COVID-19 Vaccine Safety in Cancer Patients: A Single Centre Experience
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients
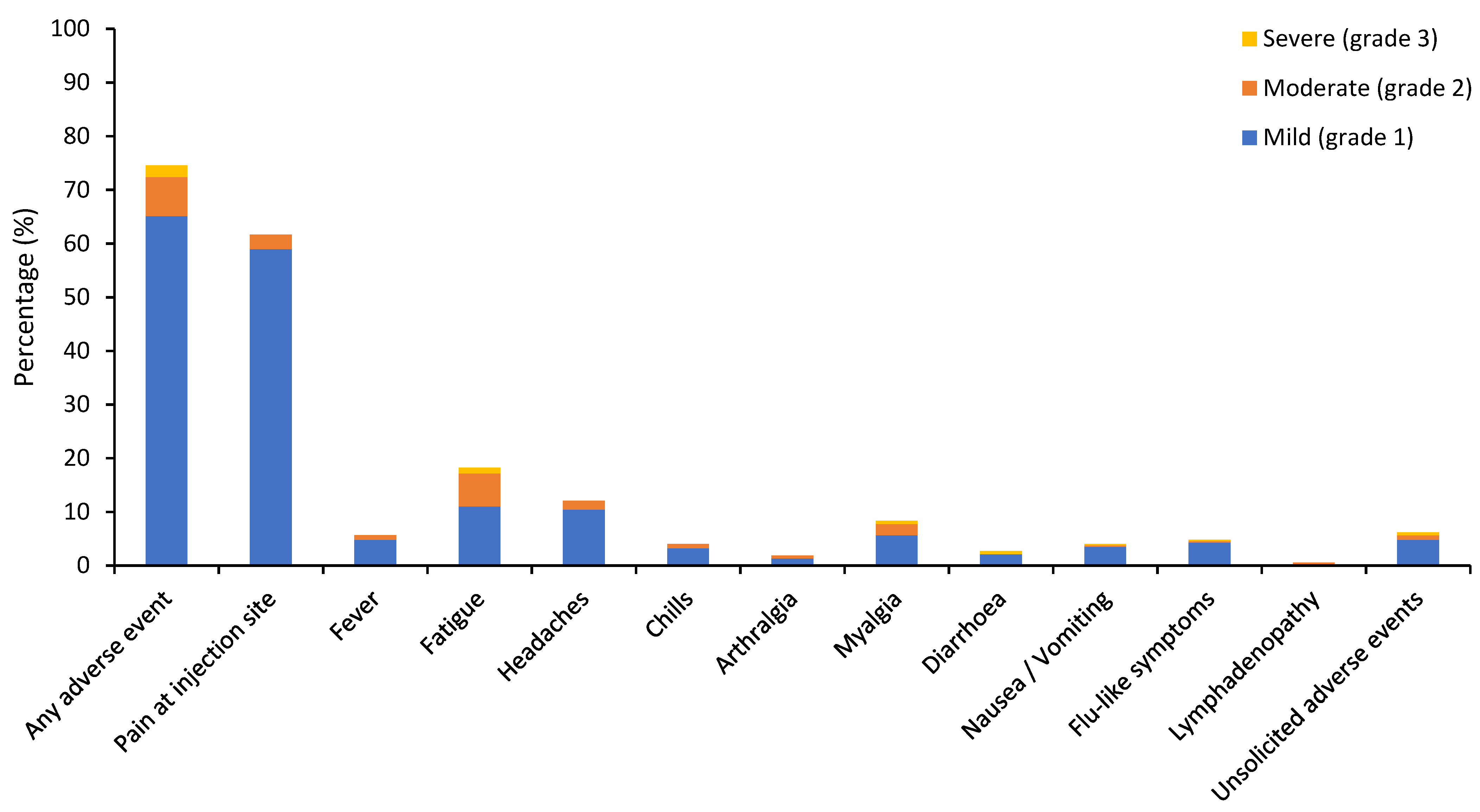
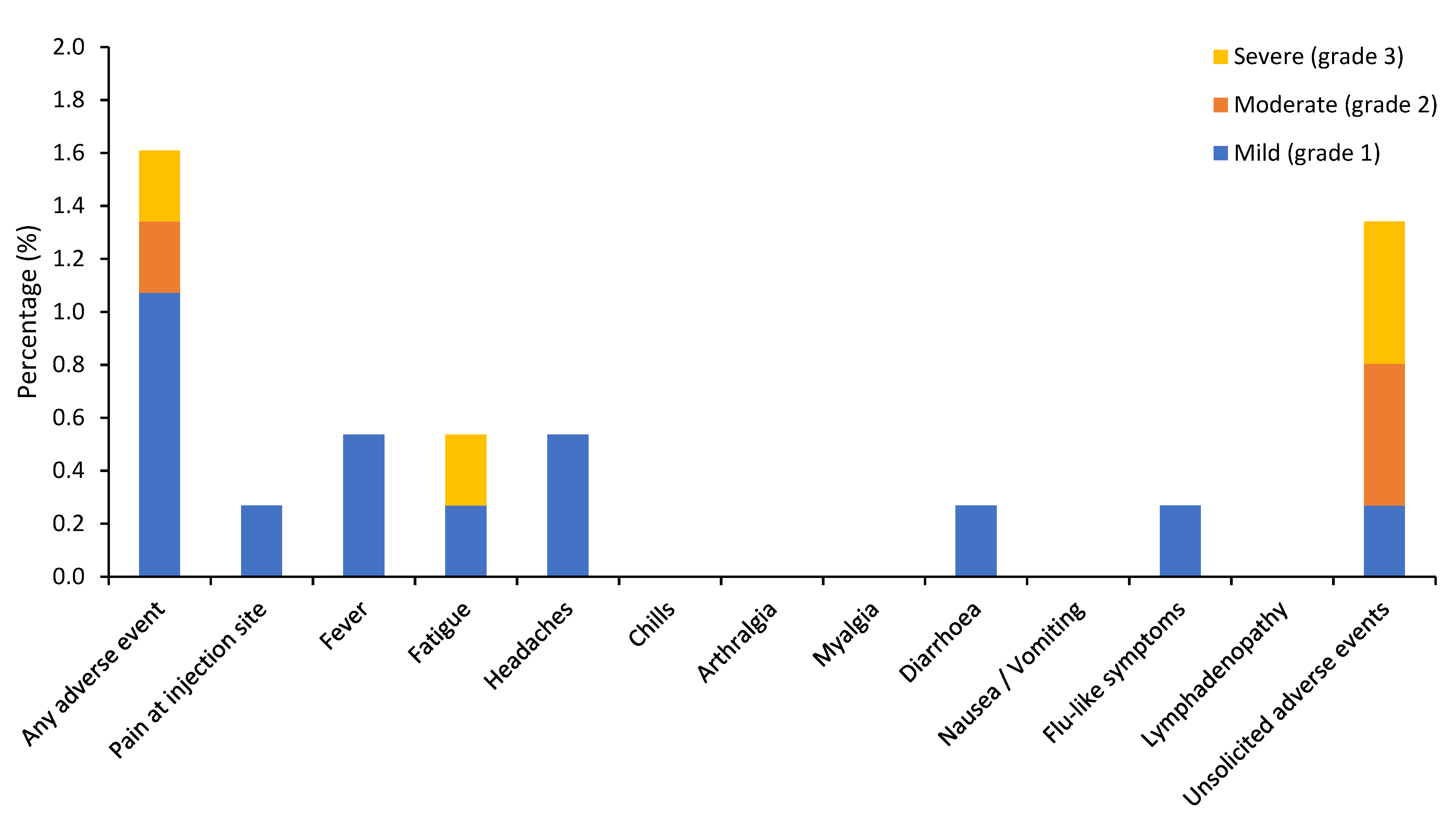
3.2. Vaccine-Related Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
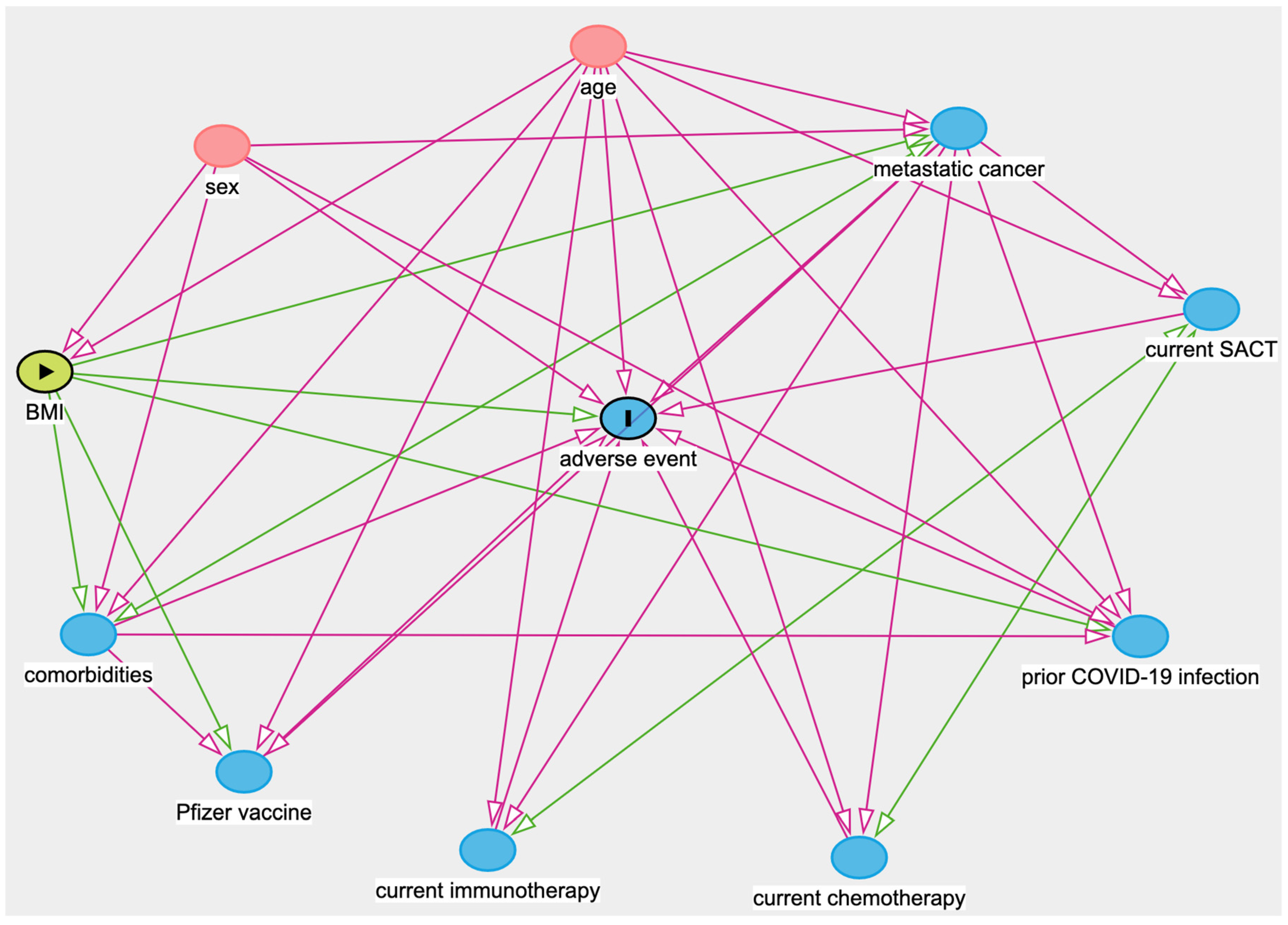
Appendix A
| Main Exposure Variable | Minimal Adjustments |
|---|---|
| Age | No adjustment is necessary to estimate the total effect of age on adverse event. |
| Sex | No adjustment is necessary to estimate the total effect of sex on adverse event. |
| BMI | Age, Sex |
| Comorbidities | Age, Sex, BMI, Metastatic cancer |
| Prior COVID-19 infection | Age, Sex, BMI, Comorbidities, Metastatic cancer |
| Metastatic cancer | Age, Sex, BMI, Comorbidities |
| Receiving active SACT | Age, Metastatic cancer, Receiving chemotherapy, Receiving immunotherapy |
| Receiving chemotherapy (within 28 days) | Age, Metastatic cancer, Receiving active SACT, Receiving immunotherapy |
| Receiving immunotherapy (within 6 months) | Age, Metastatic cancer, Receiving active SACT, Receiving chemotherapy |
| Pfizer vaccine | Age, BMI, Comorbidities, Metastatic cancer |
References
- Saini, K.S.; Tagliamento, M.; Lambertini, M.; McNally, R.; Romano, M.; Leone, M.; Curigliano, G.; de Azambuja, E. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur. J. Cancer 2020, 139, 43–50. [Google Scholar] [CrossRef]
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef]
- Jiang, C.; Robin Yabroff, K.; Deng, L.; Perimbeti, S.; Han, X. Prevalence of underlying medical conditions associated with severe COVID-19 illness in adult cancer survivors in the United States. J. Natl. Cancer Inst. 2021, djab012. [Google Scholar] [CrossRef]
- Jazieh, A.R.; Akbulut, H.; Curigliano, G.; Rogado, A.; Alsharm, A.A.; Razis, E.D.; Mula-Hussain, L.; Errihani, H.; Khattak, A.; De Guzman, R.B.; et al. International research network on COVID-19 impact on cancer care. Impact of the COVID-19 pandemic on cancer care: A global collaborative study. JCO Glob. Oncol. 2020, 6, 1428–1438. [Google Scholar] [CrossRef]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
- Sud, A.; Jones, M.E.; Broggio, J.; Loveday, C.; Torr, B.; Garrett, A.; Nicol, D.L.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020, 31, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Gruber, W.C. C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Bijker, E. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Zaks, T. COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; Del Molino Del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021, 22, 00213–00218. [Google Scholar] [CrossRef]
- Waissengrin, B.; Agbarya, A.; Safadi, E.; Padova, H.; Wolf, I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021, 22, 00155–00158. [Google Scholar] [CrossRef]
- ASCO. COVID-19 Vaccine & Patients with Cancer. 2020. Available online: https://www.asco.org/asco-coronavirusresources/covid-19-patient-care-information/covid-19-vaccine-patients-cancer (accessed on 17 May 2021).
- Garassino, M.C.; Vyas, M.; de Vries, E.G.E.; Kanesvaran, R.; Giuliani, R.; Peters, S.; European Society for Medical Oncology. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann. Oncol. 2021, 32, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Sengupta, R.; Locke, T.; Zaidi, S.K.; Campbell, K.M.; Carethers, J.M.; Jaffee, E.M.; Wherry, E.J.; Soria, J.C.; D’Souza, G. AACR COVID-19 and cancer task force. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov. 2021, 11, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.K.; Zhang, T.; Wang, A.Z.; Li, Z. COVID-19 vaccines for patients with cancer: Benefits likely outweigh risks. J. Hematol. Oncol. 2021, 14, 38. [Google Scholar] [CrossRef]
- Shaunak, N.; Nijjar, R.; Polwart, C.; Enting, D.; Rigg, A.; Harrison, C.; Wrench, D.; McLornan, D.; UK Chemotherapy Board–British Society of Haematology. Clinician FAQs and Guidance on COVID-19 Vaccine for Patients Receiving Systemic Anti-Cancer Therapy Version 3.0. Available online: https://b-s-h.org.uk/media/19241/clinician-faqs-and-guidance-on-covid19-vaccine-for-patients-receiving-sa_pdf (accessed on 17 May 2021).
- Pinato, D.J.; Scotti, L.; Gennari, A.; Colomba-Blameble, E.; Dolly, S.; Loizidou, A.; Chester, J.; Mukherjee, U.; Zambelli, A.; Aguilar-Company, J.; et al. Determinants of enhanced vulnerability to coronavirus disease 2019 in UK patients with cancer: A European study. Eur. J. Cancer 2021, 150, 190–202. [Google Scholar] [CrossRef]
- Moss, C.; Haire, A.; Cahill, F.; Enting, D.; Hughes, S.; Smith, D.; Sawyer, E.; Davies, A.; Zylstra, J.; Haire, K.; et al. Guy’s cancer cohort-real world evidence for cancer pathways. BMC Cancer 2020, 20, 187. [Google Scholar] [CrossRef] [Green Version]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Oxford COVID Vaccine Trial Group. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- CDC. Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html (accessed on 17 May 2021).
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Chapin-Bardales, J.; Gee, J.; Myers, T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA 2021. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.S.; Stuart, A.; Greenland, M.; Liu, X.; Jonathan, S.; Nguyen, V.T.; Snape, M.D. Heterologous prime-boost COVID-19 vaccination: Initial reactogenicity data. Lancet 2021. [Google Scholar] [CrossRef]
- Ariza-Heredia, E.J.; Chemaly, R.F. Practical review of immunizations in adult patients with cancer. Hum. Vaccin. Immunother. 2015, 11, 2606–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortbals, D.W.; Liebhaber, H.; Presant, C.A.; Van Amburg, A.L., III; Lee, J.Y. Influenza immunization of adult patients with malignant diseases. Ann. Intern. Med. 1977, 87, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Weitberg, A.B.; Weitzman, S.A.; Watkins, E.; Hinkle, C.; O’Rourke, S.; Dienstag, J.L. Immunogenicity of hepatitis B vaccine in oncology patients receiving chemotherapy. J. Clin. Oncol. 1985, 3, 718–722. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; Potter, D.M.; Belani, C.P.; Jacobs, S.A.; Gravenstein, S.; Lim, F.; Trump, D.L. Randomized trial of influenza vaccine with granulocyte-macrophage colony-stimulating factor or placebo in cancer patients. J. Clin. Oncol. 2002, 20, 4313–4318. [Google Scholar] [CrossRef]
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, T.C.; Casella, C.R. No pain no gain? Adjuvant effects of alum and monophosphoryl lipid A in pertussis and HPV vaccines. Curr. Opin. Immunol. 2017, 47, 17–25. [Google Scholar] [CrossRef]
- Mackay, I.R.; Goodyear, M.D.; Riglar, C.; Penschow, J.; Whittingham, S.; Russell, I.S.; Kitchen, P.R.; Collins, J.P. Effect on immunologic and other indices of adjuvant cytotoxic chemotherapy including melphalan in breast cancer. Cancer 1984, 53, 2619–2627. [Google Scholar] [CrossRef]
- Verma, R.; Foster, R.E.; Horgan, K.; Mounsey, K.; Nixon, H.; Smalle, N.; Hughes, T.A.; Carter, C.R. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. 2016, 18, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
| Patient Demographics | Total (n = 373) |
|---|---|
| Sex—no. (%) | |
| Male | 140 (37.5) |
| Female | 233 (62.5) |
| Race or ethnic group—no. (%) | |
| White | 253 (67.8) |
| Black | 54 (14.5) |
| Asian | 20 (5.4) |
| Arab | 1 (0.3) |
| Iranian | 2 (0.5) |
| Mixed | 6 (1.6) |
| Unknown * | 37 (10.2) |
| Median age—year. (range) | 56 (19–65) |
| Age groups—no. (%) | |
| 18–54 | 170 (45.6) |
| 55–65 | 203 (54.4) |
| Mean BMI—no. (range) | 28 (15.1–64.6) |
| BMI groups—no. (%) | |
| ≤18.5 (underweight) | 13 (3.5) |
| 18.5–24.9 (normal) | 112 (30.0) |
| 25–29.9 (overweight) | 138 (37.0) |
| 30–34.9 (obese stage I) | 57 (15.3) |
| 35–39.9 (obese stage II) | 29 (7.8) |
| ≥40 (obese stage III) | 18 (4.8) |
| Unknown | 6 (1.6) |
| Median IMD decile—no. (range) | 5 (1–10) |
| Patient Comorbidities | Total (n = 373) |
|---|---|
| Prior COVID-19 infection—no. (%) * | 55 (14.7) |
| Yes | 29 (7.8) |
| Suspected | 26 (7.0) |
| Respiratory disorders—no. (%) | 57 (15.3) |
| Asthma | 33 (8.9) |
| COPD | 20 (5.4) |
| Diabetes—no. (%) | 36 (9.7) |
| Insulin-dependent diabetes | 14 (3.8) |
| Non-insulin dependent diabetes | 22 (5.9) |
| Cardio/Cerebrovascular disease—no. (%) | 110 (29.5) |
| Hypertension | 95 (25.5) |
| Ischaemic heart disease | 12 (3.2) |
| Stroke | 5 (1.3) |
| Autoimmune conditions—no. (%) | 21 (5.63) |
| Inflammatory bowel disease | 6 (1.6) |
| Systemic lupus erythematosus | 3 (0.8) |
| Rheumatoid arthritis | 3 (0.8) |
| Others † | 10 (3.2) |
| Chronic viral infections—no. (%) | 6 (1.6) |
| HIV | 4 (1.1) |
| Hepatitis C | 0 |
| Hepatitis B | 2 (0.5) |
| Other cancers—no. (%) ‡ | 29 (7.8) |
| Breast | 10 (2.7) |
| Prostate | 4 (1.1) |
| Haematological | 4 (1.1) |
| Colorectal | 2 (0.5) |
| Head & Neck | 2 (0.5) |
| Urological | 2 (0.5) |
| Melanoma | 1 (0.3) |
| Endocrine | 1 (0.3) |
| Gynaecological | 1 (0.3) |
| Oncological Characteristics | Total (n = 373) |
|---|---|
| Tumour type (solid)—no. (%) | 354 (94.9) |
| Breast | 127 (34.0) |
| Lung | 50 (13.4) |
| Gynae | 38 (10.2) |
| Colorectal | 38 (10.2) |
| Urological | 34 (9.1) |
| Prostate | 23 (6.2) |
| Melanoma | 20 (5.4) |
| Others * | 24 (6.4) |
| Tumour type (haematological)—no. (%) | 19 (5.1) |
| Myeloma | 8 (2.1) |
| Lymphoma | 9 (2.4) |
| Leukaemia | 2 (0.5) |
| Cancer stage for solid tumours—no. (%) | |
| 1 | 15 (4.0) |
| 2 | 53 (14.2) |
| 3 | 69 (18.5) |
| 4 | 217 (58.2) |
| Current treatment intent—no. (%) | |
| Radical | 101 (27.1) |
| Palliative/Surveillance | 231 (61.9)/31 (8.3) |
| Unknown | 10 (2.7) |
| Current treatment regimen—no. (%) † | |
| Parenteral SACT | |
| Chemotherapy only Chemotherapy + Immunotherapy Chemotherapy + Target therapy Chemotherapy + Hormone therapy Chemotherapy + Target + Hormone Target therapy (anti-EGFR) Target therapy (anti-HER-2) Target therapy (anti-VEGF) Chemo-radiotherapy | 90 (24.1) 11 (2.9) 25 (6.7) 5 (1.3) 3 (0.8) 3 (0.8) 30 (8.0) 4 (1.1) 1 (0.3) |
| Oral SACT (continuous) | |
| Chemotherapy Mtuli-target TKIs (inc. anti-VEGF) PARP inhibitors CKD4/6 inhibitors ALK inhibitors Anti-EGFR (T790M) BRAF/MEK inhibitors mTOR inhibitors RET inhibitors ATR inhibitors | 21 (5.6) 12 (3.2) 9 (2.4) 25 (6.7) 3 (0.8) 2 (0.5) 3 (0.8) 2 (0.5) 2 (0.5) 1 (0.3) |
| Hormone therapy (total) | 88 (23.6) |
| Immunotherapy | |
| Anti-PD-1 or Anti-PD-L1 Combination Anti-PD-1/CTLA-4 | 51 (13.7) 6 (1.6) |
| Haematological SACT | 17 (4.6) ‡ |
| No active treatment | 43 (11.5) |
| Total (n = 373) | within 7 Days Post-Vaccination—No. (%) | after 7 Days Post-Vaccination—No. (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Any Grade | Grade 1 | Grade 2 | Grade 3 | Any Grade | Grade 1 | Grade 2 | Grade 3 | |
| Any adverse event | 278 (74.5) | 243 (65.1) | 27 (7.2) | 8 (2.1) | 6 (1.6) | 4 (1.1) | 1 (0.3) | 1 (0.3) |
| Local adverse events | ||||||||
| Pain at injection site/ Sore arm | 230 (61.7) | 220 (59.0) | 10 (2.7) | 0 | 1 (0.3) | 1 (0.3) | 0 | 0 |
| Erythema | 1 (0.3) | 1 (0.3) | 0 | 0 | 0 | 0 | 0 | 0 |
| Systemic adverse events | ||||||||
| Fever * | 21 (5.6) | 18 (4.8) | 3 (0.8) | 0 | 2 (0.5) | 2 (0.5) | 0 | 0 |
| Fatigue | 68 (18.2) | 41 (11.0) | 23 (6.2) | 4 (1.1) | 2 (0.5) | 1 (0.3) | 0 | 1 (0.3) |
| Headaches | 45 (12.1) | 39 (10.5) | 6 (1.6) | 0 | 2 (0.5) | 2 (0.5) | 0 | 0 |
| Chills | 15 (4.0) | 12 (3.2) | 3 (0.8) | 0 | 0 | 0 | 0 | 0 |
| Arthralgia | 7 (1.9) | 5 (1.3) | 2 (0.5) | 0 | 0 | 0 | 0 | 0 |
| Myalgia | 31 (8.3) | 21 (5.6) | 8 (2.1) | 2 (0.5) | 0 | 0 | 0 | 0 |
| Diarrhoea | 10 (2.7) | 8 (2.1) | 0 | 2 (0.5) | 1 (0.3) | 1 (0.3) | 0 | 0 |
| Nausea/Vomiting | 15 (4.0) | 13 (3.5) | 1 (0.3) | 1 (0.3) | 0 | 0 | 0 | 0 |
| Flu-like symptoms | 18 (4.8) | 16 (4.3) | 1 (0.3) | 1 (0.3) | 1 (0.3) | 1 (0.3) | 0 | 0 |
| Lymphadenopathy | 2 (0.5) | 0 | 2 (0.5) | 0 | 0 | 0 | 0 | 0 |
| Other adverse events | 28 (7.5) | 18 (4.8) | 3 (0.8) | 2 (0.5) | 5 (1.3) | 1 (0.3) | 2 (0.5) | 2 (0.5) |
| Chest pain | 1 (0.3) | 1 (0.3) | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspnoea | 4 (1.1) | 4 (1.1) | 0 | 0 | 0 | 0 | 0 | 0 |
| GORD/Gastritis | 2 (0.5) | 1 (0.3) | 1 (0.3) | 0 | 0 | 0 | 0 | 0 |
| Abdominal pain | 3 (0.8) | 3 (0.8) | 0 | 0 | 0 | 0 | 0 | 0 |
| Sore throat | 3 (0.8) | 3 (0.8) | 0 | 0 | 0 | 0 | 0 | 0 |
| Paraesthesia | 2 (0.5) | 2 (0.5) | 0 | 0 | 0 | 0 | 0 | 0 |
| Hot flushes | 2 (0.5) | 2 (0.5) | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypotension | 1 (0.3) | 0 | 1 (0.3) | 0 | 0 | 0 | 0 | 0 |
| Tumour-pain† | 2 (0.5) | 2 (0.5) | 0 | 0 | 0 | 0 | 0 | 0 |
| Transaminitis | 0 | 0 | 0 | 0 | 2 (0.5) | 0 | 1 (0.3) ‡ | 1 (0.3) § |
| Urosepsis | 1 (0.3) | 0 | 0 | 1 (0.3) ¶ | 0 | 0 | 0 | 0 |
| Anorexia | 2 (0.5) | 1 (0.3) | 1 (0.3) | 0 | 0 | 0 | 0 | 0 |
| Febrile neutropaenia | 1 (0.3) | 0 | 0 | 1 (0.3) # | 0 | 0 | 0 | 0 |
| Cough | 2 (0.5) | 2 (0.5) | 0 | 0 | 0 | 0 | 0 | 0 |
| Dizziness | 1 (0.3) | 1 (0.3) | 0 | 0 | 1 (0.3) | 1 (0.3) | 0 | 0 |
| Euphoria | 1 (0.3) | 1 (0.3) | 0 | 0 | 0 | 0 | 0 | 0 |
| Weak arm | 1 (0.3) | 1 (0.3) | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypocortisolism | 0 | 0 | 0 | 0 | 1 (0.3) | 0 | 1 (0.3) ‡ | 0 |
| VTE | 0 | 0 | 0 | 0 | 1 (0.3) | 0 | 0 | 1 (0.3) ** |
| Pruritus | 1 (0.3) | 1 (0.3) ‡ | 0 | 0 | 0 | 0 | 0 | 0 |
| Risk Factors | Adjusted OR | 95%CI (Lower) | 95%CI (Upper) | p-Value |
|---|---|---|---|---|
| Age ≥55 (ref: age <55) | 0.931 | 0.567 | 1.528 | 0.776 |
| Male (ref: female) | 0.426 | 0.259 | 0.699 | <0.001 * |
| BMI ≥30 (ref: BMI <30) | 0.935 | 0.535 | 1.632 | 0.812 |
| Comorbidities (≥1) (ref: no comorbidities) | 1.192 | 0.706 | 2.010 | 0.511 |
| Prior COVID-19 infection (ref: no prior COVID-19 infection) | 1.025 | 0.503 | 2.089 | 0.946 |
| Metastatic cancer (ref: non-metastatic cancer) | 0.848 | 0.493 | 1.458 | 0.551 |
| Receiving active systemic anti-cancer therapy (ref: not receiving active SACT) | 1.030 | 0.469 | 2.263 | 0.942 |
| Receiving chemotherapy (within 28 days) (ref: not receiving chemotherapy within 28 days) | 0.602 | 0.345 | 1.051 | 0.074 |
| Receiving immunotherapy (within 6 months) (ref: not receiving immunotherapy within 6 months) | 0.495 | 0.256 | 0.958 | 0.037 |
| Pfizer vaccine (ref: receiving non-Pfizer/BioNTech vaccine) | 0.929 | 0.522 | 1.652 | 0.801 |
| Risk Factors | Adjusted OR | 95%CI (Lower) | 95%CI (Upper) | p-Value |
|---|---|---|---|---|
| Age ≥55 (ref: age <55) | 0.481 | 0.237 | 0.974 | 0.042 |
| Male (ref: female) | 0.930 | 0.446 | 1.938 | 0.847 |
| BMI ≥30 (ref: BMI <30) | 0.797 | 0.346 | 1.835 | 0.594 |
| Comorbidities (≥1) (ref: no comorbidities) | 1.120 | 0.535 | 2.343 | 0.763 |
| Prior COVID-19 infection (ref: no prior COVID-19 infection) | 1.518 | 0.607 | 3.795 | 0.372 |
| Metastatic cancer (ref: non-metastatic cancer) | 0.493 | 0.238 | 1.021 | 0.057 |
| Receiving active systemic anti-cancer therapy (ref: not receiving active SACT) | 1.262 | 0.421 | 3.783 | 0.677 |
| Receiving chemotherapy (within 28 days) (ref: not receiving chemotherapy within 28 days) | 0.822 | 0.364 | 1.859 | 0.638 |
| Receiving immunotherapy (within 6 months) (ref: not receiving immunotherapy within 6 months) | 1.492 | 0.568 | 3.916 | 0.417 |
| Pfizer vaccine (ref: receiving non-Pfizer/BioNTech vaccine) | 0.366 | 0.177 | 0.758 | 0.007 |
| Risk Factors | Adjusted OR | 95%CI (Lower) | 95%CI (Upper) | p-Value |
|---|---|---|---|---|
| Age ≥55 (ref: age <55) | 0.803 | 0.521 | 1.240 | 0.323 |
| Male (ref: female) | 0.632 | 0.400 | 0.999 | 0.049 |
| BMI ≥30 (ref: BMI <30) | 1.065 | 0.655 | 1.733 | 0.799 |
| Comorbidities (≥1) (ref: no comorbidities) | 1.003 | 0.635 | 1.583 | 0.990 |
| Prior COVID-19 infection (ref: no prior COVID-19 infection) | 1.691 | 0.903 | 3.166 | 0.101 |
| Metastatic cancer (ref: non-metastatic cancer) | 0.548 | 0.347 | 0.867 | 0.010 |
| Receiving active systemic anti-cancer therapy (ref: not receiving active SACT) | 1.578 | 0.830 | 3.002 | 0.164 |
| Receiving chemotherapy (within 28 days) (ref: not receiving chemotherapy within 28 days) | 0.373 | 0.221 | 0.629 | <0.001 * |
| Receiving immunotherapy (within 6 months) (ref: not receiving immunotherapy within 6 months) | 0.662 | 0.345 | 1.270 | 0.215 |
| Pfizer vaccine (ref: receiving non-Pfizer/BioNTech vaccine) | 0.452 | 0.274 | 0.747 | 0.002 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, A.C.P.; McGrath, H.; Ting, J.; Srikandarajah, K.; Germanou, S.; Moss, C.; Russell, B.; Monroy-Iglesias, M.; Dolly, S.; Irshad, S.; et al. COVID-19 Vaccine Safety in Cancer Patients: A Single Centre Experience. Cancers 2021, 13, 3573. https://doi.org/10.3390/cancers13143573
So ACP, McGrath H, Ting J, Srikandarajah K, Germanou S, Moss C, Russell B, Monroy-Iglesias M, Dolly S, Irshad S, et al. COVID-19 Vaccine Safety in Cancer Patients: A Single Centre Experience. Cancers. 2021; 13(14):3573. https://doi.org/10.3390/cancers13143573
Chicago/Turabian StyleSo, Alfred Chung Pui, Harriet McGrath, Jonathan Ting, Krishnie Srikandarajah, Styliani Germanou, Charlotte Moss, Beth Russell, Maria Monroy-Iglesias, Saoirse Dolly, Sheeba Irshad, and et al. 2021. "COVID-19 Vaccine Safety in Cancer Patients: A Single Centre Experience" Cancers 13, no. 14: 3573. https://doi.org/10.3390/cancers13143573
APA StyleSo, A. C. P., McGrath, H., Ting, J., Srikandarajah, K., Germanou, S., Moss, C., Russell, B., Monroy-Iglesias, M., Dolly, S., Irshad, S., Van Hemelrijck, M., & Enting, D. (2021). COVID-19 Vaccine Safety in Cancer Patients: A Single Centre Experience. Cancers, 13(14), 3573. https://doi.org/10.3390/cancers13143573





