Cemiplimab for Locally Advanced and Metastatic Cutaneous Squamous-Cell Carcinomas: Real-Life Experience from the French CAREPI Study Group
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients
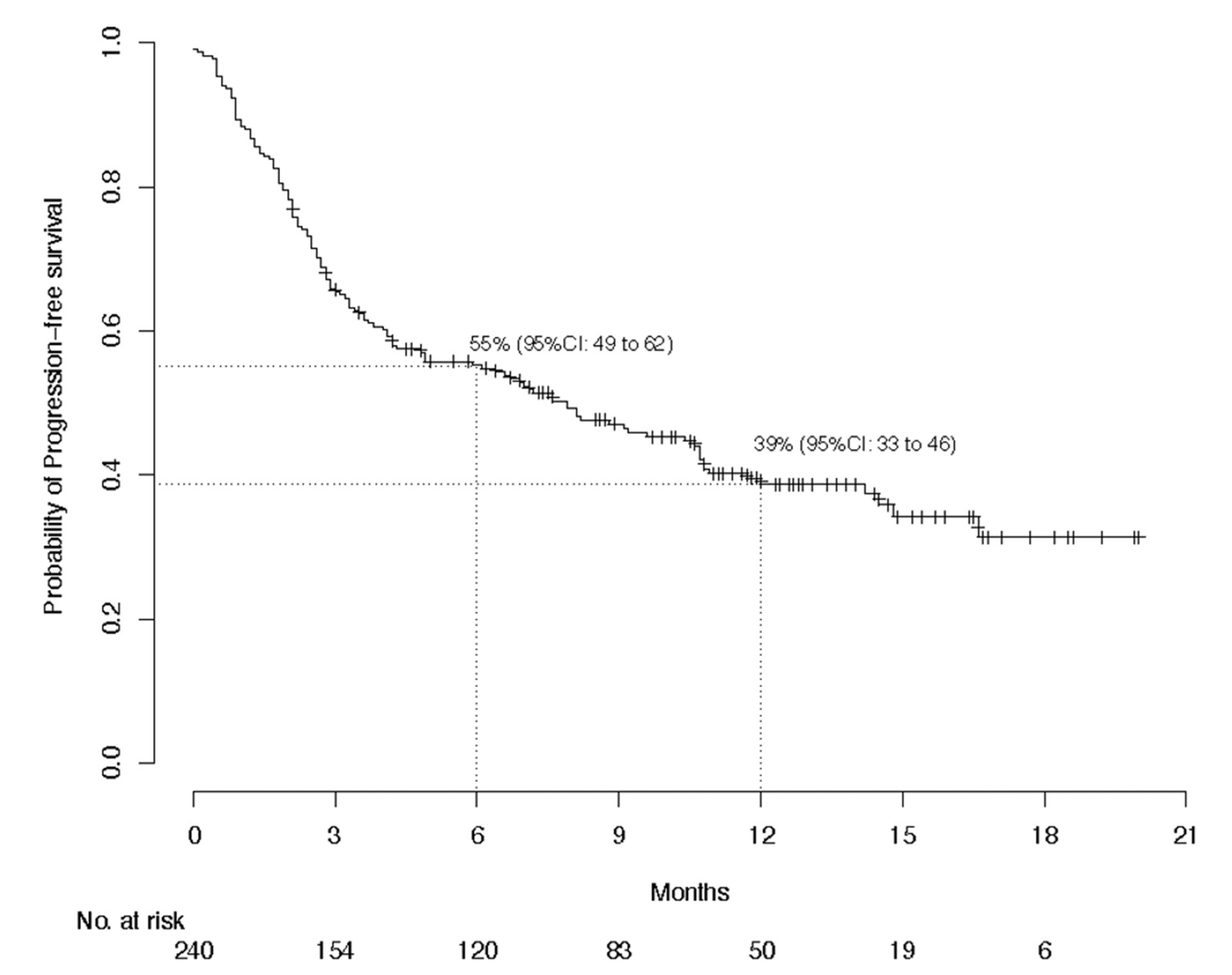
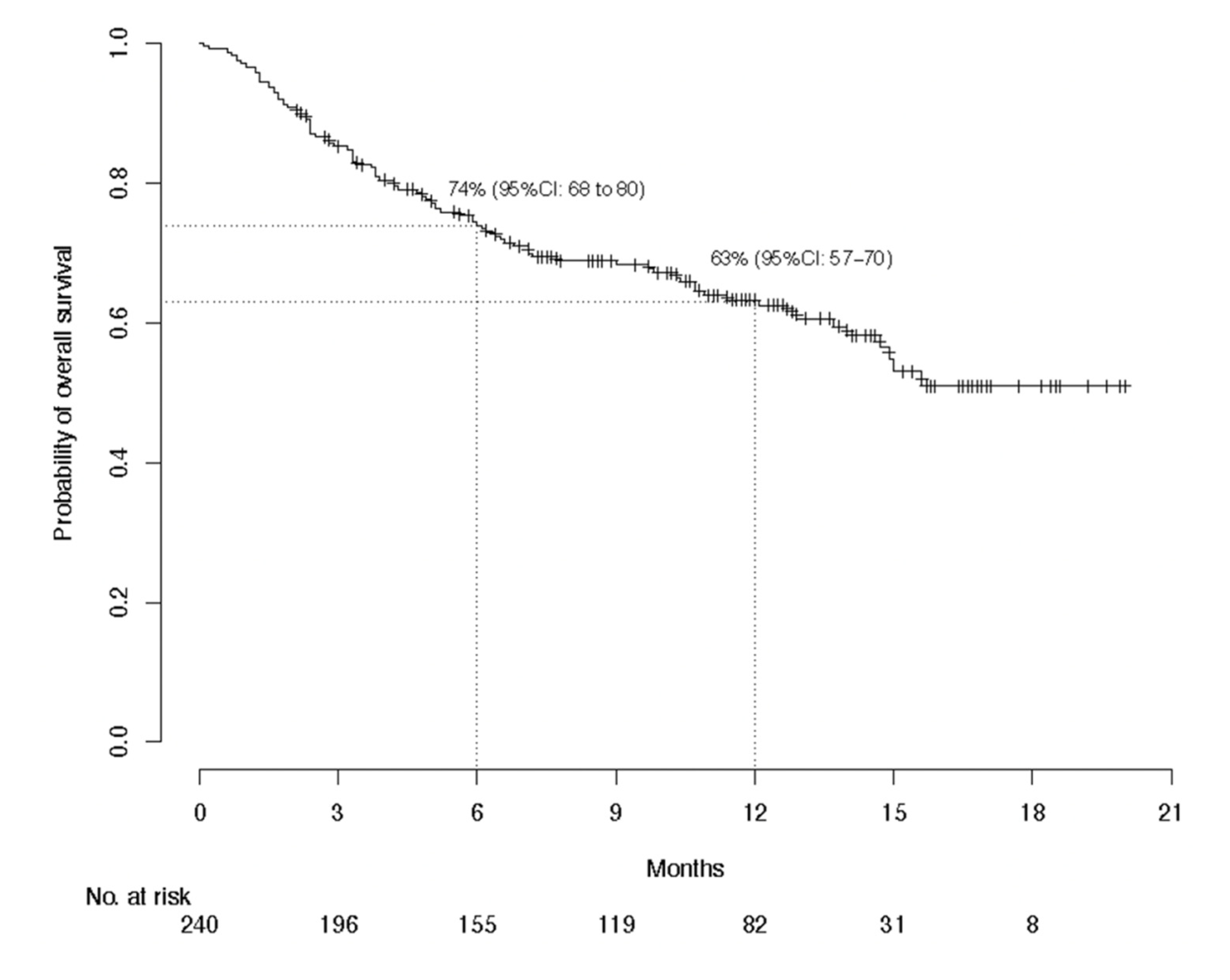
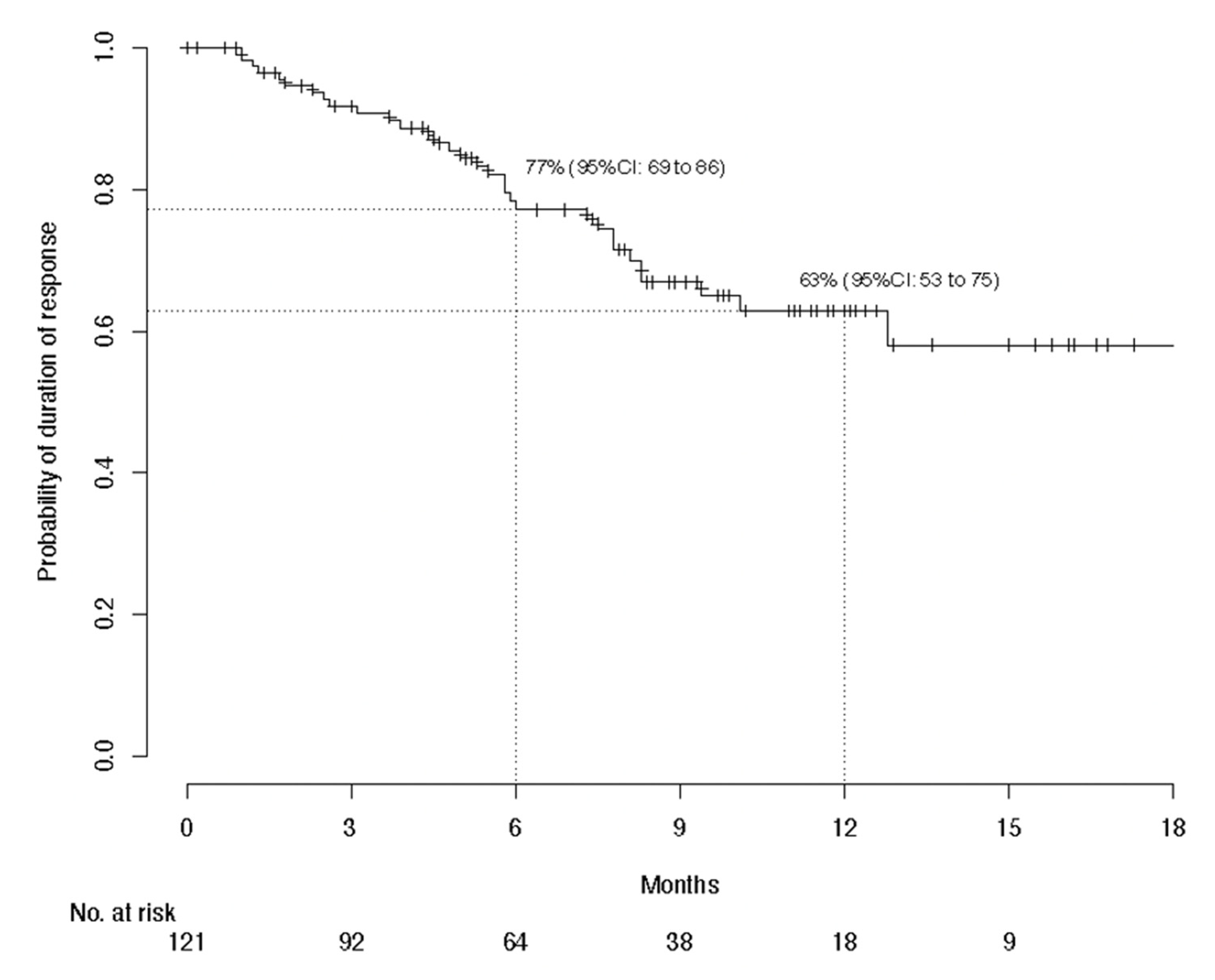
3.2. Efficacy Evaluation
3.3. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dréno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. Eur. J. Cancer 2020, 128, 60–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maubec, E. Update of the management of cutaneous squamous-cell carcinoma. Acta Dermatol. Venereol. 2020, 100, adv00143. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.B.; Ingvar, C.; Hartman, L.W.; Olsson, H.; Nielsen, K. Sunbed use increases cutaneous squamous cell carcinoma risk in women: A large-scale, prospective study in Sweden. Acta Derm. Venereol. 2019, 99, 878–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, P.; Hansen, S.; Møller, B.; Leivestad, T.; Pfeffer, P.; Geiran, O.; Fauchald, P.; Simonsen, S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J. Am. Acad. Dermatol. 1999, 40, 177–186. [Google Scholar] [CrossRef]
- Garrett, G.L.; Blanc, P.D.; Boscardin, J.; Lloyd, A.A.; Ahmed, R.L.; Anthony, T.; Bibee, K.; Breithaupt, A.; Cannon, J.; Chen, A.; et al. Incidence of and risk factors for skin cancer in organ transplant recipients in the United States. JAMA Dermatol. 2017, 153, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.M.H.; Aagnes, B.; Holdaas, H.; Gude, E.; Boberg, K.M.; Bjørtuft, Ø.; Helsing, P.; Leivestad, T.; Møller, B.; Gjersvik, P. Long-term change in the risk of skin cancer after organ transplantation: A population-based nationwide cohort study. JAMA Dermatol. 2017, 153, 1270–1277. [Google Scholar] [CrossRef] [Green Version]
- Lindelöf, B.; Sigurgeirsson, B.; Gäbel, H.; Stern, R.S. Incidence of skin cancer in 5356 patients following organ transplantation. Br. J. Dermatol. 2000, 143, 513–519. [Google Scholar]
- Grulich, A.E.; van Leeuwen, M.T.; Falster, M.O.; Vajdic, C.M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 2007, 370, 59–67. [Google Scholar] [CrossRef]
- Faust, H.; Andersson, K.; Luostarinen, T.; Gislefoss, R.E.; Dillner, J. Cutaneous human papillomaviruses and squamous cell carcinoma of the skin: Nested case-control study. Cancer Epidemiol. Biomark. Prev. 2016, 25, 721–724. [Google Scholar] [CrossRef] [Green Version]
- Torchia, D.; Massi, D.; Caproni, M.; Fabbri, P. Multiple cutaneous precanceroses and carcinomas from combined iatrogenic/professional exposure to arsenic. Int. J. Dermatol. 2008, 47, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Reed, W.B.; College, J., Jr.; Francis, M.J.O.; Zachariae, H.; Mohs, F.; Sher, M.A.; Sneddon, I.B. Epidermolysis bullosa dystrophica with epidermal neoplasms. Arch. Dermatol. 1974, 110, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E. An inherited form of large bowel cancer: Muir’s syndrome. Cancer 1980, 45, 1103–1107. [Google Scholar] [CrossRef]
- King, R.A.; Creel, D.; Cervenka, J.; Okoro, A.N.; Witkop, C.J. Albinism in Nigeria with delineation of new recessive oculocutaneous type. Clin. Genet. 1980, 17, 259–270. [Google Scholar] [CrossRef]
- Kraemer, K.H.; Lee, M.M.; Scotto, J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch. Dermatol. 1987, 123, 241–250. [Google Scholar] [CrossRef]
- Karia, P.S.; Jambusaria-Pahlajani, A.; Harrington, D.P.; Murphy, G.F.; Qureshi, A.A.; Schmults, C.D. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J. Clin. Oncol. 2014, 32, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Leibovitch, I.; Huilgol, S.C.; Selva, D.; Hill, D.; Richards, S.; Paver, R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia, I. Experience over 10 years. J. Am. Acad. Dermatol. 2005, 53, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Varra, V.; Woody, N.M.; Reddy, C.; Joshi, N.P.; Geiger, J.; Adelstein, D.J.; Burkey, B.B.; Scharpf, J.; Prendes, B.; Lamarre, E.D.; et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018, 38, 5825–5830. [Google Scholar] [CrossRef]
- Veness, M.J.; Morgan, G.J.; Palme, C.E.; Gebski, V. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: Combined treatment should be considered best practice. Laryngoscope 2005, 115, 870–875. [Google Scholar] [CrossRef]
- Veness, M.J.; Palme, C.E.; Morgan, G.J. High-risk cutaneous squamous cell carcinoma of the head and neck: Results from 266 treated patients with metastatic lymph node disease. Cancer 2006, 106, 2389–2396. [Google Scholar] [CrossRef] [Green Version]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Osterlind, A.; Hjalgrim, H.; Kulinsky, B.; Frentz, G. Skin cancer as a cause of death in Denmark. Br. J. Dermatol. 1991, 125, 580–582. [Google Scholar] [CrossRef]
- Karia, P.S.; Han, J.; Schmults, D. Cutaneous squamous cell carcinoma: Estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J. Am. Acad. Dermatol. 2013, 68, 957–966. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Grob, J.J.; Gonzalez, R.; Basset-Seguin, N.; Vornicova, O.; Schachter, J.; Joshi, A.; Meyer, N.; Grange, F.; Piulats, J.M.; Bauman, J.R.; et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: A single-arm phase II trial (KEYNOTE-629). J. Clin. Oncol. 2020, 38, 2916–2925. [Google Scholar] [CrossRef]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef]
- Maubec, E.; Boubaya, M.; Petrow, P.; Beylot-Barry, M.; Basset-Seguin, N.; Deschamps, L.; Grob, J.J.; Dréno, B.; Scheer-Senyarich, I.; Bloch-Queyrat, C.; et al. Phase II study of pembrolizumab as first-line, single-drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J. Clin. Oncol. 2020, 38, 3051–3061. [Google Scholar] [CrossRef]
- Rischin, D.; Khushalani, N.I.; Schmults, C.D.; Guminski, A.D.; Chang, A.L.; Lewis, K.D.; Lim, A.M.; Hernandez-Aya, L.F.; Hughes, B.G.M.; Schadendorf, D.; et al. Phase II study of cemiplimab in patients (pts) with advanced cutaneous squamous cell carcinoma (CSCC): Longer follow-up. J. Clin. Oncol. 2020, 38, 10018. [Google Scholar] [CrossRef]
- Rischin, D.; Migden, M.R.; Lim, A.M.; Schmults, C.D.; Khushalani, N.I.; Hughes, B.G.M.; Schadendorf, D.; Dunn, L.A.; Hernandez-Aya, L.; Chang, A.L.S.; et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: Primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J. Immunother. Cancer 2020, 8, e000775. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Ortuno, S.; Lebrun-Vignes, B.; Johnson, D.B.; Moslehi, J.J.; Hertig, A.; Salem, J.E. Transplant rejections associated with immune checkpoint inhibitors: A pharmacovigilance study and systematic literature review. Eur. J. Cancer 2021, 148, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Jansen, Y.J.L.; Rozeman, E.A.; Mason, R.; Goldinger, S.M.; Geukes Foppen, M.H.; Hoejberg, L.; Schmidt, H.; van Thienen, J.V.; Haanen, J.B.A.G.; Tiainen, L.; et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: Clinical outcomes in advanced melanoma. Ann. Oncol. 2019, 30, 1154–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libtayo 350 mg Concentrate Solution for Infusion—Summary of Product Characteristics (SmPC)—(emc) [Internet]. Available online: https://www.medicines.org.uk/emc/product/10438 (accessed on 31 March 2021).
- Dello Russo, C.; Cappoli, N.; Navarra, P. A comparison between the assessments of progression-free survival by local investigators versus blinded independent central reviews in phase III oncology trials. Eur. J. Clin. Pharmacol. 2020, 76, 1083–1092. [Google Scholar] [CrossRef]
- Matsubara, T.; Seto, T.; Takamori, S.; Fujishita, T.; Toyozawa, R.; Ito, K.; Yamaguchi, M.; Okamoto, T. Anti-PD-1 monotherapy for advanced NSCLC patients with older age or those with poor performance status. Oncol. Targets Ther. 2021, 14, 1961–1968. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef]
- Nayar, N.; Briscoe, K.; Penas, P.F. Toxic epidermal necrolysis-like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J. Immunother. 2016, 39, 149–152. [Google Scholar] [CrossRef]
- Saw, S.; Lee, H.Y.; Ng, Q.S. Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur. J. Cancer 2017, 81, 237–239. [Google Scholar] [CrossRef]
- Demirtas, S.; El Aridi, L.; Acquitter, M.; Fleuret, C.; Plantin, P. Toxic epidermal necrolysis due to anti-PD1 treatment with fatal outcome. Ann. Dermatol. Venereol. 2017, 144, 65–66. [Google Scholar] [CrossRef]
- Vivar, K.L.; Deschaine, M.; Messina, J. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J. Cutan. Pathol. 2017, 44, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Rouyer, L.; Bursztejn, A.C.; Charbit, L.; Schmutz, J.L.; Moawad, S. Stevens-Johnson syndrome associated with radiation recall dermatitis in a patient treated with nivolumab. Eur. J. Dermatol. 2018, 28, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.M.; Rancour, E.A.; Al-Omari, A.; Rahnama-Moghadam, S. Striking enhancement at the site of radiation for nivolumab-induced Stevens-Johnson syndrome. Dermatol. Online J. 2018, 24. [Google Scholar] [CrossRef]
- Haratake, N.; Tagawa, T.; Hirai, F.; Toyokawa, G.; Miyazaki, R.; Maehara, Y. Stevens-Johnson syndrome induced by pembrolizumab in a lung cancer patient. J. Thorac. Oncol. 2018, 13, 1798–1799. [Google Scholar] [CrossRef] [Green Version]
- Chirasuthat, P.; Chayavichitsilp, P. Atezolizumab-induced Stevens-Johnson syndrome in a patient with non-small cell lung carcinoma. Case Rep. Dermatol. 2018, 10, 198–202. [Google Scholar] [CrossRef]
- Griffin, L.L.; Cove-Smith, L.; Alachkar, H.; Radford, J.A.; Brooke, R.; Linton, K.M. Toxic epidermal necrolysis (TEN) associated with the use of nivolumab (PD-1 inhibitor) for lymphoma. JAAD Case Rep. 2018, 4, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Salati, M.; Pifferi, M.; Baldessari, C.; Bertolini, F.; Tomasello, C.; Cascinu, S.; Barbieri, F. Stevens-Johnson syndrome during nivolumab treatment of NSCLC. Ann. Oncol. 2018, 29, 283–284. [Google Scholar] [CrossRef]
- Hwang, A.; Iskandar, A.; Dasanu, C.A. Stevens-Johnson syndrome manifesting late in the course of pembrolizumab therapy. J. Oncol. Pharm. Pract. 2019, 25, 1520–1522. [Google Scholar] [CrossRef]
- Dasanu, C.A. Late-onset Stevens-Johnson syndrome due to nivolumab use for hepatocellular carcinoma. J. Oncol. Pharm. Pract. 2019, 25, 2052–2055. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.J.; Soria, A.; Machiels, J.P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Cai, Z.R.; Lecours, J.; Adam, J.P. Toxic epidermal necrolysis associated with pembrolizumab. J. Oncol. Pharm. Pract. 2020, 26, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Keerty, D.; Koverzhenko, V.; Belinc, D.; LaPorta, K.; Haynes, E. Immune-mediated toxic epidermal necrolysis. Cureus 2020, 12, e9587. [Google Scholar] [CrossRef]
- Cassaday, R.D.; Garcia, K.A.; Fromm, J.R. Phase 2 study of pembrolizumab for measurable residual disease in adults with acute lymphoblastic leukemia. Blood Adv. 2020, 4, 3239–3245. [Google Scholar] [CrossRef]
- Riano, I.; Cristancho, C.; Treadwell, T. Stevens-Johnson syndrome-like reaction after exposure to pembrolizumab and recombinant zoster vaccine in a patient with metastatic lung cancer. J. Investig. Med. High Impact Case Rep. 2020, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maloney, N.J.; Ravi, V.; Cheng, K.; Bach, D.Q.; Worswick, S. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: A systematic review. Int. J. Dermatol. 2020, 59, e183–e188. [Google Scholar] [CrossRef]
- Dodiuk-Gad, R.P.; Chung, W.H.; Valeyrie-Allanore, L.; Shear, N.H. Stevens-Johnson syndrome and toxic epidermal necrolysis: An update. Am. J. Clin. Dermatol. 2015, 16, 475–493. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
| Characteristic | Value |
|---|---|
| Age, years | 77.1 ± 13.3 |
| Male sex | 178 (73) |
| ECOG performance status | |
| 0 | 60 (25) |
| 1 | 118 (48) |
| ≥2 | 66 (27) |
| Unknown | 1 (0.4) |
| Immunocompromised | 59 (24) |
| Human immunodeficiency virus-positive | 8 (3) |
| Organ transplant | 7 (3) |
| Chronic lymphocytic leukemia | 20 (8) |
| Other blood disorders a | 18 (7) |
| Immunosuppressive drugs | 6 (3) |
| Genodermatosis | 8 (3) |
| Inherited epidermolysis bullosa | 2 (0.8) |
| Muir–Torre syndrome | 2 (0.8) |
| Xeroderma pigmentosum | 1 (0.4) |
| Ichthyosis | 2 (0.8) |
| Epidermodysplasia verruciformis | 1 (0.4) |
| Chronic dermatitis | 28 (11) |
| Burns | 4 (1.6) |
| Scars | 2 (0.8) |
| Lichen planus | 2 (0.8) |
| Chronic wounds | 9 (4) |
| Warts/condylomas | 4 (1.6) |
| Arsenic keratosis | 2 (0.8) |
| Radiodermatitis | 3 (1.2) |
| Others b | 2 (0.8) |
| ≥3 primary CSCCs | 80 (33) |
| Primary CSCC site | |
| Head-and-neck c | 164 (70) |
| Trunk | 9 (4) |
| Anorectal and/or genital | 12 (5) |
| Arm or leg | 58 (24) |
| Unknown | 3 (1.2) |
| Histopathological characteristics | |
| Poor differentiation | 57 (23) |
| Perineural invasion | 26 (11) |
| Both | 9 (4) |
| None | 69 (28) |
| Unknown | 84 (34) |
| CSCC stage | |
| Localized | 85 (35) |
| Regional | 95 (39) |
| Distant metastases | 64 (26) |
| Unknown | 1 (0.4) |
| Outcome | n (%) |
|---|---|
| Complete response | 51 (21) |
| Confirmed | 36 (15) |
| Unconfirmed | 15 (6) |
| Partial response | 70 (29) |
| Confirmed | 41 (17) |
| Unconfirmed | 29 (12) |
| Stable disease | 22 (9) |
| Progressive disease | 84 (35) |
| Not assessable | 13 (5) |
| Best overall response rate, n (% [95% CI]) | 121 (50.4 [43.9–56.9]) |
| Confirmed | 77 (32) |
| Unconfirmed | 44 (18) |
| Best overall disease control rate, n (% [95% CI]) | 143 (59.6 [53.1–65.8]) |
| Factor | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Progression-free survival | ||||
| Age | 1.00 (0.98–1.01) | 0.62 | 1.00 (0.98–1.01) | 0.63 |
| Male sex | 0.79 (0.55–1.15) | 0.22 | 0.91 (0.61–1.37) | 0.66 |
| Immunocompromised | 1.03 (0.7–1.51) | 0.89 | 1.15 (0.76–1.76) | 0.5 |
| ECOG PS ≥ 2 | ||||
| ≤6 months | 2.3 (1.53–3.44) | <0.0001 | 2.33 (1.52–3.55) | 0.0001 |
| 6 months | 0.88 (0.31–2.51) | 0.81 | 0.85 (0.3–2.46) | 0.77 |
| Chronic dermatitis | 1.67 (1.02–2.71) | 0.04 | 1.07 (0.61–1.87) | 0.8 |
| Primary head-or-neck CSCC | 0.58 (0.41–0.81) | 0.0002 | 0.52 (0.34–0.79) | 0.0025 |
| Localized disease | 1.16 (0.82–1.64) | 0.41 | 0.72 (0.49–1.05) | 0.09 |
| Previous systemic treatment | 0.88 (0.62–1.23) | 0.44 | 1.03 (0.71–1.50) | 0.88 |
| Overall survival | ||||
| Age | 1.00 (0.99–1.02) | 0.81 | 0.99 (0.98–1.01) | 0.46 |
| Male sex | 0.9 (0.56–1.44) | 0.66 | 1.01 (0.61–1.67) | 0.97 |
| Immunocompromised | 0.82 (0.49–1.35) | 0.43 | 0.91 (0.53–1.56) | 0.72 |
| ECOG PS ≥ 2 | ||||
| ≤6 months | 4.39 (2.62–7.33) | <0.0001 | 4.56 (2.64–7.85) | 0.0001 |
| >6 months | 1.61 (0.61–4.27) | 0.34 | 1.69 (0.63–4.52) | 0.3 |
| Chronic dermatitis | 0.98 (0.49–1.95) | 0.95 | 0.7 (0.32–1.51) | 0.36 |
| Primary head-or-neck CSCC | 0.76 (0.49–1.18) | 0.22 | 0.67 (0.4–1.13) | 0.13 |
| Localized disease | 1.02 (0.66–1.58) | 0.94 | 0.74 (0.45–1.2) | 0.22 |
| Previous systemic treatment | 0.76 (0.5–1.17) | 0.21 | 1.09 (0.68–1.76) | 0.72 |
| Adverse Event | Any Grade | Grade ≥ 3 |
|---|---|---|
| Any | 75 (31) | 22 (9) |
| Led to cemiplimab discontinuation | 16 (7) | 12 (5) |
| Fatigue | 21 (9) | 4 (2) |
| Arthralgias/myalgias | 17 (7) | 2 (1) |
| Cholestasis/cytolysis/hepatitis | 10 (4) | 5 (2) |
| Diarrhea | 7 (3) | 0 |
| Pruritus | 6 (3) | 0 |
| Rash | 5 (2) | 0 |
| Hypothyroidism | 5 (2) | 0 |
| Renal failure | 5 (2) | 3 (1) |
| Hyperthyroidism | 4 (2) | 0 |
| Lymphopenia | 3 (1) | 0 |
| Decreased appetite | 3 (1) | 1 (0.4) |
| Peripheral neuropathy | 3 (1) | 0 |
| Anemia | 2 (1) | 0 |
| Neutropenia | 2 (1) | 0 |
| Myocarditis | 2 (1) | 1 (0.4) |
| Corticotropic insufficiency | 2 (1) | 0 |
| Colitis | 2 (1) | 2 (1) |
| Vomiting | 2 (1) | 1 (0.4) |
| Loss of weight | 2 (1) | 0 |
| Balance disorder | 2 (1) | 0 |
| Transplant rejection | 2 (1) | 2 (1) |
| Adverse Event | Severity Grade | |||
|---|---|---|---|---|
| Any | Grade 3 | Grade 4 | Grade 5 | |
| Cholestasis/cytolysis/hepatitis | 5 (2) | 3 | 2 | 0 |
| Fatigue | 4 (2) | 4 | 0 | 0 |
| Renal impairment | 3 (1) | 2 | 1 | 0 |
| Arthralgias/myalgias | 2 (1) | 2 | 0 | 0 |
| Colitis | 2 (1) | 2 | 0 | 0 |
| Transplant rejection | 2 (1) | 1 | 1 | 0 |
| Decreased appetite | 1 (0.4) | 1 | 0 | 0 |
| Myocarditis | 1 (0.4) | 1 | 0 | 0 |
| Vomiting | 1 (0.4) | 1 | 0 | 0 |
| Acute pancreatitis | 1 (0.4) | 1 | 0 | 0 |
| Interstitial lung disease | 1 (0.4) | 1 | 0 | 0 |
| Drug reaction with eosinophilia and systemic symptoms | 1 (0.4) | 0 | 1 | 0 |
| Toxic epidermal necrolysis | 1 (0.4) | 0 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hober, C.; Fredeau, L.; Pham-Ledard, A.; Boubaya, M.; Herms, F.; Celerier, P.; Aubin, F.; Beneton, N.; Dinulescu, M.; Jannic, A.; et al. Cemiplimab for Locally Advanced and Metastatic Cutaneous Squamous-Cell Carcinomas: Real-Life Experience from the French CAREPI Study Group. Cancers 2021, 13, 3547. https://doi.org/10.3390/cancers13143547
Hober C, Fredeau L, Pham-Ledard A, Boubaya M, Herms F, Celerier P, Aubin F, Beneton N, Dinulescu M, Jannic A, et al. Cemiplimab for Locally Advanced and Metastatic Cutaneous Squamous-Cell Carcinomas: Real-Life Experience from the French CAREPI Study Group. Cancers. 2021; 13(14):3547. https://doi.org/10.3390/cancers13143547
Chicago/Turabian StyleHober, Candice, Lisa Fredeau, Anne Pham-Ledard, Marouane Boubaya, Florian Herms, Philippe Celerier, François Aubin, Nathalie Beneton, Monica Dinulescu, Arnaud Jannic, and et al. 2021. "Cemiplimab for Locally Advanced and Metastatic Cutaneous Squamous-Cell Carcinomas: Real-Life Experience from the French CAREPI Study Group" Cancers 13, no. 14: 3547. https://doi.org/10.3390/cancers13143547
APA StyleHober, C., Fredeau, L., Pham-Ledard, A., Boubaya, M., Herms, F., Celerier, P., Aubin, F., Beneton, N., Dinulescu, M., Jannic, A., Meyer, N., Duval-Modeste, A.-B., Cesaire, L., Neidhardt, È.-M., Archier, É., Dréno, B., Lesage, C., Berthin, C., Kramkimel, N., ... Maubec, È. (2021). Cemiplimab for Locally Advanced and Metastatic Cutaneous Squamous-Cell Carcinomas: Real-Life Experience from the French CAREPI Study Group. Cancers, 13(14), 3547. https://doi.org/10.3390/cancers13143547







