Consistent Major Differences in Sex- and Age-Specific Diagnostic Performance among Nine Faecal Immunochemical Tests Used for Colorectal Cancer Screening
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Selection of Study Participants
2.3. Data/Sample Collection and Processing
2.4. Test Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Population
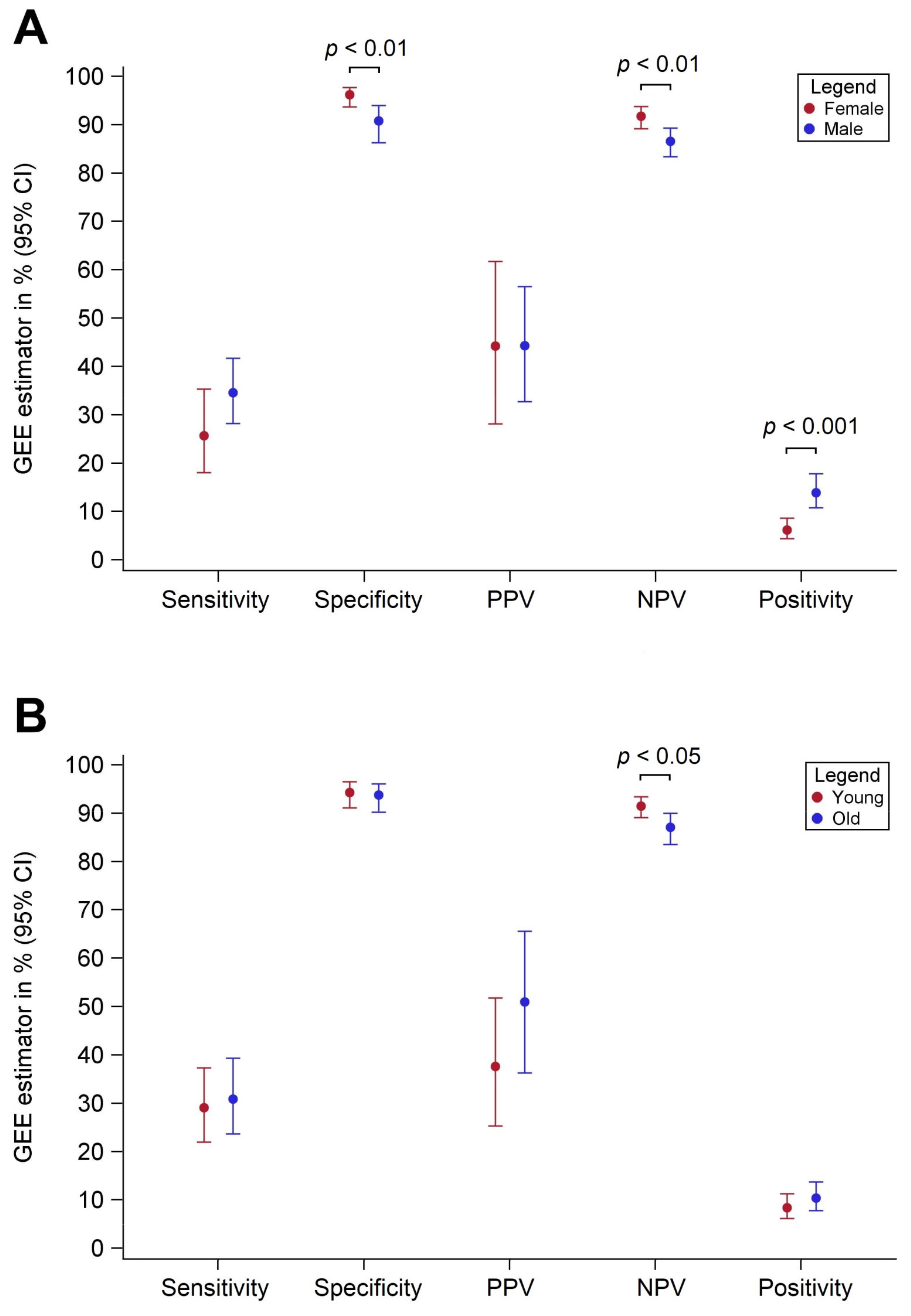
3.2. Diagnostic Performance by Sex
3.3. Diagnostic Performance by Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- European Colorectal Cancer Screening Guidelines Working Group: European guidelines for quality assurance in colorectal cancer screening and diagnosis: Overview and introduction to the full Supplement publication. Endoscocpy 2012, 45, 51–59. [CrossRef] [Green Version]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.-C.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.Y.; Young, G.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Senore, C.; Basu, P.; Anttila, A.; Ponti, A.; Tomatis, M.; Vale, D.B.; Ronco, G.; Soerjomataram, I.; Primic-Žakelj, M.; Riggi, E.; et al. Performance of colorectal cancer screening in the European Union Member States: Data from the second European screening report. Gut 2018, 68, 1232–1244. [Google Scholar] [CrossRef]
- Gies, A.; Bhardwaj, M.; Stock, C.; Schrotz-King, P.; Brenner, H. Quantitative fecal immunochemical tests for colorectal cancer screening. Int. J. Cancer 2018, 143, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Imperiale, T.F.; Gruber, R.N.; Stump, T.E.; Emmett, T.W.; Monahan, P.O. Performance Characteristics of Fecal Immunochemical Tests for Colorectal Cancer and Advanced Adenomatous Polyps: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2019, 170, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Selby, K.; Levine, E.H.; Doan, C.; Gies, A.; Brenner, H.; Quesenberry, C.; Lee, J.K.; Corley, D.A. Effect of Sex, Age, and Positivity Threshold on Fecal Immunochemical Test Accuracy: A Systematic Review and Meta-analysis. Gastroenterology 2019, 157, 1494–1505. [Google Scholar] [CrossRef] [Green Version]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, E.D.; Gatsonis, A.C.; Glasziou, P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; De Vet, H.C.W.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef] [Green Version]
- Fraser, C.G.; Allison, J.E.; Young, G.P.; Halloran, S.P.; Seaman, H.E. Improving the reporting of evaluations of faecal immunochemical tests for haemoglobin. Eur. J. Cancer Prev. 2015, 24, 24–26. [Google Scholar] [CrossRef]
- Gies, A.; Cuk, K.; Schrotz-King, P.; Brenner, H. Direct Comparison of Diagnostic Performance of 9 Quantitative Fecal Immunochemical Tests for Colorectal Cancer Screening. Gastroenterology 2018, 154, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Gies, A.; Cuk, K.; Schrotz-King, P.; Brenner, H. Combination of Different Fecal Immunochemical Tests in Colorectal Cancer Screening: Any Gain in Diagnostic Performance? Cancers 2019, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Hundt, S.; Haug, U.; Brenner, H. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann. Intern. Med. 2009, 150, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Tao, S.; Haug, U. Low-Dose Aspirin Use and Performance of Immunochemical Fecal Occult Blood Tests. JAMA 2010, 304, 2513–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, H.; Tao, S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur. J. Cancer 2013, 49, 3049–3054. [Google Scholar] [CrossRef] [PubMed]
- Gies, A.; Niedermaier, T.; Weigl, K.; Schrotz-King, P.; Hoffmeister, M.; Brenner, H. Effect of long-term frozen storage and thawing of stool samples on faecal haemoglobin concentration and diagnostic performance of faecal immunochemical tests. Clin. Chem. Lab. Med. 2019, 58, 390–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenfield, R.E.; Kochwa, S.; Kaczera, Z.; Maimon, J. Nonuniform Distribution of Occult Blood in Feces. Am. J. Clin. Pathol. 1979, 71, 204–209. [Google Scholar] [CrossRef]
- Fraser, C.G.; Allison, E.J.; Halloran, S.P.; Young, G. A Proposal to Standardize Reporting Units for Fecal Immunochemical Tests for Hemoglobin. J. Natl. Cancer Inst. 2012, 104, 810–814. [Google Scholar] [CrossRef] [Green Version]
- Brenner, H.; Haug, U.; Hundt, S. Sex Differences in Performance of Fecal Occult Blood Testing. Am. J. Gastroenterol. 2010, 105, 2457–2464. [Google Scholar] [CrossRef]
- Bakker, C.A.K.-D.; Jonkers, D.M.; Sanduleanu-Dascalescu, S.; De Bruïne, A.P.; Meijer, G.A.; Janssen, J.B.; Van Engeland, M.; Stockbrügger, R.W.; Masclee, A.A. Test Performance of Immunologic Fecal Occult Blood Testing and Sigmoidoscopy Compared with Primary Colonoscopy Screening for Colorectal Advanced Adenomas. Cancer Prev. Res. 2011, 4, 1563–1571. [Google Scholar] [CrossRef] [Green Version]
- Grobbee, E.J.; Wieten, E.; Hansen, B.E.; Stoop, E.M.; De Wijkerslooth, T.R.; Lansdorp-Vogelaar, I.; Bossuyt, P.M.; Dekker, E.; Kuipers, E.J.; Spaander, M.C.W. Fecal immunochemical test-based colorectal cancer screening: The gender dilemma. United Eur. Gastroenterol. J. 2017, 5, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Brenner, H.; Qian, J.; Werner, S. Variation of diagnostic performance of fecal immunochemical testing for hemoglobin by sex and age: Results from a large screening cohort. Clin. Epidemiol. 2018, 10, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Alonso, L.R.; Moranta, F.R.; Arajol, C.; Gilabert, P.; Serra, K.; Martin-Cardona, A.; Ibáñez-Sanz, G.; Moreno, V.; Guardiola, J. Proton pump inhibitors reduce the accuracy of faecal immunochemical test for detecting advanced colorectal neoplasia in symptomatic patients. PLoS ONE 2018, 13, e0203359. [Google Scholar] [CrossRef]
- Ibáñez-Sanz, G.; Milà, N.; de la Peña-Negro, L.C.; Garcia, M.; Vidal, C.; Rodríguez-Alonso, L.; Binefa, G.; Rodríguez-Moranta, F.; Moreno, V. Proton-pump inhibitors are associated with a high false-positivity rate in faecal immunochemical testing. J. Gastroenterol. 2021, 56, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Chandrapalan, S.; Hee, S.W.; Widlak, M.M.; Farrugia, A.; Alam, M.T.; Smith, S.; Arasaradnam, R.P. Performance of the faecal immunochemical test for the detection of colorectal neoplasms and the role of proton pump inhibitors in their diagnostic accuracy. Colorectal Dis. 2021. [Google Scholar] [CrossRef]
- Arnal, D.M.J.; Garcia Mateo, S.; Hermoso-Duran, S.; Abad, D.; Carrera-Lasfuentes, P.; Velazquez-Campoy, A.; Abian Franco, O.; Lanas, A. False-positive fecal immunochemical test results in colorectal cancer screening and gastrointestinal drug use. Int. J. Colorectal Dis. 2021. [Google Scholar] [CrossRef]
- Brenner, H.; Niedermaier, T.; Chen, H. Strong subsite-specific variation in detecting advanced adenomas by fecal immunochemical testing for hemoglobin. Int. J. Cancer 2017, 140, 2015–2022. [Google Scholar] [CrossRef] [Green Version]
- Niedermaier, T.; Weigl, K.; Hoffmeister, M.; Brenner, H. Diagnostic performance of flexible sigmoidoscopy combined with fecal immunochemical test in colorectal cancer screening: Meta-analysis and modeling. Eur. J. Epidemiol. 2017, 32, 481–493. [Google Scholar] [CrossRef]
- Brenner, H.; Calderazzo, S.; Seufferlein, T.; Ludwig, L.; Dikopoulos, N.; Mangold, J.; Böck, W.; Stolz, T.; Eisenbach, T.; Block, T.; et al. Effect of a Single Aspirin Dose Prior to Fecal Immunochemical Testing on Test Sensitivity for Detecting Advanced Colorectal Neoplasms: A Randomized Clinical Trial. JAMA 2019, 321, 1686–1692. [Google Scholar] [CrossRef]
- Sadik, R.; Abrahamsson, H.; Stotzer, P.-O. Gender Differences in Gut Transit Shown with a Newly Developed Radiological Procedure. Scand. J. Gastroenterol. 2003, 38, 36–42. [Google Scholar] [CrossRef]
- Kim, N.H.; Park, J.H.; Park, D.I.; Sohn, C.I.; Choi, K.; Jung, Y.S. The fecal immunochemical test has high accuracy for detecting advanced colorectal neoplasia before age 50. Dig. Liver Dis. 2017, 49, 557–561. [Google Scholar] [CrossRef]
| Characteristic | n | Col % | FIT Measurements | ||
|---|---|---|---|---|---|
| n | Row % | ||||
| Sex | Women | 806 | 48.4 | ||
| Men | 861 | 51.6 | |||
| Age | 50–64 | 1014 | 60.8 | ||
| 65–79 | 653 | 39.2 | |||
| Advanced Neoplasia | Yes | 230 | 13.8 | 216 * | 93.9 * |
| Colorectal cancer | 16 | 1.0 | 16 * | ||
| Advanced adenoma | 214 | 12.8 | 200 * | ||
| No | 1437 | 86.2 | 300 ** | 20.9 ** | |
| FIT Brand | Sensitivity (%) | Specificity (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Diff. | p | Female | Male | Diff. | p | |
| At original thresholds recommended by the manufacturers | ||||||||
| IDK Hb ELISA | 40.5 | 49.3 | −8.8 | 0.22 | 90.3 | 80.7 | +9.6 | 0.02 |
| QuantOn Hem | 36.5 | 48.6 | −12.1 | 0.09 | 89.0 | 82.1 | +6.9 | 0.09 |
| immoCARE-C | 33.8 | 41.1 | −7.3 | 0.29 | 92.9 | 86.9 | +6.0 | 0.09 |
| CAREprime | 28.4 | 38.0 | −9.6 | 0.16 | 95.5 | 86.9 | +8.6 | 0.01 |
| RIDASCREEN Hb | 32.4 | 43.0 | −10.6 | 0.13 | 94.8 | 86.2 | +8.6 | 0.01 |
| Eurolyser FOB test | 17.6 | 25.4 | −7.8 | 0.20 | 98.7 | 95.2 | +3.5 | 0.09 |
| OC-Sensor | 17.6 | 23.9 | −6.3 | 0.28 | 99.4 | 95.9 | +3.5 | 0.08 |
| QuikRead go iFOBT | 14.9 | 25.4 | −10.5 | 0.08 | 98.7 | 94.5 | +4.2 | 0.06 |
| SENTiFIT-FOB Gold | 17.6 | 23.9 | −6.3 | 0.28 | 98.7 | 93.8 | +4.9 | 0.04 |
| GEE-Model | 25.7 | 34.6 | −8.9 | 0.12 | 96.2 | 90.8 | +5.4 | 0.005 |
| At adjusted thresholds yielding 95% specificity among all study participants | ||||||||
| IDK Hb ELISA | 25.7 | 33.8 | −8.1 | 0.22 | 96.8 | 93.1 | +3.7 | 0.15 |
| QuantOn Hem | 24.3 | 27.5 | −3.2 | 0.62 | 95.5 | 94.5 | +1.0 | 0.69 |
| immoCARE-C | 27.0 | 34.0 | −7.0 | 0.29 | 96.8 | 93.1 | +3.7 | 0.15 |
| CAREprime | 18.9 | 28.2 | −9.3 | 0.14 | 98.1 | 91.7 | +6.4 | 0.02 |
| RIDASCREEN Hb | 25.7 | 36.6 | −10.9 | 0.11 | 96.1 | 93.8 | +2.3 | 0.36 |
| Eurolyser FOB test | 21.6 | 28.2 | −6.6 | 0.30 | 98.1 | 91.7 | +6.4 | 0.02 |
| OC-Sensor | 20.3 | 28.9 | −8.6 | 0.17 | 97.4 | 92.4 | +5.0 | 0.06 |
| QuikRead go iFOBT | 14.9 | 25.4 | −10.5 | 0.08 | 98.7 | 94.5 | +4.2 | 0.06 |
| SENTiFIT-FOB Gold | 23.0 | 28.9 | −5.9 | 0.35 | 98.1 | 91.7 | +6.4 | 0.02 |
| GEE-Model | 22.4 | 30.2 | −7.8 | 0.18 | 97.3 | 93.0 | +4.3 | 0.04 |
| At adjusted thresholds yielding 95% specificity among women and among men, respectively | ||||||||
| IDK Hb ELISA | 28.4 | 24.6 | 3.8 | 0.55 | 94.8 | 95.2 | −0.4 | 0.89 |
| QuantOn Hem | 29.7 | 25.4 | 4.3 | 0.49 | 94.8 | 95.2 | −0.4 | 0.89 |
| immoCARE-C | 32.4 | 23.4 | 9.0 | 0.16 | 94.8 | 95.2 | −0.4 | 0.89 |
| CAREprime | 31.1 | 23.9 | 7.2 | 0.26 | 94.8 | 95.2 | −0.4 | 0.89 |
| RIDASCREEN Hb | 32.4 | 24.6 | 7.8 | 0.22 | 94.8 | 95.2 | −0.4 | 0.89 |
| Eurolyser FOB test | 25.7 | 26.1 | −0.4 | 0.95 | 96.1 | 95.2 | +0.9 | 0.68 |
| OC-Sensor | 27.0 | 26.1 | 0.9 | 0.88 | 94.8 | 95.2 | −0.4 | 0.89 |
| QuikRead go iFOBT | 14.9 | 25.4 | −10.5 | 0.08 | 98.7 | 95.1 | +3.6 | 0.09 |
| SENTiFIT-FOB Gold | 24.3 | 23.9 | 0.4 | 0.95 | 96.8 | 95.2 | +1.6 | 0.48 |
| GEE-Model | 27.3 | 24.8 | 2.5 | 0.66 | 95.6 | 95.2 | +0.4 | 0.82 |
| FIT Brand | PPV (% (95% CI)) | NPV (% (95% CI)) | ||||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Diff. | p | Female | Male | Diff. | p | |
| At original thresholds recommended by the manufacturers | ||||||||
| IDK Hb ELISA | 31.9 | 35.1 | −3.2 | 0.75 | 92.8 | 88.0 | 4.8 | 0.10 |
| QuantOn Hem | 27.4 | 36.7 | −9.3 | 0.37 | 92.3 | 88.1 | 4.2 | 0.16 |
| immoCARE-C | 35.5 | 39.7 | −4.2 | 0.73 | 92.3 | 87.3 | 5.0 | 0.09 |
| CAREprime | 41.6 | 38.6 | 3.0 | 0.83 | 92.0 | 86.7 | 5.3 | 0.08 |
| RIDASCREEN Hb | 41.1 | 40.1 | 1.0 | 0.94 | 92.3 | 87.5 | 4.8 | 0.10 |
| Eurolyser FOB test | 63.9 | 56.9 | 7.0 | 0.75 | 91.1 | 85.6 | 5.5 | 0.06 |
| OC-Sensor | 76.6 | 57.1 | 19.5 | 0.41 | 91.2 | 85.5 | 5.7 | 0.06 |
| QuikRead go iFOBT | 57.9 | 51.3 | 6.6 | 0.77 | 90.9 | 85.5 | 5.4 | 0.08 |
| SENTiFIT-FOB Gold | 63.1 | 47.4 | 15.7 | 0.47 | 91.1 | 85.2 | 5.9 | 0.05 |
| GEE-Model | 44.2 | 44.3 | −0.1 | 1.00 | 91.8 | 86.6 | 5.2 | 0.01 |
| At adjusted thresholds yielding 95% specificity among all study participants | ||||||||
| IDK Hb ELISA | 46.8 | 50.9 | −4.1 | 0.80 | 92.1 | 87.0 | 5.1 | 0.08 |
| QuantOn Hem | 37.1 | 51.8 | −14.7 | 0.36 | 91.8 | 86.2 | 5.6 | 0.05 |
| immoCARE-C | 48.2 | 50.4 | −2.2 | 0.89 | 92.2 | 87.1 | 5.1 | 0.07 |
| CAREprime | 51.8 | 40.7 | 11.1 | 0.56 | 91.5 | 85.9 | 5.6 | 0.05 |
| RIDASCREEN Hb | 42.3 | 55.2 | −12.9 | 0.41 | 92.0 | 87.6 | 4.4 | 0.12 |
| Eurolyser FOB test | 55.3 | 41.1 | 14.2 | 0.44 | 91.8 | 85.9 | 5.9 | 0.04 |
| OC-Sensor | 46.4 | 44.3 | 2.1 | 0.91 | 91.6 | 86.1 | 5.5 | 0.06 |
| QuikRead go iFOBT | 55.8 | 48.1 | 7.7 | 0.73 | 91.2 | 85.8 | 5.4 | 0.06 |
| SENTiFIT-FOB Gold | 56.9 | 41.3 | 15.6 | 0.39 | 91.9 | 86.0 | 5.9 | 0.04 |
| GEE-Model | 47.6 | 46.9 | 0.7 | 0.96 | 91.8 | 86.4 | 5.4 | 0.005 |
| At adjusted thresholds yielding 95% specificity among women and among men, respectively | ||||||||
| IDK Hb ELISA | 37.8 | 50.9 | −13.1 | 0.41 | 92.2 | 85.7 | 6.5 | 0.03 |
| QuantOn Hem | 38.8 | 52.8 | −14.0 | 0.37 | 92.3 | 85.9 | 6.4 | 0.03 |
| immoCARE-C | 41.0 | 49.4 | −8.4 | 0.59 | 92.6 | 85.6 | 7.0 | 0.02 |
| CAREprime | 41.0 | 50.2 | −9.2 | 0.52 | 92.5 | 85.6 | 6.9 | 0.02 |
| RIDASCREEN Hb | 41.1 | 50.9 | −9.8 | 0.53 | 92.6 | 85.7 | 6.9 | 0.02 |
| Eurolyser FOB test | 42.4 | 51.7 | −8.3 | 0.58 | 92.0 | 86.0 | 6.0 | 0.04 |
| OC-Sensor | 36.6 | 51.7 | −15.1 | 0.34 | 92.1 | 86.0 | 6.1 | 0.04 |
| QuikRead go iFOBT | 55.8 | 51.6 | 4.2 | 0.85 | 91.2 | 85.9 | 5.3 | 0.07 |
| SENTiFIT-FOB Gold | 45.6 | 49.0 | −3.4 | 0.84 | 91.9 | 85.6 | 6.3 | 0.03 |
| GEE-Model | 40.9 | 50.9 | −10.0 | 0.44 | 92.1 | 85.8 | 6.3 | 0.001 |
| FIT Brand | Sensitivity (% (95% CI)) | Specificity (% (95% CI)) | ||||||
|---|---|---|---|---|---|---|---|---|
| 50–64 Years | 65–79 Years | Diff. | p | 50–64 Years | 65–79 Years | Diff. | p | |
| At original thresholds recommended by the manufacturers | ||||||||
| IDK Hb ELISA | 44.0 | 48.6 | −4.6 | 0.50 | 88.4 | 81.5 | 6.9 | 0.10 |
| QuantOn Hem | 43.1 | 45.8 | −2.7 | 0.69 | 86.2 | 84.9 | 1.3 | 0.75 |
| immoCARE-C | 37.0 | 40.2 | −3.2 | 0.64 | 90.6 | 89.1 | 1.5 | 0.67 |
| CAREprime | 32.1 | 37.4 | −5.3 | 0.42 | 91.2 | 91.6 | −0.4 | 0.90 |
| RIDASCREEN Hb | 35.8 | 43.0 | −7.2 | 0.28 | 93.4 | 86.6 | 6.8 | 0.05 |
| Eurolyser FOB test | 21.1 | 24.3 | −3.2 | 0.58 | 96.1 | 98.3 | −2.2 | 0.29 |
| OC-Sensor | 21.1 | 22.4 | −1.3 | 0.81 | 97.2 | 98.3 | −1.1 | 0.55 |
| QuikRead go iFOBT | 22.0 | 21.5 | 0.5 | 0.93 | 96.1 | 97.5 | −1.4 | 0.53 |
| SENTiFIT-FOB Gold | 22.9 | 20.6 | 2.3 | 0.67 | 95.6 | 97.5 | −1.9 | 0.40 |
| GEE-Model | 29.1 | 30.9 | −1.8 | 0.73 | 94.3 | 93.8 | 0.5 | 0.76 |
| At adjusted thresholds yielding 95% specificity among all study participants | ||||||||
| IDK Hb ELISA | 28.4 | 33.6 | −5.2 | 0.41 | 95.6 | 94.1 | 1.5 | 0.57 |
| QuantOn Hem | 24.8 | 28.0 | −3.2 | 0.59 | 95.6 | 94.1 | 1.5 | 0.57 |
| immoCARE-C | 28.7 | 34.6 | −5.9 | 0.35 | 95.6 | 94.1 | 1.5 | 0.57 |
| CAREprime | 22.9 | 27.1 | −4.2 | 0.48 | 94.5 | 95.8 | −1.3 | 0.61 |
| RIDASCREEN Hb | 30.3 | 35.5 | −5.2 | 0.41 | 95.6 | 94.1 | 1.5 | 0.57 |
| Eurolyser FOB test | 23.9 | 28.0 | −4.1 | 0.48 | 95.0 | 95.0 | 0.0 | 0.98 |
| OC-Sensor | 25.7 | 26.2 | −0.5 | 0.94 | 96.1 | 93.3 | 2.8 | 0.27 |
| QuikRead go iFOBT | 22.0 | 21.5 | 0.5 | 0.93 | 96.1 | 97.5 | −1.4 | 0.53 |
| SENTiFIT-FOB Gold | 23.9 | 29.9 | −6.0 | 0.32 | 94.5 | 95.8 | −1.3 | 0.61 |
| GEE-Model | 25.6 | 29.4 | −3.8 | 0.49 | 95.4 | 94.9 | 0.5 | 0.80 |
| At adjusted thresholds yielding 95% specificity among younger and older participants, respectively | ||||||||
| IDK Hb ELISA | 30.3 | 21.5 | 8.8 | 0.14 | 95.0 | 95.0 | 0.0 | 0.98 |
| QuantOn Hem | 25.7 | 27.1 | −1.4 | 0.81 | 95.0 | 95.0 | 0.0 | 0.98 |
| immoCARE-C | 29.6 | 28.0 | 1.6 | 0.80 | 95.0 | 95.0 | 0.0 | 0.98 |
| CAREprime | 22.9 | 28.0 | −5.1 | 0.39 | 95.0 | 95.0 | 0.0 | 0.98 |
| RIDASCREEN Hb | 31.2 | 22.4 | 8.8 | 0.15 | 95.0 | 95.0 | 0.0 | 0.98 |
| Eurolyser FOB test | 23.9 | 33.6 | 9.7 | 0.11 | 95.0 | 95.0 | 0.0 | 0.98 |
| OC-Sensor | 27.5 | 23.4 | 4.1 | 0.48 | 95.0 | 95.0 | 0.0 | 0.98 |
| QuikRead go iFOBT | 22.0 | 21.5 | 0.5 | 0.93 | 96.1 | 97.5 | −1.4 | 0.53 |
| SENTiFIT-FOB Gold | 23.9 | 34.6 | −10.7 | 0.08 | 95.0 | 95.0 | 0.0 | 0.98 |
| GEE-Model | 26.3 | 26.7 | −0.4 | 0.95 | 95.2 | 95.2 | 0.0 | 0.97 |
| FIT Brand | PPV (% (95% CI)) | NPV (% (95% CI)) | ||||||
|---|---|---|---|---|---|---|---|---|
| 50–64 Years | 65–79 Years | Diff. | p | 50–64 Years | 65–79 Years | Diff. | p | |
| At original thresholds recommended by the manufacturers | ||||||||
| IDK Hb ELISA | 31.7 | 35.4 | −3.7 | 0.70 | 92.6 | 88.4 | 4.2 | 0.15 |
| QuantOn Hem | 26.7 | 37.5 | −10.8 | 0.26 | 92.2 | 88.2 | 4.0 | 0.17 |
| immoCARE-C | 32.3 | 43.1 | −10.8 | 0.35 | 92.0 | 87.8 | 4.2 | 0.14 |
| CAREprime | 31.5 | 49.4 | −17.9 | 0.15 | 91.4 | 87.6 | 3.8 | 0.20 |
| RIDASCREEN Hb | 40.3 | 40.9 | −0.6 | 0.96 | 92.0 | 87.9 | 4.1 | 0.15 |
| Eurolyser FOB test | 41.8 | 76.5 | −34.7 | 0.07 | 90.6 | 86.3 | 4.3 | 0.14 |
| OC-Sensor | 54.7 | 78.3 | −23.6 | 0.21 | 90.8 | 86.1 | 4.7 | 0.11 |
| QuikRead go iFOBT | 42.8 | 65.9 | −23.1 | 0.22 | 90.7 | 85.7 | 5.0 | 0.09 |
| SENTiFIT-FOB Gold | 42.9 | 67.2 | −24.3 | 0.19 | 90.8 | 85.7 | 5.1 | 0.08 |
| GEE-Model | 37.6 | 51.0 | −13.4 | 0.16 | 91.5 | 87.1 | 4.4 | 0.02 |
| At adjusted thresholds yielding 95% specificity among all study participants | ||||||||
| IDK Hb ELISA | 44.4 | 55.0 | −10.6 | 0.48 | 91.2 | 86.9 | 4.3 | 0.13 |
| QuantOn Hem | 41.8 | 50.4 | −8.6 | 0.58 | 90.8 | 85.9 | 4.9 | 0.10 |
| immoCARE-C | 44.1 | 55.5 | −11.4 | 0.45 | 91.3 | 87.0 | 4.3 | 0.14 |
| CAREprime | 33.3 | 57.7 | −24.4 | 0.13 | 90.5 | 85.9 | 4.6 | 0.13 |
| RIDASCREEN Hb | 46.0 | 56.1 | −10.1 | 0.50 | 91.4 | 87.2 | 4.2 | 0.14 |
| Eurolyser FOB test | 36.6 | 54.2 | −17.6 | 0.26 | 90.7 | 86.0 | 4.7 | 0.12 |
| OC-Sensor | 44.6 | 45.2 | −0.6 | 0.97 | 91.0 | 85.5 | 5.5 | 0.07 |
| QuikRead go iFOBT | 40.8 | 64.3 | −23.5 | 0.21 | 90.6 | 85.3 | 5.3 | 0.08 |
| SENTiFIT-FOB Gold | 34.2 | 60.2 | −26 | 0.10 | 90.6 | 86.4 | 4.2 | 0.16 |
| GEE-Model | 40.5 | 54.9 | −14.4 | 0.25 | 90.9 | 86.2 | 4.7 | 0.02 |
| At adjusted thresholds yielding 95% specificity among younger and older participants, respectively | ||||||||
| IDK Hb ELISA | 43.0 | 54.3 | −11.3 | 0.26 | 91.4 | 84.9 | 6.5 | 0.03 |
| QuantOn Hem | 39.6 | 53.3 | −13.7 | 0.38 | 90.9 | 85.9 | 5.0 | 0.09 |
| immoCARE-C | 41.9 | 53.9 | −12.0 | 0.44 | 91.4 | 86.0 | 5.4 | 0.07 |
| CAREprime | 35.7 | 54.1 | −18.4 | 0.24 | 90.6 | 86.0 | 4.6 | 0.13 |
| RIDASCREEN Hb | 43.7 | 48.6 | −4.9 | 0.76 | 91.5 | 85.1 | 6.4 | 0.03 |
| Eurolyser FOB test | 36.6 | 58.7 | −22.1 | 0.15 | 90.7 | 87.0 | 3.7 | 0.21 |
| OC-Sensor | 40.7 | 49.5 | −8.8 | 0.58 | 91.1 | 85..2 | 5.9 | 0.05 |
| QuikRead go iFOBT | 40.8 | 64.3 | −23.5 | 0.21 | 90.6 | 85.3 | 5.3 | 0.08 |
| SENTiFIT-FOB Gold | 36.6 | 59.4 | −22.8 | 0.14 | 90.7 | 87.1 | 3.6 | 0.23 |
| GEE-Model | 39.9 | 54.3 | −14.4 | 0.26 | 91.0 | 85.8 | 5.2 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gies, A.; Niedermaier, T.; Alwers, E.; Hielscher, T.; Weigl, K.; Heisser, T.; Schrotz-King, P.; Hoffmeister, M.; Brenner, H. Consistent Major Differences in Sex- and Age-Specific Diagnostic Performance among Nine Faecal Immunochemical Tests Used for Colorectal Cancer Screening. Cancers 2021, 13, 3574. https://doi.org/10.3390/cancers13143574
Gies A, Niedermaier T, Alwers E, Hielscher T, Weigl K, Heisser T, Schrotz-King P, Hoffmeister M, Brenner H. Consistent Major Differences in Sex- and Age-Specific Diagnostic Performance among Nine Faecal Immunochemical Tests Used for Colorectal Cancer Screening. Cancers. 2021; 13(14):3574. https://doi.org/10.3390/cancers13143574
Chicago/Turabian StyleGies, Anton, Tobias Niedermaier, Elizabeth Alwers, Thomas Hielscher, Korbinian Weigl, Thomas Heisser, Petra Schrotz-King, Michael Hoffmeister, and Hermann Brenner. 2021. "Consistent Major Differences in Sex- and Age-Specific Diagnostic Performance among Nine Faecal Immunochemical Tests Used for Colorectal Cancer Screening" Cancers 13, no. 14: 3574. https://doi.org/10.3390/cancers13143574
APA StyleGies, A., Niedermaier, T., Alwers, E., Hielscher, T., Weigl, K., Heisser, T., Schrotz-King, P., Hoffmeister, M., & Brenner, H. (2021). Consistent Major Differences in Sex- and Age-Specific Diagnostic Performance among Nine Faecal Immunochemical Tests Used for Colorectal Cancer Screening. Cancers, 13(14), 3574. https://doi.org/10.3390/cancers13143574






