Effects of and Lessons Learned from an Internet-Based Physical Activity Support Program (with and without Physiotherapist Telephone Counselling) on Physical Activity Levels of Breast and Prostate Cancer Survivors: The PABLO Randomized Controlled Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
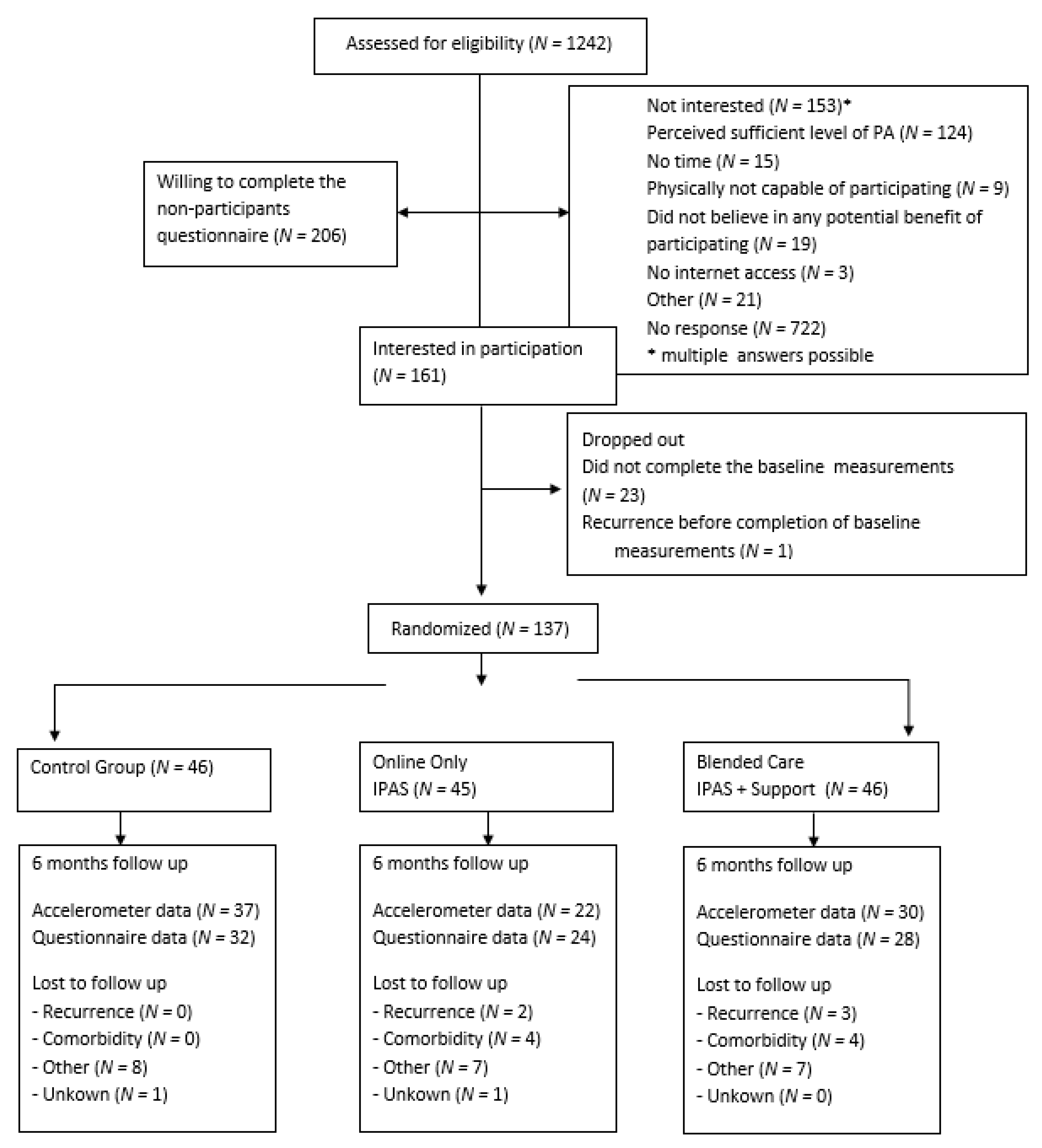
2.1. Research Design and Study Population
2.2. Procedure
2.3. Trial Arms
2.4. Measurements
2.5. Primary Outcome
2.6. Secondary Outcomes
2.7. Statistical Analyses
2.7.1. Sociodemographic and Clinical Data
2.7.2. Primary Outcome
2.7.3. Secondary Outcomes
2.7.4. Adherence
3. Results
3.1. Effect on Primary Outcome
3.2. Effect on Secondary Outcomes
3.3. Actual Use and Adherence
3.4. Per Protocol Analysis of the Primary Outcome
3.5. Evaluation of the Intervention
3.6. Cost-Analysis
4. Discussion
4.1. Intervention Design
4.2. Recruitment
4.3. Adherence
4.4. Physical Activity Support by Blended Care
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvão, D.A.; Alfano, C.M.; et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Caballero-García, A.; Córdova Martínez, A.; Lázaro Asensio, M.P.; Fernández-Lázaro, C.I. Physical activity in oncology patients with breast cancer: Nonpharmacological sports-based medical therapy? Systematic review. Arch. Med. Deporte 2020, 37, 266–274. [Google Scholar]
- Lee, J. A Meta-analysis of the Association Between Physical Activity and Breast Cancer Mortality. Cancer Nurs. 2019, 42, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Mctiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity In Cancer Prevention And Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Haberlin, C.; O’Dwyer, T.; Mockler, D.; Moran, J.; O’Donnell, D.M.; Broderick, J. The use of eHealth to promote physical activity in cancer survivors: A systematic review. Support. Care Cancer 2018, 26, 3323–3336. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.R.; Steed, L.; Quirk, H.; Greasley, R.U.; Saxton, J.M.; Taylor, S.J.; Rosario, D.J.; Thaha, M.A.; Bourke, L. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst. Rev. 2018, 9, Cd010192. [Google Scholar] [CrossRef]
- Dorri, S.; Asadi, F.; Olfatbakhsh, A.; Kazemi, A. A Systematic Review of Electronic Health (eHealth) interventions to improve physical activity in patients with breast cancer. Breast Cancer 2020, 27, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Golsteijn, R.H.J.; Bolman, C.; Volders, E.; Peels, D.A.; de Vries, H.; Lechner, L. Short-Term efficacy of a computer-tailored physical activity intervention for prostate and colorectal cancer patients and survivors: A randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.L.; Baima, J.; Swisher, A.K.; Winters-Stone, K.M.; Welsh, J. A Systematic Review of Exercise Systematic Reviews in the Cancer Literature (2005–2017). PM R. 2017, 9, 347–384. [Google Scholar] [CrossRef]
- Groen, W.G.; van Harten, W.H.; Vallance, J.K. Systematic review and meta-analysis of distance-based physical activity interventions for cancer survivors (2013–2018): We still haven’t found what we’re looking for. Cancer Treat. Rev. 2018, 69, 188–203. [Google Scholar] [CrossRef]
- Erbe, D.; Eichert, H.C.; Riper, H.; Ebert, D.D. Blending Face-to-Face and Internet-Based Interventions for the Treatment of Mental Disorders in Adults: Systematic Review. J. Med. Internet Res. 2017, 19, 306. [Google Scholar] [CrossRef]
- Kloek, C.; Bossen, D.; de Bakker, D.H.; Veenhof, C.; Dekker, J. Blended Interventions to Change Behavior in Patients With Chronic Somatic Disorders: Systematic Review. J. Med. Internet Res. 2017, 19, 418. [Google Scholar] [CrossRef]
- Willems, R.A.; Bolman, C.A.W.; Lechner, L.; Mesters, I.; Gunn, K.M.; Skrabal Ross, X.; Olver, I. Online interventions aimed at reducing psychological distress in cancer patients: Evidence update and suggestions for future directions. Curr. Opin. Support. Palliat. Care 2020, 14, 27–39. [Google Scholar] [CrossRef]
- Kuijpers, W.; Groen, W.G.; Oldenburg, H.S.; Wouters, M.W.; Aaronson, N.K.; van Harten, W.H. Development of MijnAVL, an Interactive Portal to Empower Breast and Lung Cancer Survivors: An Iterative, Multi-Stakeholder Approach. J. Med. Internet Res. Protoc. 2015, 4, 14. [Google Scholar] [CrossRef][Green Version]
- Prochaska, J.; DiClemente, C. Self change processes, self efficacy and decisional balance across five stages of smoking cessation. Prog. Clin. Biol. 1984, 156, 131. [Google Scholar]
- Ajzen, I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- Bandura, A. Social Foundations of Thought and Action: A Social Cognitive Theory; Prentice-Hall: Englewood Cliffs, NJ, USA; Hoboken, NJ, USA, 1986. [Google Scholar]
- Kuijpers, W.; Groen, W.G.; Oldenburg, H.S.; Wouters, M.W.; Aaronson, N.K.; van Harten, W.H. Ehealth for Breast Cancer Survivors: Use, Feasibility and Impact of an Interactive Portal. J. Med. Internet Res. Cancer 2016, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Meneses, K.; Pisu, M.; Azuero, A.; Benz, R.; Su, X.; McNees, P. A telephone-based education and support intervention for Rural Breast Cancer Survivors: A randomized controlled trial comparing two implementation strategies in rural Florida. J. Cancer Surviv. 2020, 14, 494–503. [Google Scholar] [CrossRef]
- Atema, V.; van Leeuwen, M.; Kieffer, J.M.; Oldenburg, H.S.A.; van Beurden, M.; Gerritsma, M.A.; Kuenen, M.A.; Plaisier, P.W.; Lopes Cardozo, A.M.F.; van Riet, Y.E.A.; et al. Efficacy of Internet-Based Cognitive Behavioral Therapy for Treatment-Induced Menopausal Symptoms in Breast Cancer Survivors: Results of a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 809–822. [Google Scholar] [CrossRef]
- van de Wiel, H.J.; Stuiver, M.M.; May, A.M.; van Grinsven, S.; Aaronson, N.K.; Retel, V.P.; Oldenburg, H.S.A.; van der Poel, H.G.; Horenblas, S.; van Harten, W.H.; et al. (Cost-)effectiveness of an internet-based physical activity support program (with and without physiotherapy counselling) on physical activity levels of breast and prostate cancer survivors: Design of the PABLO trial. BMC Cancer 2018, 18, 1073. [Google Scholar] [CrossRef]
- ALEA Tools for clinical trials, 2018 ALEA Clinical, Hollandse Kade 1, 1391 JD, Abcoude, The Netherlands. Available online: https://www.aleaclinical.eu/ (accessed on 25 March 2020).
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef]
- Eysenbach, G. CONSORT-EHEALTH: Improving and standardizing evaluation reports of Web-based and mobile health interventions. J. Med. Internet Res. 2011, 13, 126. [Google Scholar] [CrossRef]
- Gezondheidsraad. Beweegrichtlijnen 2017. Available online: https://www.gezondheidsraad.nl/binaries/gezondheidsraad/documenten/adviezen/2017/08/22/beweegrichtlijnen-2017/kernadvies-Beweegrichtlijnen-2017.pdf (accessed on 25 March 2020).
- Bassett, D.R., Jr.; Ainsworth, B.E.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; King, G.A. Validity of four motion sensors in measuring moderate intensity physical activity. Med. Sci. Sports Exerc. 2000, 32, 471–480. [Google Scholar] [CrossRef]
- Peddle-McIntyre, C.J.; Cavalheri, V.; Boyle, T.; McVeigh, J.A.; Jeffery, E.; Lynch, B.M.; Vallance, J.K. A Review of Accelerometer-based Activity Monitoring in Cancer Survivorship Research. Med. Sci. Sports Exerc. 2018, 50, 1790–1801. [Google Scholar] [CrossRef]
- Santos-Lozano, A.; Santin-Medeiros, F.; Cardon, G.; Torres-Luque, G.; Bailon, R.; Bergmeir, C.; Ruiz, J.R.; Lucia, A.; Garatachea, N. Actigraph GT3X: Validation and determination of physical activity intensity cut points. Int. J. Sports Med. 2013, 34, 975–982. [Google Scholar] [CrossRef]
- Carr, L.J.; Mahar, M.T. Accuracy of intensity and inclinometer output of three activity monitors for identification of sedentary behavior and light-intensity activity. J. Obes. 2012, 460271. [Google Scholar] [CrossRef] [PubMed]
- IPAQ. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)–Short and Long Forms. 2005. Available online: https://www.researchgate.net/file.PostFileLoader.html?id=5641f4c36143250eac8b45b7&assetKey=AS%3A294237418606593%401447163075131 (accessed on 25 March 2020).
- Smets, E.M.A.; Garssen, B.; Bonke, B.; De Haes, J.C.J.M. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- McNair, D.; Lorr, M.; Droppleman, L. Manual: Profile of Mood States (POMS); EDITS /Educational and Industrial Testing Service Inc.: San Diego, CA, USA, 1971. [Google Scholar]
- McHorney, C.A.; Ware, J.E., Jr.; Raczek, A.E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 1993, 31, 247–263. [Google Scholar] [CrossRef]
- Foundation ER. Manual: EQ-5D-5L-English-User-Guide_version-3.0-Sept-2019-secured.pdf. 2019. Available online: https://euroqol.org/publications/user-guides/ (accessed on 16 March 2021).
- Marcus, B.H.; Selby, V.C.; Niaura, R.S.; Rossi, J.S. Self-Efficacy and the stages of exercise behavior change. Res. Q. Exerc. Sport. 1992, 63, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Friedenreich, C.M. Utility of the theory of planned behavior for understanding exercise during breast cancer treatment. Psycho-Oncology 1999, 8, 112–122. [Google Scholar] [CrossRef]
- van Waart, H.; van Harten, W.H.; Buffart, L.M.; Sonke, G.S.; Stuiver, M.M.; Aaronson, N.K. Why do patients choose (not) to participate in an exercise trial during adjuvant chemotherapy for breast cancer? Psycho-Oncology 2016, 25, 964–970. [Google Scholar] [CrossRef]
- van der Ploeg, H.P.; van der Beek, A.J.; van der Woude, L.H.; van Mechelen, W. Physical activity for people with a disability: A conceptual model. Sports Med. 2004, 34, 639–649. [Google Scholar] [CrossRef]
- Sallis, J.F.; Calfas, K.J.; Alcaraz, J.E.; Gehrman, C.; Johnson, M.F. Potential mediators of change in a physical activity promotion course for university students: Project GRAD. Ann. Behav. Med. 1999, 21, 149–158. [Google Scholar] [CrossRef] [PubMed]
- CAO Ziekenhuizen. 2020. Available online: https://cao-ziekenhuizen.nl/salarisschalen-premies-vergoedingen (accessed on 16 March 2021).
- Dutch Healthcare Authority (Nederlandse Zorgautoriteit [NZa]). Pricelist Healthcare Products 2016 (Tarievenlijst DBC-Zorgproducten en Overige Producten 2016). 2016. Available online: https://puc.overheid.nl/nza/ (accessed on 16 March 2021).
- RstudioTeam. RStudio: Integrated Development for R. RStudio. 2018. Available online: http://www.rstudio.com/ (accessed on 16 March 2021).
- de Vries, H.J.; Kloek, C.J.J.; de Bakker, D.H.; Dekker, J.; Bossen, D.; Veenhof, C. Determinants of Adherence to the Online Component of a Blended Intervention for Patients with Hip and/or Knee Osteoarthritis: A Mixed Methods Study Embedded in the e-Exercise Trial. Telemed. J. e-Health 2017, 23, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Bossen, D.; Buskermolen, M.; Veenhof, C.; de Bakker, D.; Dekker, J. Adherence to a Web-Based Physical Activity Intervention for Patients With Knee and/or Hip Osteoarthritis: A Mixed Method Study. J. Med. Internet Res. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Furness, K.; Sarkies, M.N.; Huggins, C.E.; Croagh, D.; Haines, T.P. Impact of the Method of Delivering Electronic Health Behavior Change Interventions in Survivors of Cancer on Engagement, Health Behaviors, and Health Outcomes: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, 16112. [Google Scholar] [CrossRef]
- Bessell, T.L.; McDonald, S.; Silagy, C.A.; Anderson, J.N.; Hiller, J.E.; Sansom, L.N. Do Internet interventions for consumers cause more harm than good? A systematic review. Health Expect. 2002, 5, 28–37. [Google Scholar] [CrossRef]
- van den Berg, M.H.; Schoones, J.W.; Vliet Vlieland, T.P.M. Internet-Based Physical Activity Interventions: A Systematic Review of the Literature. J. Med. Internet Res. 2007, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Schroé, H.; Van Dyck, D.; De Paepe, A.; Poppe, A.; Wei Loh, W.; Verloigne, M.; Loeys, T.; De Bourdeaudhuij, I.; Grombez, G. Which behaviour change techniques are effective to promote physical activity and reduce sedentary behaviour in adults: A factorial randomized trial of an e- and m-health intervention. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 127. [Google Scholar] [CrossRef]
- Berg, M.; Linden, K.; Adolfsson, A.; Sparud Lundin, C.; Ranerup, A. Web-Based Intervention for Women With Type 1 Diabetes in Pregnancy and Early Motherhood: Critical Analysis of Adherence to Technological Elements and Study Design. J. Med. Internet Res. 2018, 20, 160. [Google Scholar] [CrossRef]
- Groen, W.G.; Kuijpers, W.; Oldenburg, H.S.; Wouters, M.W.; Aaronson, N.K.; van Harten, W.H. Supporting Lung Cancer Patients With an Interactive Patient Portal: Feasibility Study. J. Med. Internet Res. Cancer 2017, 3, 10. [Google Scholar] [CrossRef]
- Courneya, K.S.; McKenzie, D.C.; Mackey, J.R.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Cook, D.; Jespersen, D.; Proulx, C.; et al. Effects of exercise dose and type during breast cancer chemotherapy: Multicenter randomized trial. J. Natl. Cancer Inst. 2013, 105, 1821–1832. [Google Scholar] [CrossRef]
- Sears, S.R.; Stanton, A.L.; Kwan, L.; Krupnick, J.L.; Rowland, J.H.; Meyerowitz, B.E.; Ganz, P.A. Recruitment and retention challenges in breast cancer survivorship research: Results from a multisite, randomized intervention trial in women with early stage breast cancer. Cancer epidemiology, biomarkers & prevention: A publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. Cancer Epidemiol. Prev. Biomark. 2003, 12, 1087–1090. [Google Scholar]
- Kampshoff, C.S.; van Mechelen, W.; Schep, G.; Nijziel, M.R.; Witlox, L.; Bosman, L.; Chinapaw, M.J.; Brug, J.; Buffart, L.M. Participation in and adherence to physical exercise after completion of primary cancer treatment. Int J. Behav. Nutr. Phys. Act. 2016, 13, 100. [Google Scholar] [CrossRef]
- Services USDoHaH. Physical Activity Guidelines Advisory Committee Scientific Report. 2018. Available online: https://healthgov/sites/default/files/2019-09/PAG_Advisory_Committee_Reportpdf (accessed on 25 March 2020).
- Kelders, S.M.; Kok, R.N.; Ossebaard, H.C.; Van Gemert-Pijnen, J.E. Persuasive system design does matter: A systematic review of adherence to web-based interventions. J. Med. Internet Res. 2012, 14, 152. [Google Scholar] [CrossRef]
- Gaskin, C.J.; Craike, M.; Mohebbi, M.; Salmon, J.; Courneya, K.S.; Broadbent, S.; Livingston, P.M. Associations of objectively measured moderate-to-vigorous physical activity and sedentary behavior with quality of life and psychological well-being in prostate cancer survivors. Cancer Causes Control 2016, 27, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Sieverink, F.; Kelders, S.M.; van Gemert-Pijnen, J.E.W.C. Clarifying the Concept of Adherence to eHealth Technology: Systematic Review on When Usage Becomes Adherence. J. Med. Internet Res. 2017, 19, 402. [Google Scholar] [CrossRef] [PubMed]
- Eysenbach, G. Issues in evaluating health websites in an Internet-based randomized controlled trial. J. Med. Internet Res. 2002, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Schaap, L.A.; Wijnhoven, H.A.H. Self-Reported Impact of the COVID-19 Pandemic on Nutrition and Physical Activity Behaviour in Dutch Older Adults Living Independently. Nutrients 2020, 12, 3708. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.; To, Q.G.; Khalesi, S.; Williams, S.L.; Alley, S.J.; Thwaite, T.L.; Fenning, A.S.; Vandelanotte, C. Depression, Anxiety and Stress during COVID-19: Associations with Changes in Physical Activity, Sleep, Tobacco and Alcohol Use in Australian Adults. Int. J. Environ. Res. Public Health 2020, 17, 4065. [Google Scholar] [CrossRef]
- Castañeda-Babarro, A.; Arbillaga-Etxarri, A.; Gutiérrez-Santamaría, B.; Coca, A. Physical Activity Change during COVID-19 Confinement. Int. J. Environ. Res. Public Health 2020, 17, 6878. [Google Scholar] [CrossRef] [PubMed]
- Lynch, B.M.; Nguyen, N.H.; Moore, M.M.; Reeves, M.M.; Rosenberg, D.E.; Boyle, T.; Vallance, J.K.; Milton, S.; Friedenreich, C.M.; English, D.R. A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: The ACTIVATE Trial. Cancer 2019, 125, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.M.; Stein, K.; Dunsiger, S. Peers Promoting Physical Activity among Breast Cancer Survivors: A Randomized Control. Trial. Health Psychol. 2015, 34, 463–472. [Google Scholar] [CrossRef]
- Gal, R.; May, A.M.; van Overmeeren, E.J.; Simons, M.; Monninkhof, E.M. The Effect of Physical Activity Interventions Comprising Wearables and Smartphone Applications on Physical Activity: A Systematic Review and Meta-analysis. Sports Med. 2018, 1, 42. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Control Group N = 46 | Online Only (IPAS) N = 45 | Blended Care (IPAS + Support) N = 46 |
|---|---|---|---|
| Tumor type/sexN (%) Prostate cancer/Men Breast cancer/Women | 24 (52) 22 (48) | 22 (49) 23 (51) | 24 (52) 22 (48) |
| CenterN (%) AVL Rijnstate UMCU a | 42 (91) 3 (7) 1 (2) | 42 (93) 3 (7) 0 (0) | 41 (89) 3 (7) 2 (4) |
| TreatmentN (%) b | |||
| Radiotherapy Prostate cancer Breast cancer | 8 (35) 16 (73) | 5 (23) 16 (70) | 6 (25) 16 (73) |
| Chemotherapy (only in breast cancer) (Neo)adjuvant Adjuvant | 14 (31) 4 (9) | 6 (13) 3 (7) | 5 (11) 3 (7) |
| Endocrine therapy Prostate cancer Breast cancer | 2 (9) 13 (59) | 1 (5) 10 (44) | 2 (8) 9 (41) |
| Breast-conserving surgery c | 15 (68) | 14 (61) | 12 (55) |
| Mastectomy c | 7 (32) | 7 (30) | 10 (46) |
| Breast reconstruction c | 8 (36) | 8 (35) | 8 (36) |
| Prostatectomy d | 16 (70) | 20 (91) | 18 (77) |
| Brachytherapy d | 0 (0) | 0 (0) | 2 (8) |
| Treatment duration in months (median, IQR) Prostate Breast | 3 (2.5–9) 6 (5–8.5) | 2.5 (2–5) 4 (4–8) | 3 (3–6.5) 7 (5.5–9.5) |
| Age in years (mean, SD) | 59.2 (14.4) | 59.3 (11.3) | 59.8 (11.7) |
| Living situation (%) | |||
| Single | 7 (15) | 6 (13) | 10 (22) |
| Living together | 36 (78) | 38 (84) | 34 (74) |
| With partner, not living together | 2 (4) | 1 (2) | 2 (4) |
| Missing | 1 (2) | - | - |
| Education levelN (%) | |||
| Primary school | 1 (2) | 0 (0) | 1 (2) |
| High School | 12 (26) | 14 (31) | 19 (41) |
| College/University | 31 (68) | 31 (69) | 25 (54) |
| Missing | 2 (4) | - | 1 (2) |
| Working situationN (%) e | |||
| Paid Job | 15 (33) | 21 (47) | 22 (48) |
| Retired | 17 (37) | 13 (29) | 15 (33) |
| On disability | 8 (17) | 4 (9) | 5 (11) |
| Smoking behaviorN (%) | |||
| Never | 24 (52) | 18 (40) | 20 (44) |
| Previous | 18 (39) | 25 (56) | 21 (46) |
| Current | 3 (7) | 2 (4) | 5 (11) |
| Missing | 1 (2) | - | - |
| Alcohol consumptionN (%) | |||
| No | 12 (26) | 13 (29) | 10 (22) |
| Yes | 33 (72) | 32 (71) | 36 (78) |
| Missing | 1 (2) | - | - |
| Computer useN (%) | |||
| Sometimes | 4 (9) | 2 (4) | 1 (2) |
| Often | 41 (89) | 43 (96) | 45 (98) |
| Missing | 1 (2) | - | - |
| Computer skills N (%) | |||
| Poor | 3 (7) | 5 (11) | 3 (7) |
| Moderate | 15 (33) | 8 (18) | 14 (30) |
| Good | 27 (58) | 32 (71) | 29 (63) |
| Missing | 1 (2) | - | - |
| Physical activity levels before diagnosis in days per week (median, IQR) | |||
| Moderate f | 5 (4–5) | 5 (4–6) | 6 (4–6) |
| Vigorous g | 2 (1- 2) | 2 (2–3) | 1 (1–3) |
| T0 (N = 84) | T1 (N = 84) | Between Group Differences | ||
|---|---|---|---|---|
| Measure | Mean (SD) | Mean (SD) | AMD † (95% CI) | p-Value |
| Primary Outcome | ||||
| MVPA/week Control)‡ (N = 34) Online Only (N = 21) Blended (N = 28) | 289.5 (128.2) 309.4 (152.6) 245.6 (147.4) | 294.8 (162.7) 291.9 (142.1) 240.3 (156.8) | REF −15.42 (−30.5:37.6) 5.70 (−30.5:37.6) | 0.39 0.75 |
| Online Only N = 22 * (Mean, SD) | Blended N = 25 * (Mean, SD) | |
|---|---|---|
| Technical issues (N, % yes) | 2 (8) | 9 (36) |
| I experienced IPAS as useful a | 2.8 (1.2) | 3.2 (1.1) |
| I experienced the role of physiotherapist as useful a | NA | 3.7 (0.7) |
| I experienced IPAS helpful in becoming more physically active a | 2.3 (1.1) | 3.3 (1.0) |
| The physiotherapist helped me to become more physically active a | NA | 3.5 (1.0) |
| I am satisfied about IPAS a | 2.7 (0.9) | 2.9 (0.9) |
| I am satisfied about the role of physiotherapist a | NA | 3.5 (0.8) |
| IPAS and the physiotherapist strengthen each other’s effect a | NA | 3.3 (0.8) |
| I would value IPAS on a scale from 1 till 10 b | 5.4 (2.5) | 6.1 (1.5) |
| I would recommend IPAS to other cancer survivors (N, %) Yes Maybe No | 3 (14) 15 (68) 4 (18) | 13 (52) 10 (40) 2 (8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van de Wiel, H.J.; Stuiver, M.M.; May, A.M.; van Grinsven, S.; Aaronson, N.K.; Oldenburg, H.S.A.; van der Poel, H.G.; Koole, S.N.; Retèl, V.P.; van Harten, W.H.; et al. Effects of and Lessons Learned from an Internet-Based Physical Activity Support Program (with and without Physiotherapist Telephone Counselling) on Physical Activity Levels of Breast and Prostate Cancer Survivors: The PABLO Randomized Controlled Trial. Cancers 2021, 13, 3665. https://doi.org/10.3390/cancers13153665
van de Wiel HJ, Stuiver MM, May AM, van Grinsven S, Aaronson NK, Oldenburg HSA, van der Poel HG, Koole SN, Retèl VP, van Harten WH, et al. Effects of and Lessons Learned from an Internet-Based Physical Activity Support Program (with and without Physiotherapist Telephone Counselling) on Physical Activity Levels of Breast and Prostate Cancer Survivors: The PABLO Randomized Controlled Trial. Cancers. 2021; 13(15):3665. https://doi.org/10.3390/cancers13153665
Chicago/Turabian Stylevan de Wiel, H. J., M. M. Stuiver, A. M. May, S. van Grinsven, N. K. Aaronson, H. S. A. Oldenburg, H. G. van der Poel, S. N. Koole, V. P. Retèl, W. H. van Harten, and et al. 2021. "Effects of and Lessons Learned from an Internet-Based Physical Activity Support Program (with and without Physiotherapist Telephone Counselling) on Physical Activity Levels of Breast and Prostate Cancer Survivors: The PABLO Randomized Controlled Trial" Cancers 13, no. 15: 3665. https://doi.org/10.3390/cancers13153665
APA Stylevan de Wiel, H. J., Stuiver, M. M., May, A. M., van Grinsven, S., Aaronson, N. K., Oldenburg, H. S. A., van der Poel, H. G., Koole, S. N., Retèl, V. P., van Harten, W. H., & Groen, W. G. (2021). Effects of and Lessons Learned from an Internet-Based Physical Activity Support Program (with and without Physiotherapist Telephone Counselling) on Physical Activity Levels of Breast and Prostate Cancer Survivors: The PABLO Randomized Controlled Trial. Cancers, 13(15), 3665. https://doi.org/10.3390/cancers13153665






