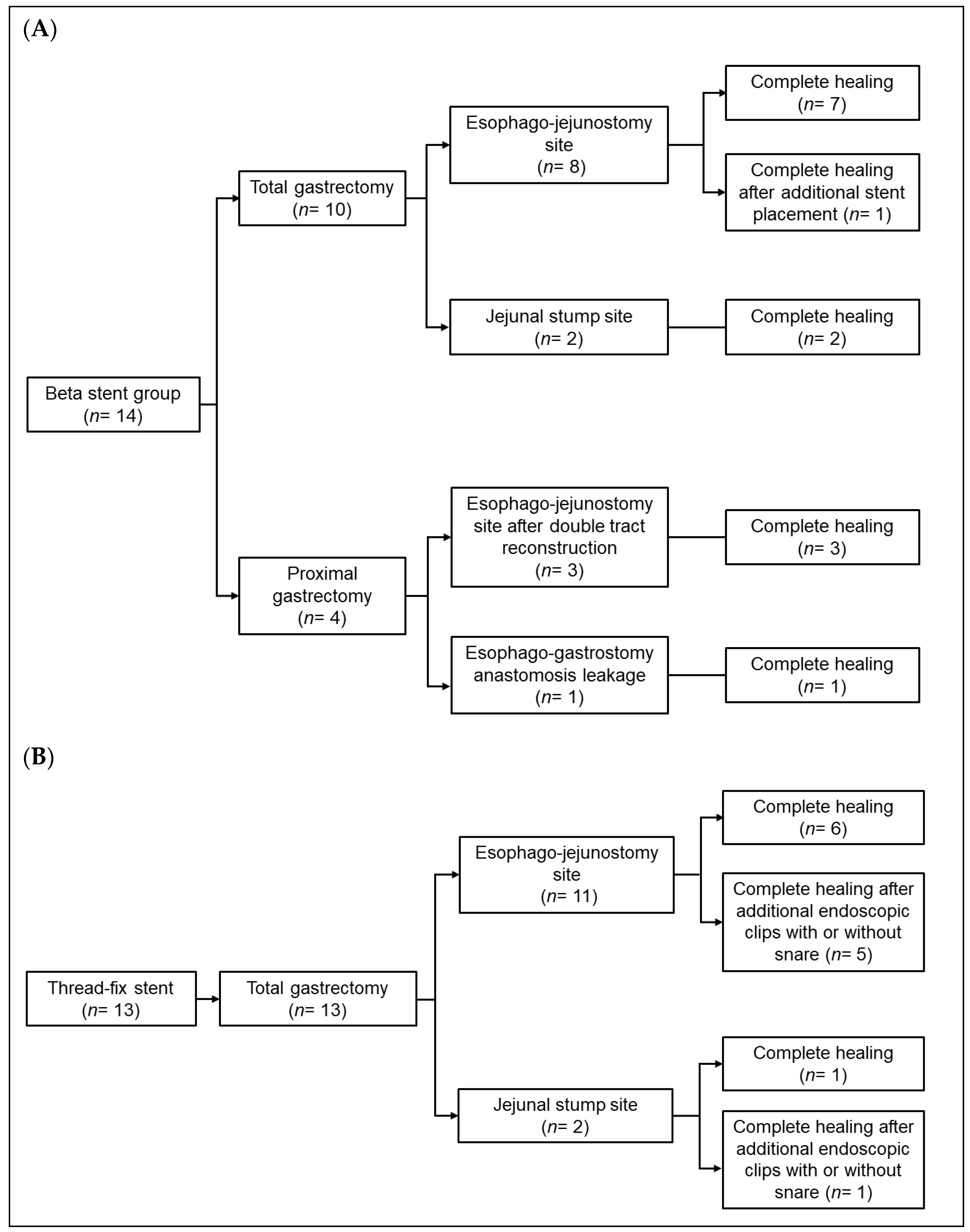
3.3. Treatment Outcomes After Stent Placement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauvanet, A.; Mariette, C.; Thomas, P.; Lozac’h, P.; Segol, P.; Tiret, E.; Delpero, J.-R.; Collet, D.; Leborgne, J.; Pradere, B.; et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: Predictive factors. J. Am. Coll. Surg. 2005, 201, 253–262. [Google Scholar] [CrossRef]
- Yoo, H.M.; Lee, H.H.; Shim, J.H.; Jeon, H.M.; Park, C.H.; Song, K.Y. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer. J. Surg. Oncol. 2011, 104, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Sierzega, M.; Kolodziejczyk, P.; Kulig, J. Impact of anastomotic leakage on long-term survival after total gastrectomy for carcinoma of the stomach. Br. J. Surg. 2010, 97, 1035–1042. [Google Scholar] [CrossRef]
- Inokuchi, M.; Otsuki, S.; Fujimori, Y.; Sato, Y.; Nakagawa, M.; Kojima, K. Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. World J. Gastroenterol. 2015, 21, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Lee, Y.; Kim, D.W.; Park, Y.S.; Ahn, S.-H.; Park, D.J.; Kim, H.-H. Laparoscopic proximal gastrectomy with double tract reconstruction is superior to laparoscopic total gastrectomy for proximal early gastric cancer. Surg. Endosc. 2017, 31, 3961–3969. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, T.; Okabe, H.; Tsunoda, S.; Shinohara, H.; Obama, K.; Hosogi, H.; Hisamori, S.; Miyazaki, K.; Nakayama, T.; Sakai, Y. Superiority of laparoscopic proximal gastrectomy with hand-sewn esophagogastrostomy over total gastrectomy in improving postoperative body weight loss and quality of life. Surg. Endosc. 2017, 31, 3664–3672. [Google Scholar] [CrossRef] [PubMed]
- Hayami, M.; Hiki, N.; Nunobe, S.; Mine, S.; Ohashi, M.; Kumagai, K.; Ida, S.; Watanabe, M.; Sano, T.; Yamaguchi, T. Clinical Outcomes and Evaluation of Laparoscopic Proximal Gastrectomy with Double-Flap Technique for Early Gastric Cancer in the Upper Third of the Stomach. Ann. Surg. Oncol. 2017, 24, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Choi, W.B.; Song, J.; Hyung, W.J.; Choi, S.H.; Noh, S.H. Complications requiring reoperation after gastrectomy for gastric cancer: 17 years experience in a single institute. J. Gastrointest. Surg. 2009, 13, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ahn, J.Y.; Jung, H.Y.; Lee, J.H.; Choi, K.-S.; Kim, D.H.; Choi, K.D.; Song, H.J.; Lee, G.H.; Kim, J.-H.; et al. Clinical Outcomes of Postoperative Upper Gastrointestinal Leakage According to Treatment Modality. Dig. Dis. Sci. 2016, 61, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Piso, P.; Stukenborg, C.; Raab, R.; Jähne, J. Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur. J. Surg. Oncol. 2000, 26, 168–171. [Google Scholar] [CrossRef]
- Messager, M.; Warlaumont, M.; Renaud, F.; Marin, H.; Branche, J.; Piessen, G.; Mariette, C. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur. J. Surg. Oncol. 2017, 43, 258–269. [Google Scholar] [CrossRef]
- Feith, M.; Gillen, S.; Schuster, T.; Theisen, J.; Friess, H.; Gertler, R. Healing occurs in most patients that receive endoscopic stents for anastomotic leakage; dislocation remains a problem. Clin. Gastroenterol. Hepatol. 2011, 9, 202–210. [Google Scholar] [CrossRef]
- Shim, C.N.; Kim, H.I.; Hyung, W.J.; Noh, S.H.; Song, M.K.; Kang, D.R.; Park, J.C.; Lee, H.; Shin, S.K.; Lee, Y.C.; et al. Self-expanding metal stents or nonstent endoscopic therapy: Which is better for anastomotic leaks after total gastrectomy? Surg. Endosc. 2014, 28, 833–840. [Google Scholar] [CrossRef]
- Jung, G.M.; Lee, S.H.; Myung, D.S.; Lee, W.S.; Joo, Y.E.; Jung, M.R.; Ryu, S.Y.; Park, Y.G.; Cho, S.B. Novel Endoscopic Stent for Anastomotic Leaks after Total Gastrectomy Using an Anchoring Thread and Fully Covering Thick Membrane: Prevention of Embedding and Migration. J. Gastric Cancer. 2018, 18, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, C.S.; Cho, Y.D.; Moon, J.H.; Kim, J.O.; Cho, J.Y.; Kim, Y.S.; Lee, J.S.; Lee, M.S. Fixation of a modified covered esophageal stent: Its clinical usefulness for preventing stent migration. Endoscopy 2001, 33, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tringali, A.; Bove, V.; Perri, V.; Landi, R.; Familiari, P.; BošKoski, I.; Costamagna, G. Endoscopic treatment of post-laparoscopic sleeve gastrectomy leaks using a specifically designed metal stent. Endoscopy 2017, 49, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Boerlage, T.C.C.; Houben, G.P.M.; Groenen, M.J.M.; Linde, K.; Laar, A.W.J.M.; Emous, M.; Fockens, P.; Voermans, R.P. A novel fully covered double-bump stent for staple line leaks after bariatric surgery: A retrospective analysis. Surg. Endosc. 2018, 32, 3174–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, A.; Curcio, G.; Ligresti, D.; Tarantino, I.; Barresi, L.; Solina, G.; Traina, M. Overtube-assisted over-the-wire stent placement to treat a post-surgical duodenal leak. Endoscopy 2016, 48 (Suppl. 1), E220–E221. [Google Scholar] [CrossRef] [Green Version]
- Vanbiervliet, G.; Filippi, J.; Karimdjee, B.S.; Venissac, N.; Iannelli, A.; Rahili, A.; Benizri, E.; Pop, D.; Staccini, P.; Tran, A.; et al. The role of clips in preventing migration of fully covered metallic esophageal stents: A pilot comparative study. Surg. Endosc. 2012, 26, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kantsevoy, S.V.; Bitner, M. Esophageal stent fixation with endoscopic suturing device (with video). Gastrointest. Endosc. 2012, 76, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Law, R.; Prabhu, A.; Fujii-Lau, L.; Shannon, C.; Singh, S. Stent migration following endoscopic suture fixation of esophageal self-expandable metal stents: A systematic review and meta-analysis. Surg. Endosc. 2018, 32, 675–681. [Google Scholar] [CrossRef] [PubMed]


| Total (n = 27) | Beta Stent Group | Thread-Fix Stent Group | p | |
|---|---|---|---|---|
| (n = 14) | (n = 13) | |||
| Age (years), median (IQR) | 66 (59–73) | 71 (60–76) | 64 (59–68) | 0.576 |
| Sex, n (%) | 0.29 | |||
| Female | 1 (3.7) | 0 (0) | 1 (7.7) | |
| Male | 26 (96.3) | 14 (100) | 12 (92.3) | |
| Body mass index (kg/m2), median (IQR) | 24.0 (22.3–26.5) | 24.0 (22.3–25.9) | 24.7 (23.0–26.6) | 0.382 |
| Smoking status, n (%) | 0.275 | |||
| Non-smoker | 4 (14.8) | 2 (14.3) | 2 (15.3) | |
| Ex-smoker | 18 (66.7) | 11 (78.6) | 7 (53.8) | |
| Current smoker | 5 (18.5) | 1 (7.1) | 4 (30.8) | |
| Alcohol drinking, n (%) | 0.883 | |||
| No | 10 (37.0) | 5 (35.7) | 5 (38.5) | |
| Yes | 17 (63.0) | 9 (64.3) | 8 (61.5) | |
| ASA score, n (%) | 0.531 | |||
| 1 | 4 (14.8) | 2 (14.3) | 2 (15.4) | |
| 2 | 21 (77.8) | 10 (71.4) | 11 (84.6) | |
| 3 | 2 (7.4) | 2 (14.3) | 0 (0) | |
| Gastric cancer type, n (%) | 0.516 | |||
| Early gastric cancer | 10 (37.0) | 6 (42.9) | 4 (30.8) | |
| Advanced gastric cancer | 17 (63.0) | 8 (57.1) | 9 (69.2) | |
| Type of surgery, n (%) | 0.037 | |||
| Total gastrectomy | 23 (85.2) | 10 (71.4) | 13 (100) | |
| Proximal gastrectomy | 4 (14.8) | 4 (28.6) | 0 (0) | |
| Surgical approach, n (%) | 0.31 | |||
| Open | 16 (59.3) | 7 (50.0) | 9 (69.2) | |
| Laparoscopy | 11 (40.7) | 7 (50.0) | 4 (30.8) | |
| Pathological tumor stage, n (%) | 0.59 | |||
| Stage I | 13 (48.1) | 8 (57.1) | 5 (38.5) | |
| Stage II | 5 (18.5) | 3 (21.4) | 2 (15.4) | |
| Stage III | 7 (25.9) | 2 (14.3) | 5 (38.5) | |
| Stage IV | 2 (7.4) | 1 (7.1) | 1 (7.7) |
| Beta Stent Group | Thread-Fix Stent Group | p | |
|---|---|---|---|
| (n = 14) | (n = 13) | ||
| Interval between surgery and diagnosis (days), median (IQR) | 8 (5–11) | 7 (6–7) | 0.77 |
| Leakage site, n (%) | 0.936 | ||
| Esophageal anastomosis site * | 12 (85.7) | 11 (84.6) | |
| Jejunal stump | 2 (14.3) | 2 (15.4) | |
| Leakage size (mm), median (IQR) | 12 (8–20) | 18 (12–25) | 0.091 |
| Leakage management before stent insertion, n (%) | |||
| Percutaneous drainage | 7 (50.0) | 7 (53.8) | 0.842 |
| Endoscopic procedures † | 3 (21.4) | 2 (15.4) | 0.686 |
| Time interval between diagnosis and stent placement (days), median (IQR) | 3 (1–7) | 1 (1–7) | 0.676 |
| Beta Stent Group | Thread-Fix Stent Group | p | |
|---|---|---|---|
| (n = 14) | (n = 13) | ||
| Complete leakage healing on the first stent placement, n (%) | 13 (92.9) | 7 (53.8) | 0.021 |
| Additional endoscopic procedures, n (%) | 1 (7.1) | 6 (46.2) | 0.021 |
| Second stent placement | 1 | 0 | |
| Endoscopic clips with/without detachable snare | 0 | 6 | |
| Interval between stent insertion and diet initiation (days), median (IQR) | |||
| Sips of water | 3 (1–6) | 4 (2–9) | 0.606 |
| Soft diet | 6 (3–12) | 14 (6–25) | 0.118 |
| Discharge with stent maintenance status, n (%) | 7 (50.0) | 0 (0) | 0.003 |
| Hospitalization after stent placement * (days), median (IQR) | 16 (14–31) | 32 (21–67) | 0.037 |
| Duration of stent maintenance (days), median (IQR) | 28 (21–42) | 18 (16–20) | 0.006 |
| Interval between leakage diagnosis and complete healing (days), median (IQR) | 35 (22–48) | 31 (21–54) | 0.942 |
| Complications related to stent placement, n (%) | |||
| Erosion | 7 (50.0) | 5 (38.5) | 0.547 |
| Reflux symptoms | 5 (35.7) | 5 (38.5) | 0.883 |
| Migration | 1 (7.1) | 0 (0) | 0.326 |
| Stent site stricture requiring balloon dilatation | 0 (0) | 3 (23.1) | 0.057 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-I.; Kim, C.G.; Lee, J.Y.; Choi, I.J.; Eom, B.W.; Yoon, H.M.; Ryu, K.W.; Kim, Y.-W. Effectiveness of a Novel Covered Stent without External Thread Fixation for Anastomotic Leakage after Total or Proximal Gastrectomy for Gastric Cancer. Cancers 2021, 13, 3720. https://doi.org/10.3390/cancers13153720
Kim Y-I, Kim CG, Lee JY, Choi IJ, Eom BW, Yoon HM, Ryu KW, Kim Y-W. Effectiveness of a Novel Covered Stent without External Thread Fixation for Anastomotic Leakage after Total or Proximal Gastrectomy for Gastric Cancer. Cancers. 2021; 13(15):3720. https://doi.org/10.3390/cancers13153720
Chicago/Turabian StyleKim, Young-Il, Chan Gyoo Kim, Jong Yeul Lee, Il Ju Choi, Bang Wool Eom, Hong Man Yoon, Keun Won Ryu, and Young-Woo Kim. 2021. "Effectiveness of a Novel Covered Stent without External Thread Fixation for Anastomotic Leakage after Total or Proximal Gastrectomy for Gastric Cancer" Cancers 13, no. 15: 3720. https://doi.org/10.3390/cancers13153720
APA StyleKim, Y.-I., Kim, C. G., Lee, J. Y., Choi, I. J., Eom, B. W., Yoon, H. M., Ryu, K. W., & Kim, Y.-W. (2021). Effectiveness of a Novel Covered Stent without External Thread Fixation for Anastomotic Leakage after Total or Proximal Gastrectomy for Gastric Cancer. Cancers, 13(15), 3720. https://doi.org/10.3390/cancers13153720






