Primary Central Nervous System Lymphoma in Elderly Patients: Management and Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
2. Clinical Aspects
3. Diagnosis
4. Treatment
4.1. Achieving a High Rate of Remission: Induction Treatment
Intrathecal Chemotherapy
4.2. Achieving Long-Term Remission
4.2.1. Consolidation Treatment
Whole-Brain Radiation Therapy (WBRT)
High-Dose Chemotherapy Conditioning with Autologous Stem Cell Transplantation (HDC-ASCT)
Nonmyeloablative Intensive Chemotherapy
4.2.2. Maintenance Treatment
4.3. Salvage Treatment
4.4. The Oldest Patients
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shiels, M.S.; Pfeiffer, R.M.; Besson, C.; Clarke, C.A.; Morton, L.M.; Nogueira, L.; Pawlish, K.; Yanik, E.L.; Suneja, G.; Engels, E.A. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br. J. Haematol. 2016, 174, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Eloranta, S.; Brånvall, E.; Celsing, F.; Papworth, K.; Ljungqvist, M.; Enblad, G.; Ekström-Smedby, K. Increasing incidence of primary central nervous system lymphoma but no improvement in survival in Sweden 2000–2013. Eur. J. Haematol. 2018, 100, 61–68. [Google Scholar] [CrossRef]
- Mendez, J.S.; Ostrom, Q.T.; Gittleman, H.; Kruchko, C.; DeAngelis, L.M.; Barnholtz-Sloan, J.S.; Grommes, C. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro. Oncol. 2018, 20, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, M.; Dinmohamed, A.G.; Visser, O.; Doorduijn, J.K.; Bromberg, J.E.C. Improved survival in primary central nervous system lymphoma up to age 70 only: A population-based study on incidence, primary treatment and survival in the Netherlands, 1989–2015. Leukemia 2017, 31, 1822–1825. [Google Scholar] [CrossRef] [PubMed]
- Villano, J.L.; Koshy, M.; Shaikh, H.; Dolecek, T.A.; McCarthy, B.J. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br. J. Cancer. 2011, 105, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Houillier, C.; Soussain, C.; Ghesquières, H.; Soubeyran, P.; Chinot, O.; Taillandier, L.; Lamy, T.; Choquet, S.; Ahle, G.; Damaj, G.; et al. Management and outcome of primary CNS lymphoma in the modern era: An LOC network study. Neurology 2020, 94, e1027–e1039. [Google Scholar] [CrossRef]
- Kasenda, B.; Ferreri, A.J.; Marturano, E.; Forst, D.; Bromberg, J.; Ghesquieres, H.; Ferlay, C.; Blay, J.Y.; Hoang-Xuan, K.; Pulczynski, E.J.; et al. First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)—A systematic review and individual patient data meta-analysis. Ann. Oncol. 2015, 26, 1305–1313. [Google Scholar] [CrossRef]
- Roth, P.; Martus, P.; Kiewe, P.; Möhle, R.; Klasen, H.; Rauch, M.; Röth, A.; Kaun, S.; Thiel, E.; Korfel, A.; et al. Outcome of elderly patients with primary CNS lymphoma in the G-PCNSL-SG-1 trial. Neurology 2012, 79, 890–896. [Google Scholar] [CrossRef]
- Hoang-Xuan, K.; Taillandier, L.; Chinot, O.; Soubeyran, P.; Bogdhan, U.; Hildebrand, J.; Frenay, M.; De Beule, N.; Delattre, J.Y.; Baron, B.; et al. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: A multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J. Clin. Oncol. 2003, 21, 2726–2731. [Google Scholar] [CrossRef]
- Roth, P.; Hoang-Xuan, K. Challenges in the treatment of elderly patients with primary central nervous system lymphoma. Curr. Opin. Neurol. 2014, 27, 697–701. [Google Scholar] [CrossRef]
- Houillier, C.; Ghesquières, H.; Chabrot, C.; Soussain, C.; Ahle, G.; Choquet, S.; Nicolas-Virelizier, E.; Bay, J.O.; Vargaftig, J.; Gaultier, C.; et al. Rituximab, methotrexate, procarbazine, vincristine and intensified cytarabine consolidation for primary central nervous system lymphoma (PCNSL) in the elderly: A LOC network study. J. Neurooncol. 2017, 133, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Siegal, T.; Bairey, O. Primary CNS Lymphoma in the Elderly: The Challenge. Acta Haematol. 2019, 141, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Illerhaus, G.; Kasenda, B.; Ihorst, G.; Egerer, G.; Lamprecht, M.; Keller, U.; Wolf, H.H.; Hirt, C.; Stilgenbauer, S.; Binder, M.; et al. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: A prospective, single-arm, phase 2 trial. Lancet Haematol. 2016, 3, e388–e397. [Google Scholar] [CrossRef]
- Velasco, R.; Mercadal, S.; Vidal, N.; Alañá, M.; Barceló, M.I.; Ibáñez-Juliá, M.J.; Bobillo, S.; Caldú-Agud, R.; García-Molina, E.; Martínez, P.; et al. Diagnostic delay and outcome in immunocompetent patients with primary central nervous system lymphoma in Spain: A multicentric study. J. Neurooncol. 2020, 148, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Ney, D.E.; Reiner, A.S.; Panageas, K.S.; Brown, H.S.; DeAngelis, L.M.; Abrey, L.E. Characteristics and outcomes of elderly patients with primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center experience. Cancer 2010, 116, 4605–4612. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.; Blay, J.Y.; Reni, M.; Pasini, F.; Spina, M.; Ambrosetti, A.; Calderoni, A.; Rossi, A.; Vavassori, V.; Conconi, A.; et al. Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experience. J. Clin. Oncol. 2003, 21, 266–272. [Google Scholar] [CrossRef]
- Abrey, L.E.; Ben-Porat, L.; Panageas, K.S.; Yahalom, J.; Berkey, B.; Curran, W.; Schultz, C.; Leibel, S.; Nelson, D.; Mehta, M.; et al. Primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center prognostic model. J. Clin. Oncol. 2006, 24, 5711–5715. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Ahn, H.J.; Yoon, D.H.; Hong, J.Y.; Yoo, C.; Kim, S.; Huh, J.; Suh, C. Primary central nervous system lymphoma: A new prognostic model for patients with diffuse large B-cell histology. Blood Res. 2017, 52, 285–292. [Google Scholar] [CrossRef]
- Welch, M.R.; Omuro, A.; Deangelis, L.M. Outcomes of the oldest patients with primary CNS lymphoma treated at Memorial Sloan-Kettering Cancer Center. Neuro. Oncol. 2012, 14, 1304–1311. [Google Scholar] [CrossRef]
- Cerqua, R.; Balestrini, S.; Perozzi, C.; Cameriere, V.; Renzi, S.; Lagalla, G.; Mancini, G.; Montanari, M.; Leoni, P.; Scerrati, M.; et al. Diagnostic delay and prognosis in primary central nervous system lymphoma compared with glioblastoma multiforme. Neurol. Sci. 2016, 37, 23–29. [Google Scholar] [CrossRef]
- Laude, M.C.; Julia, E.; Nicolas-Virelizier, E.; Antherieu, G.; Safar, V.; Rey, P.; Ferrant, E.; Traverse-Glehen, A.; Chassagne-Clément, C.; Meyronet, D.; et al. Diagnosis-to-Treatment Interval Is an Important Prognostic Factor with a Time-Dependent Effect Predicting Event-Free Survival after 12 Months from First-Line Treatment in Newly Diagnosed Diffuse Large B-Cell Primary CNS Lymphoma. Blood 2020, 136 (Suppl. 1), 43–45. [Google Scholar] [CrossRef]
- Omuro, A.; Chinot, O.; Taillandier, L.; Ghesquieres, H.; Soussain, C.; Delwail, V.; Lamy, T.; Gressin, R.; Choquet, S.; Soubeyran, P.; et al. Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: An intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol. 2015, 2, e251–e259. [Google Scholar] [CrossRef]
- Van der Meulen, M.; Dirven, L.; Bakunina, K.; van den Bent, M.J.; Issa, S.; Doorduijn, J.K.; Bromberg, J.E.C. MMSE is an independent prognostic factor for survival in primary central nervous system lymphoma. J. Neurooncol. 2021, 152, 357–362. [Google Scholar] [CrossRef]
- Schorb, E.; Kasenda, B.; Ihorst, G.; Scherer, F.; Wendler, J.; Isbell, L.; Fricker, H.; Finke, J.; Illerhaus, G. High-dose chemotherapy and autologous stem cell transplant in elderly patients with primary CNS lymphoma: A pilot study. Blood Adv. 2020, 4, 3378–3381. [Google Scholar] [CrossRef] [PubMed]
- Hoang-Xuan, K.; Bessell, E.; Bromberg, J.; Hottinger, A.F.; Preusser, M.; Rudà, R.; Schlegel, U.; Siegal, T.; Soussain, C.; Abacioglu, U.; et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: Guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015, 16, e322–e332. [Google Scholar] [CrossRef]
- Fox, C.P.; Phillips, E.H.; Smith, J.; Linton, K.; Gallop-Evans, E.; Hemmaway, C.; Auer, D.P.; Fuller, C.; Davies, A.J.; McKay, P.; et al. Guidelines for the diagnosis and management of primary central nervous system diffuse large B-cell lymphoma. Br. J. Haematol. 2019, 184, 348–363. [Google Scholar] [CrossRef]
- Burton, A.F.; Storr, J.M.; Dunn, W.L. Cytolytic action of corticosteroids on thymus and lymphoma cells in vitro. Can. J. Biochem. 1967, 45, 289–297. [Google Scholar] [CrossRef]
- Helmberg, A.; Auphan, N.; Caelles, C.; Karin, M. Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J. 1995, 14, 452–460. [Google Scholar] [CrossRef]
- Ayroldi, E.; Zollo, O.; Bastianelli, A.; Marchetti, C.; Agostini, M.; Di Virgilio, R.; Riccardi, C. GILZ mediates the antiproliferative activity of glucocorticoids by negative regulation of Ras signaling. J. Clin. Investig. 2007, 117, 1605–1615. [Google Scholar] [CrossRef]
- Roth, P.; Wick, W.; Weller, M. Steroids in neurooncology: Actions, indications, side-effects. Curr. Opin. Neurol. 2010, 23, 597–602. [Google Scholar] [CrossRef]
- Grander, D.; Kharaziha, P.; Laane, E.; Pokrovskaja, K.; Panaretakis, T. Autophagy as the main means of cytotoxicity by glucocorticoids in hematological malignancies. Autophagy 2009, 5, 1198–1200. [Google Scholar] [CrossRef] [PubMed]
- Laane, E.; Tamm, K.P.; Buentke, E.; Ito, K.; Kharaziha, P.; Oscarsson, J.; Corcoran, M.; Björklund, A.C.; Hultenby, K.; Lundin, J.; et al. Cell death induced by dexamethasone in lymphoid leukemia is mediated through initiation of autophagy. Cell Death Differ. 2009, 16, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Woodworth, G.F.; Coon, A.L.; Frazier, J.M.; Amundson, E.; Garonzik, I.; Olivi, A.; Weingart, J.D. Independent predictors of morbidity after image-guided stereotactic brain biopsy: A risk assessment of 270 cases. J. Neurosurg. 2005, 102, 897–901. [Google Scholar] [CrossRef]
- Malikova, H.; Liscak, R.; Latnerova, I.; Guseynova, K.; Syrucek, M.; Pytlik, R. Complications of MRI-guided stereotactic biopsy of brain lymphoma. Neuro. Endocrinol. Lett. 2014, 35, 613–618. [Google Scholar]
- Morell, A.A.; Shah, A.H.; Cavallo, C.; Eichberg, D.G.; Sarkiss, C.A.; Benveniste, R.; Ivan, M.E.; Komotar, R.J. Diagnosis of primary central nervous system lymphoma: A systematic review of the utility of CSF screening and the role of early brain biopsy. Neurooncol. Pract. 2019, 6, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Kellermann, S.G.; Hamisch, C.A.; Rueß, D.; Blau, T.; Goldbrunner, R.; Treuer, H.; Grau, S.J.; Ruge, M.I. Stereotactic biopsy in elderly patients: Risk assessment and impact on treatment decision. J. Neurooncol. 2017, 134, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.; Wong, V.; Kadoch, C.; Gao, H.X.; Barajas, R.; Chen, L.; Josephson, A.; Scott, B.; Douglas, V.; Maiti, M.; et al. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood 2013, 121, 4740–4748. [Google Scholar] [CrossRef]
- Baraniskin, A.; Kuhnhenn, J.; Schlegel, U.; Chan, A.; Deckert, M.; Gold, R.; Maghnouj, A.; Zöllner, H.; Reinacher-Schick, A.; Schmiegel, W.; et al. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood 2011, 117, 3140–3146. [Google Scholar] [CrossRef]
- Viaccoz, A.; Ducray, F.; Tholance, Y.; Barcelos, G.K.; Thomas-Maisonneuve, L.; Ghesquières, H.; Meyronet, D.; Quadrio, I.; Cartalat-Carel, S.; Louis-Tisserand, G.; et al. CSF neopterin level as a diagnostic marker in primary central nervous system lymphoma. Neuro. Oncol. 2015, 17, 1497–1503. [Google Scholar] [CrossRef]
- Muñiz, C.; Martín-Martín, L.; López, A.; Sánchez-González, B.; Salar, A.; Almeida, J.; Sancho, J.M.; Ribera, J.M.; Heras, C.; Peñalver, F.J.; et al. Contribution of cerebrospinal fluid sCD19 levels to the detection of CNS lymphoma and its impact on disease outcome. Blood 2014, 123, 1864–1869. [Google Scholar] [CrossRef]
- Poulain, S.; Boyle, E.M.; Roumier, C.; Demarquette, H.; Wemeau, M.; Geffroy, S.; Herbaux, C.; Bertrand, E.; Hivert, B.; Terriou, L.; et al. MYD88 L265P mutation contributes to the diagnosis of Bing Neel syndrome. Br. J. Haematol. 2014, 167, 506–513. [Google Scholar] [CrossRef]
- Hiemcke-Jiwa, L.S.; Minnema, M.C.; Radersma-van Loon, J.H.; Jiwa, N.M.; de Boer, M.; Leguit, R.J.; de Weger, R.A.; Huibers, M.M.H. The use of droplet digital PCR in liquid biopsies: A highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid. Hematol. Oncol. 2018, 36, 429–435. [Google Scholar] [CrossRef]
- Geng, M.; Song, Y.; Xiao, H.; Wu, Z.; Deng, X.; Chen, C.; Wang, G. Clinical significance of interleukin-10 concentration in the cerebrospinal fluid of patients with primary central nervous system lymphoma. Oncol. Lett. 2021, 21, 2. [Google Scholar]
- Shao, J.; Chen, K.; Li, Q.; Ma, J.; Ma, Y.; Lin, Z.; Kang, H.; Chen, B. High Level of IL-10 in Cerebrospinal Fluid is Specific for Diagnosis of Primary Central Nervous System Lymphoma. Cancer Manag. Res. 2020, 12, 6261–6268. [Google Scholar] [CrossRef]
- Nguyen-Them, L.; Costopoulos, M.; Tanguy, M.L.; Houillier, C.; Choquet, S.; Benanni, H.; Elias-Shamieh, R.; Armand, M.; Faivre, G.; Glaisner, S.; et al. The CSF IL-10 concentration is an effective diagnostic marker in immunocompetent primary CNS lymphoma and a potential prognostic biomarker in treatment-responsive patients. Eur. J. Cancer 2016, 61, 69–76. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, W.; Zhang, L.; Wu, W.; Zhang, Y.; Han, X.; Yang, C.; Zhang, L.; Zhou, D. Cerebrospinal Fluid IL-10 and IL-10/IL-6 as Accurate Diagnostic Biomarkers for Primary Central Nervous System Large B-cell Lymphoma. Sci. Rep. 2016, 6, 38671. [Google Scholar] [CrossRef]
- Sasayama, T.; Nakamizo, S.; Nishihara, M.; Kawamura, A.; Tanaka, H.; Mizukawa, K.; Miyake, S.; Taniguchi, M.; Hosoda, K.; Kohmura, E. Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro. Oncol. 2012, 14, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Hiemcke-Jiwa, L.S.; Ten Dam-van Loon, N.H.; Leguit, R.J.; Nierkens, S.; Ossewaarde-van Norel, J.; de Boer, J.H.; Roholl, F.F.; de Weger, R.A.; Huibers, M.M.H.; de Groot-Mijnes, J.D.F.; et al. Potential Diagnosis of Vitreoretinal Lymphoma by Detection of MYD88 Mutation in Aqueous Humor With Ultrasensitive Droplet Digital Polymerase Chain Reaction. JAMA Ophthalmol. 2018, 136, 1098–1104. [Google Scholar] [CrossRef]
- Yonese, I.; Takase, H.; Yoshimori, M.; Onozawa, E.; Tsuzura, A.; Miki, T.; Mochizuki, M.; Miura, O.; Arai, A. CD79B mutations in primary vitreoretinal lymphoma: Diagnostic and prognostic potential. Eur. J. Haematol. 2019, 102, 191–196. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Calimeri, T.; Lopedote, P.; Francaviglia, I.; Daverio, R.; Iacona, C.; Belloni, C.; Steffanoni, S.; Gulino, A.; Anghileri, E.; et al. MYD88 L265P mutation and interleukin-10 detection in cerebrospinal fluid are highly specific discriminating markers in patients with primary central nervous system lymphoma: Results from a prospective study. Br. J. Haematol. 2021, 193, 497–505. [Google Scholar] [CrossRef]
- Howard, S.C.; McCormick, J.; Pui, C.H.; Buddington, R.K.; Harvey, R.D. Preventing and Managing Toxicities of High-Dose Methotrexate. Oncologist 2016, 21, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Chowdhary, S.; Lombardi, K.M.; Chalmers, L.M.; Chamberlain, M. Clinical utility and pharmacology of high-dose methotrexate in the treatment of primary CNS lymphoma. Expert Rev. Neurother. 2006, 6, 635–652. [Google Scholar] [CrossRef]
- Omuro, A.M.; Taillandier, L.; Chinot, O.; Carnin, C.; Barrie, M.; Hoang-Xuan, K. Temozolomide and methotrexate for primary central nervous system lymphoma in the elderly. J. Neurooncol. 2007, 85, 207–211. [Google Scholar] [CrossRef]
- Zhu, J.J.; Gerstner, E.R.; Engler, D.A.; Mrugala, M.M.; Nugent, W.; Nierenberg, K.; Hochberg, F.H.; Betensky, R.A.; Batchelor, T.T. High-dose methotrexate for elderly patients with primary CNS lymphoma. Neuro. Oncol. 2009, 11, 211–215. [Google Scholar] [CrossRef]
- Illerhaus, G.; Marks, R.; Müller, F.; Ihorst, G.; Feuerhake, F.; Deckert, M.; Ostertag, C.; Finke, J. High-dose methotrexate combined with procarbazine and CCNU for primary CNS lymphoma in the elderly: Results of a prospective pilot and phase II study. Ann. Oncol. 2009, 20, 319–325. [Google Scholar] [CrossRef]
- Fritsch, K.; Kasenda, B.; Hader, C.; Nikkhah, G.; Prinz, M.; Haug, V.; Haug, S.; Ihorst, G.; Finke, J.; Illerhaus, G. Immunochemotherapy with rituximab, methotrexate, procarbazine, and lomustine for primary CNS lymphoma (PCNSL) in the elderly. Ann. Oncol. 2011, 22, 2080–2085. [Google Scholar] [CrossRef]
- Olivier, G.; Clavert, A.; Lacotte-Thierry, L.; Gardembas, M.; Escoffre-Barbe, M.; Brion, A.; Cumin, I.; Legouffe, E.; Solal-Celigny, P.; Chabin, M.; et al. A phase 1-dose escalation study of idarubicin combined with methotrexate, vindesine, and prednisolone for untreated elderly patients with primary central nervous system lymphoma. The GOELAMS LCP 99 trial. Am. J. Hematol. 2014, 89, 1024–1029. [Google Scholar] [CrossRef]
- Pulczynski, E.J.; Kuittinen, O.; Erlanson, M.; Hagberg, H.; Fosså, A.; Eriksson, M.; Nordstrøm, M.; Østenstad, B.; Fluge, Ø.; Leppä, S.; et al. Successful change of treatment strategy in elderly patients with primary central nervous system lymphoma by de-escalating induction and introducing temozolomide maintenance: Results from a phase II study by the Nordic Lymphoma Group. Haematologica 2015, 100, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Schorb, E.; Fox, C.P.; Fritsch, K.; Isbell, L.; Neubauer, A.; Tzalavras, A.; Witherall, R.; Choquet, S.; Kuittinen, O.; De-Silva, D.; et al. High-dose thiotepa-based chemotherapy with autologous stem cell support in elderly patients with primary central nervous system lymphoma: A European retrospective study. Bone Marrow Transplant. 2017, 52, 1113–1119. [Google Scholar] [CrossRef]
- Fritsch, K.; Kasenda, B.; Schorb, E.; Hau, P.; Bloehdorn, J.; Möhle, R.; Löw, S.; Binder, M.; Atta, J.; Keller, U.; et al. High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia 2017, 31, 846–852. [Google Scholar] [CrossRef]
- Faivre, G.; Butler, M.J.; Le, I.; Brenner, A. Temozolomide as a Single Agent Maintenance Therapy in Elderly Patients With Primary CNS Lymphoma. Clin. Lymphoma Myeloma Leuk. 2019, 19, 665–669. [Google Scholar] [CrossRef]
- Vu, K.; Mannis, G.; Hwang, J.; Geng, H.; Rubenstein, J.L. Low-dose lenalidomide maintenance after induction therapy in older patients with primary central nervous system lymphoma. Br. J. Haematol. 2019, 186, 180–183. [Google Scholar] [CrossRef]
- Jahnke, K.; Korfel, A.; Martus, P.; Weller, M.; Herrlinger, U.; Schmittel, A.; Fischer, L.; Thiel, E.; German Primary Central Nervous System Lymphoma Study Group (G-PCNSL-SG). High-dose methotrexate toxicity in elderly patients with primary central nervous system lymphoma. Ann. Oncol. 2005, 16, 445–449. [Google Scholar] [CrossRef]
- Schaff, L.; Lobbous, M.; Sener, U.; Gavrilovic, I.; Miller, A.; Stone, J.; Piotrowski, A.; Skakodub, A.; Madzsar, J.; Schumpp, A.; et al. Pilot study of repeated planned glucarpidase following high dose methotrexate (HD-MTX) in CNS Lymphoma (CNSL). Neuro. Oncol. 2020, 22 (Suppl. 2), ii56. [Google Scholar]
- Schmitt, A.M.; Herbrand, A.K.; Fox, C.P.; Bakunina, K.; Bromberg, J.E.C.; Cwynarski, K.; Doorduijn, J.K.; Ferreri, A.J.M.; Illerhaus, G.; Issa, S.; et al. Rituximab in primary central nervous system lymphoma—A systematic review and meta-analysis. Hematol. Oncol. 2019, 37, 548–557. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Cwynarski, K.; Pulczynski, E.; Ponzoni, M.; Deckert, M.; Politi, L.S.; Torri, V.; Fox, C.P.; Rosée, P.L.; Schorb, E.; et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016, 3, e217–e227. [Google Scholar] [CrossRef]
- Bromberg, J.E.C.; Issa, S.; Bakunina, K.; Minnema, M.C.; Seute, T.; Durian, M.; Cull, G.; Schouten, H.C.; Stevens, W.B.C.; Zijlstra, J.M.; et al. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): A randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2019, 20, 216–228. [Google Scholar] [CrossRef]
- Seidel, S.; Kowalski, T.; Margold, M.; Baraniskin, A.; Schroers, R.; Martus, P.; Schlegel, U. HDMTX-based polychemotherapy including intraventricular therapy in elderly patients with primary CNS lymphoma: A single center series. Ther. Adv. Neurol. Disord. 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Pels, H.; Schmidt-Wolf, I.G.; Glasmacher, A.; Schulz, H.; Engert, A.; Diehl, V.; Zellner, A.; Schackert, G.; Reichmann, H.; Kroschinsky, F.; et al. Primary central nervous system lymphoma: Results of a pilot and phase II study of systemic and in- traventricular chemotherapy with deferred radiotherapy. J. Clin. Oncol. 2003, 21, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Sierra Del Rio, M.; Ricard, D.; Houillier, C.; Navarro, S.; Gonzalez-Aguilar, A.; Idbaih, A.; Kaloshi, G.; Elhallani, S.; Omuro, A.; Choquet, S.; et al. Prophylactic intrathecal chemotherapy in primary CNS lymphoma. J. Neurooncol. 2012, 106, 143–146. [Google Scholar] [CrossRef]
- Nelson, D.F.; Martz, K.L.; Bonner, H.; Nelson, J.S.; Newall, J.; Kerman, H.D.; Thomson, J.W.; Murray, K.J. Non-Hodgkin’s lymphoma of the brain: Can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 9–17. [Google Scholar] [CrossRef]
- Abrey, L.E.; DeAngelis, L.M.; Yahalom, J. Long-term survival in primary CNS lymphoma. J. Clin. Oncol. 1998, 16, 859–863. [Google Scholar] [CrossRef]
- Bessell, E.M.; Graus, F.; López-Guillermo, A.; Villá, S.; Verger, E.; Petit, J.; Holland, I.; Byrne, P. CHOD/BVAM regimen plus radiotherapy in patients with primary CNS non-Hodgkin’s lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 457–464. [Google Scholar] [CrossRef]
- Omuro, A.M.P.; Ben-Porat, L.S.; Panageas, K.S.; Kim, A.K.; Correa, D.D.; Yahalom, J.; Deangelis, L.M.; Abrey, L.E. Delayed Neurotoxicity in Primary Central Nervous System Lymphoma. Arch. Neurol. 2005, 62, 1595–1600. [Google Scholar] [CrossRef]
- DeAngelis, L.M.; Delattre, J.Y.; Posner, J.B. Radiation-induced dementia in patients cured of brain metastases. Neurology 1989, 39, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Crossen, J.R.; Garwood, D.; Glatstein, E.; Neuwelt, E.A. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J. Clin. Oncol. 1994, 12, 627–642. [Google Scholar] [CrossRef]
- Duffey, P.; Chari, G.; Cartlidge, N.E.F.; Shaw, P.J. Progressive deterioration of intellect and motor function occurring several decades after cranial irradiation. Arch. Neurol. 1996, 53, 814–818. [Google Scholar] [CrossRef]
- Morris, P.G.; Correa, D.D.; Yahalom, J.; Raizer, J.J.; Schiff, D.; Grant, B.; Grimm, S.; Lai, R.K.; Reiner, A.S.; Panageas, K.; et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: Final results and long- term outcome. J. Clin. Oncol. 2013, 31, 3971–3979. [Google Scholar] [CrossRef]
- Soussain, C.; Hoang-Xuan, K.; Taillandier, L.; Fourme, E.; Choquet, S.; Witz, F.; Casasnovas, O.; Dupriez, B.; Souleau, B.; Taksin, A.L.; et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J. Clin. Oncol. 2008, 26, 2512–2518. [Google Scholar] [CrossRef]
- Omuro, A.; Correa, D.D.; DeAngelis, L.M.; Moskowitz, C.H.; Matasar, M.J.; Kaley, T.J.; Gavrilovic, I.T.; Nolan, C.; Pentsova, E.; Grommes, C.C.; et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015, 125, 1403–1410. [Google Scholar] [CrossRef]
- Houillier, C.; Taillandier, L.; Dureau, S.; Lamy, T.; Laadhari, M.; Chinot, O.; Moluçon-Chabrot, C.; Soubeyran, P.; Gressin, R.; Choquet, S.; et al. Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J. Clin. Oncol. 2019, 37, 823–833. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Cwynarski, K.; Pulczynski, E.; Fox, C.P.; Schorb, E.; La Rosée, P.; Binder, M.; Fabbri, A.; Torri, V.; Minacapelli, E.; et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: Results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017, 4, e510–e523. [Google Scholar] [PubMed]
- Correa, D.D.; Braun, E.; Kryza-Lacombe, M.; Ho, K.W.; Reiner, A.S.; Panageas, K.S.; Yahalom, J.; Sauter, C.S.; Abrey, L.E.; DeAngelis, L.M.; et al. Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J. Neurooncol. 2019, 144, 553–562. [Google Scholar] [CrossRef]
- Rubenstein, J.L.; His, E.D.; Johnson, J.L.; Jung, S.H.; Nakashima, M.O.; Grant, B.; Cheson, B.D.; Kaplan, L.D. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J. Clin. Oncol. 2013, 31, 3061–3068. [Google Scholar] [CrossRef]
- Birsen, R.; Willems, L.; Pallud, J.; Blanc, E.; Burroni, B.; Legoff, M.; Le Ray, E.; Pilorge, S.; Deau, B.; Franchi, P.; et al. Efficacy and safety of high-dose etoposide cytarabine as consolidation following rituximab methotrexate temozolomide induction in newly diagnosed primary central nervous system lymphoma in immunocompetent patients. Haematologica 2018, 103, e296–e299. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.; Giri, S.; Ruppert, A.S.; Bartlett, N.L.; Hsi, E.D.; Cheson, B.D.; Nayak, L.; Leonard, J.P.; Rubenstein, J.L. Myeloablative versus non-myeloablative consolidative chemotherapy for newly diagnosed primary central nervous system lymphoma: Results of CALGB 51101 (Alliance). J. Clin. Oncol. 2021, 39 (Suppl. 15), 7506. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Betensky, R.A.; Hochberg, F.H.; Grossman, S.A.; Lesser, G.J.; Nabors, L.B.; Chon, B.; Batchelor, T.T. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin. Cancer Res. 2004, 10, 5643–5646. [Google Scholar] [CrossRef]
- Pentsova, E.; Deangelis, L.M.; Omuro, A. Methotrexate re-challenge for recurrent primary central nervous system lymphoma. J. Neurooncol. 2014, 117, 161–165. [Google Scholar] [CrossRef]
- Herrlinger, U.; Brugger, W.; Bamberg, M.; Kuker, W.; Dichgans, J.; Weller, M. PCV salvage chemotherapy for recurrent primary CNS lymphoma. Neurology 2000, 54, 1707–1708. [Google Scholar] [CrossRef]
- Reni, M.; Zaja, F.; Mason, W.; Perry, J.; Mazza, E.; Spina, M.; Bordonaro, R.; Ilariucci, F.; Faedi, M.; Corazzelli, G.; et al. Temozolomide as salvage treatment in primary brain lymphomas. Br. J. Cancer 2007, 96, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Enting, R.H.; Demopoulos, A.; DeAngelis, L.M.; Abrey, L.E. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology 2004, 63, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Abrey, L.E.; Drappatz, J.; Gilbert, M.R.; Reardon, D.A.; Wen, P.Y.; Prados, M.; Deangelis, L.M.; Omuro, A. North American Brain Tumor Consortium. Multicenter phase II study of rituximab and temozolomide in recurrent primary central nervous system lymphoma. Leuk. Lymphoma 2013, 54, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Lee, E.Q.; Nayak, L.; Doherty, L.; Kesari, S.; Muzikansky, A.; Norden, A.D.; Chen, H.; Wen, P.Y.; Drappatz, J. Retrospective study of pemetrexed as salvage therapy for central nervous system lymphoma. J. Neurooncol. 2013, 115, 71–77. [Google Scholar] [CrossRef]
- Han, S.; Wang, M.; Liu, B.; Yu, J. Pemetrexed for primary central nervous system lymphoma in the elderly. Clin. Transl. Oncol. 2016, 18, 138–143. [Google Scholar] [CrossRef]
- Raizer, J.J.; Rademaker, A.; Evens, A.M.; Rice, L.; Schwartz, M.; Chandler, J.P.; Getch, C.C.; Tellez, C.; Grimm, S.A. Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer 2012, 118, 3743–3748. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Han, S.; Xing, B.; Li, H.; Zhu, Y.; Zhou, S.; Wang, X.; Xu, J.; Tao, R. Efficacy and safety of pemetrexed on recurrent primary central nervous system lymphomas in China: A prospective study. Onco. Targets Ther. 2017, 10, 2595–2600. [Google Scholar] [CrossRef]
- Fischer, L.; Thiel, E.; Klasen, H.A.; Birkmann, J.; Jahnke, K.; Martus, P.; Korfel, A. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann. Oncol. 2006, 17, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Voloschin, A.D.; Betensky, R.; Wen, P.Y.; Hochberg, F.; Batchelor, T. Topotecan as salvage therapy for relapsed or refractory primary central nervous system lymphoma. J. Neurooncol. 2008, 86, 211–215. [Google Scholar] [CrossRef]
- Sierra del Rio, M.; Choquet, S.; Hoang-Xuan, K.; Glaisner, S.; Fourme, E.; Janvier, M.; Soussain, C. Platine and cytarabine-based salvage treatment for primary central nervous system lymphoma. J. Neurooncol. 2011, 105, 409–414. [Google Scholar] [CrossRef]
- Arellano, R.; Lopez, G.; Bessell, E.M.; Nomdedeu, B.; Montserrat, E.; Graus, F. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (ara-C) for patients with recurrent primary central nervous system lymphoma. Eur. J. Haematol. 2003, 70, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Choquet, S.; Grenier, A.; Houillier, C.; Soussain, C.; Moles, M.; Gastinne, T.; Cassoux, N.; Beral, H.; Weil, D.; Leblond, V.; et al. Very high efficiency of ICE (Ifosfamide-Carboplatin-Etoposide) in Relapse/Refractory (R/R) Primary Central Nervous System (PCNSL) and Vitreo-Retinal (VRL) Non Hodgkin Lymphoma. A LOC network multicenter retrospective study on 58 cases. Blood 2015, 126, 1524. [Google Scholar] [CrossRef]
- Lopez-Girona, A.; Mendy, D.; Ito, T.; Miller, K.; Gandhi, A.K.; Kang, J.; Karasawa, S.; Carmel, G.; Jackson, P.; Abbasian, M.; et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012, 26, 2326–2335. [Google Scholar] [CrossRef]
- Gribben, J.G.; Fowler, N.; Morschhauser, F. Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma. J. Clin. Oncol. 2015, 33, 2803–2811. [Google Scholar] [CrossRef]
- Houillier, C.; Choquet, S.; Touitou, V.; Martin-Duverneuil, N.; Navarro, S.; Mokhtari, K.; Soussain, C.; Hoang-Xuan, K. Lenalidomide monotherapy as salvage treatment for recurrent primary CNS lymphoma. Neurology 2015, 84, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.; Geng, H.; Fraser, E.J.; Formaker, P.; Chen, L.; Sharma, J.; Killea, P.; Choi, K.; Ventura, J.; Kurhanewicz, J.; et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018, 2, 1595–1607. [Google Scholar] [CrossRef]
- Ghesquieres, H.; Chevrier, M.; Laadhari, M.; Chinot, O.; Choquet, S.; Moluçon-Chabrot, C.; Beauchesne, P.; Gressin, R.; Morschhauser, F.; Schmitt, A.; et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: A multicenter prospective ‘proof of concept’ phase II study of the french oculo-cerebral lymphoma (LOC) network and the lymphoma study association (LYSA) dagger. Ann. Oncol. 2019, 30, 621–628. [Google Scholar] [PubMed]
- Tun, H.W.; Johnston, P.B.; DeAngelis, L.M.; Atherton, P.J.; Pederson, L.D.; Koenig, P.A.; Reeder, C.B.; Omuro, A.M.P.; Schiff, D.; O’Neill, B.; et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood 2018, 132, 2240–2248. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Dunleavy, K.; Roschewski, M.; Widemann, B.C.; Butman, J.A.; Schmitz, R.; Yang, Y.; Cole, D.E.; Melani, C.; Higham, C.S.; et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell 2017, 31, 833–843.e5. [Google Scholar] [CrossRef]
- Grommes, C.; Pastore, A.; Palaskas, N.; Tang, S.S.; Campos, C.; Schartz, D.; Codega, P.; Nichol, D.; Clark, O.; Hsieh, W.Y.; et al. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov. 2017, 7, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Soussain, C.; Choquet, S.; Blonski, M.; Leclercq, D.; Houillier, C.; Rezai, K.; Bijou, F.; Houot, R.; Boyle, E.; Gressin, R.; et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur. J. Cancer 2019, 117, 121–130. [Google Scholar]
- Grommes, C.; Tang, S.S.; Wolfe, J.; Kaley, T.J.; Daras, M.; Pentsova, E.I.; Piotrowski, A.F.; Stone, J.; Lin, A.; Nolan, C.P.; et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood 2019, 133, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Iwamoto, F.M.; LaCasce, A.; Mukundan, S.; Roemer, M.G.M.; Chapuy, B.; Armand, P.; Rodig, S.J.; Shipp, M.A. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood 2017, 129, 3071–3073. [Google Scholar] [CrossRef] [PubMed]
- Ambady, P.; Szidonya, L.; Firkins, J.; James, J.; Johansson, K.; White, T.; Jezierski, C.; Doolittle, N.D.; Neuwelt, E.A. Combination immunotherapy as a non-chemotherapy alternative for refractory or recurrent CNS lymphoma. Leuk. Lymphoma 2019, 60, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Hoang-Xuan, K.; Houot, R.; Soussain, C.; Blonski, M.; Schmitt, A.; Delwail, V.; Damaj, G.L.; Ghesquieres, H.; Peyrade, F.; Tempescul, A.; et al. First Results of the Acsé Pembrolizumab Phase II in the Primary CNS Lymphoma (PCNSL) Cohort. Blood 2020, 136 (Suppl. 1), 15–16. [Google Scholar] [CrossRef]
- Maarek, A.; Maucort-Boulch, D.; Houillier, C.; Hoang-Xuan, K.; Soussain, C.; Ghesquières, H. P14.45 Primary diffuse large B-cell CNS lymphoma over 80 years: An analysis of 110 patients from the french Oculo-Cerebral Lymphoma (LOC) network. Neuro. Oncol. 2019, 21 (Suppl. 3), iii77. [Google Scholar] [CrossRef]
- Kurzwelly, D.; Glas, M.; Roth, P.; Weimann, E.; Lohner, H.; Waha, A.; Schabet, M.; Reifenberger, G.; Weller, M.; Herrlinger, U. Primary CNS lymphoma in the elderly: Temozolomide therapy and MGMT status. J. Neurooncol. 2010, 97, 389–392. [Google Scholar] [CrossRef][Green Version]
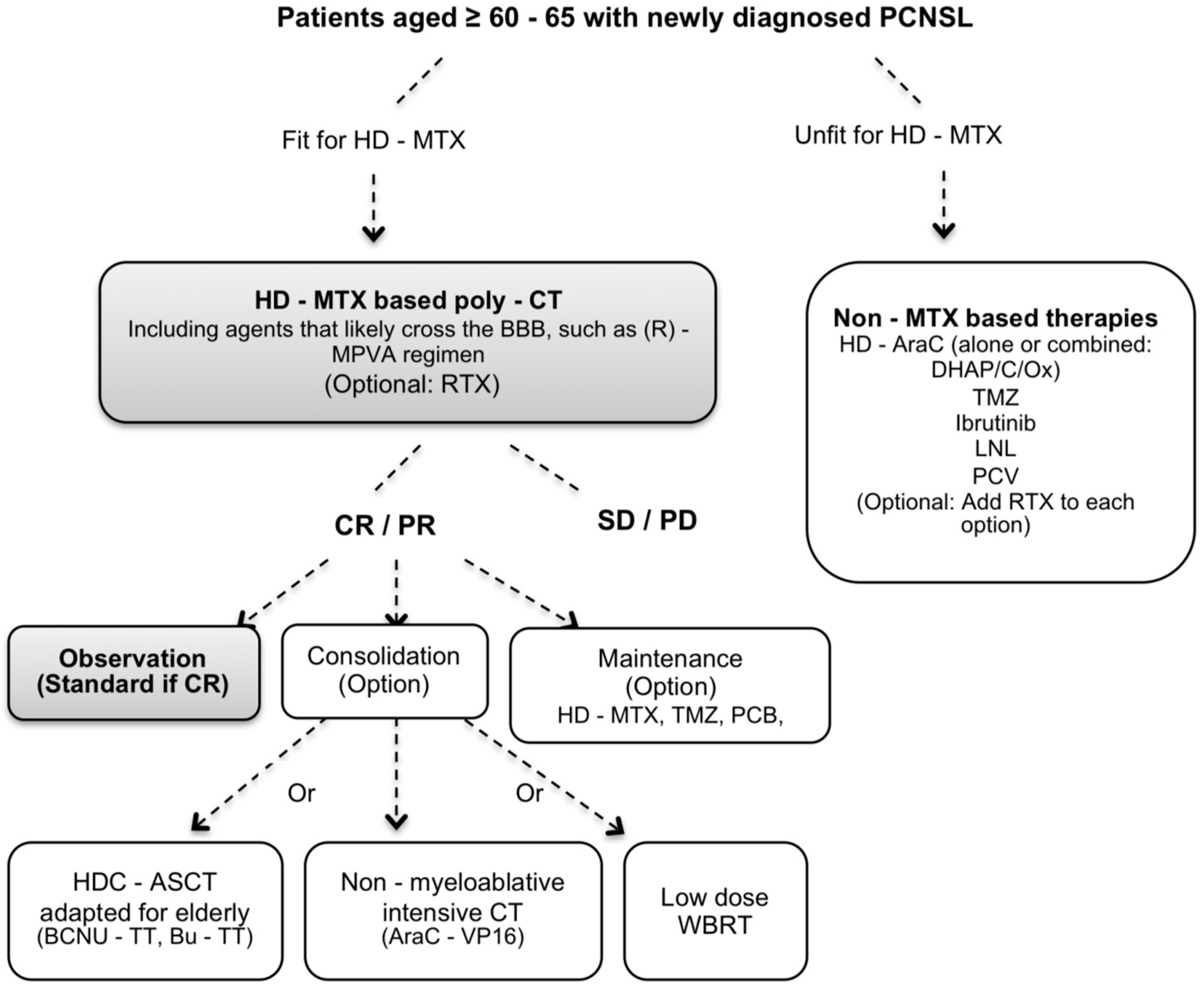
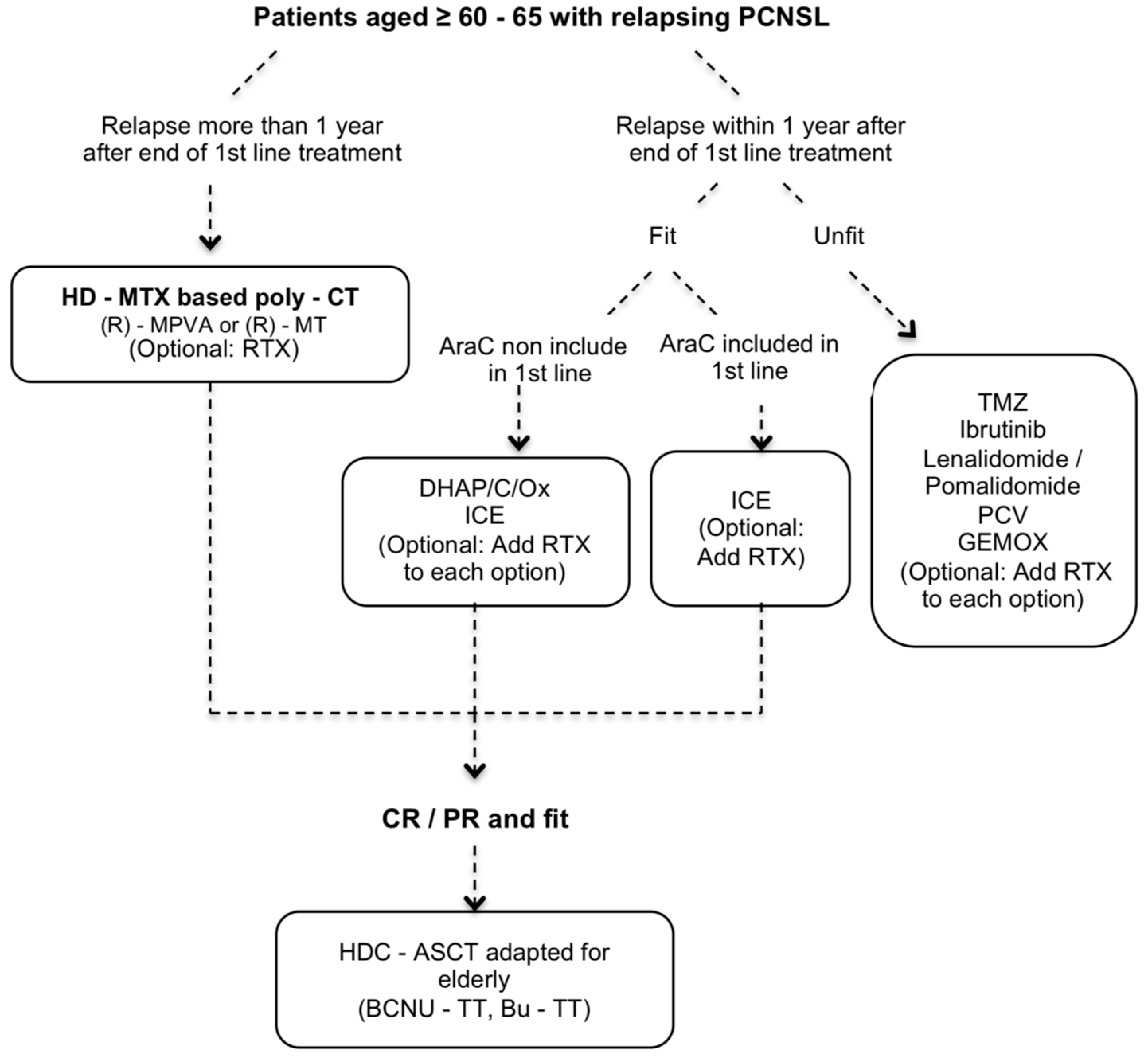
| Author | Type | N | Median Age (Range) | Induction | Consolidation | Maintenance | CR % I/M or C 1 | PFS OS mo | Toxic Deaths % |
|---|---|---|---|---|---|---|---|---|---|
| Hoang-Xuan 2003 [9] | Phase II | 50 | 72 (60–81) | HD-MTX IV + IT, IT ARAC, CCNU, PCB | None | HD-MTX IV + IT, IT ARAC, CCNU, PCB | 42 | 7 14.3 | 4 |
| Omuro 2007 [53] | Retrospective | 23 | 68 (60–79) | HD-MTX, TMZ | None | HD-MTX, TMZ | 30/61 | 8 35 | 4 |
| Zhu 2009 [54] | Retrospective | 31 | 74 (70–85) | HD-MTX | None | HD-MTX | 60 | 7.1 37 | 0 |
| Illerhaus 2009 [55] | Phase II | 30 | 70 (57–79) | HD-MTX, CCNU, PCB | None | None | 44.4 | 5.9 15.4 | 6 |
| Fritsch 2011 [56] | Phase II | 28 | 75 (65–83) | HD-MTX, RTX, PCB, CCNU | None | None | 64 | 16 18 | 7 |
| Roth 2012 [8] | Retrospective | 66 | ≥70 (NR) | HD-MTX based CT | None | None | 64 | 13.9 26.7 | 9 |
| WBRT | 75 | 24.1 29.3 | |||||||
| Olivier 2014 [57] | Phase I | 35 | 65 (61–70) | MTX, VIND, IDA | None | None | 17 | 13 19 | 8.5 |
| Omuro 2015 [22] | Phase II randomized | 48 | 73 (60–85) | HD-MTX, TMZ | None None | None | 38 | 6 14 | 10 |
| 47 | 72 (60–84) | HD-MTX, PCB, VCR, ARAC | 53 | 9.5 31 | 6 | ||||
| Pulczynski 2015 [58] 2 | Phase II | 27 | 70 (66–75) | RTX, HD-MTX, TMZ, IFOS, IV + IT ARAC, VIND | None | TMZ | 69/58 | 14 NA | 15 |
| Schorb 2017 [59] | Retrospective | 15 | 70 (66–75) | HD-MTX based CT | HDC-ASCT (BCNU-TT, Bu-TT + Cy, TT) | None | 27/73 | NA NA | 4 |
| Fritsch 2017 [60] | Phase II | 107 | 73 (66–85) | HD-MTX, RTX, PCB + CCNU | None | PCB | 35.5 | 10.3 20.7 | 8 |
| Houillier 2017 [11] | Retrospective | 90 | 68 (60–87) | RTX, HD-MTX, PCB, VCR | 3 cycles ARAC | None | 55 | 10 28.1 | 6 |
| Faivre 2019 [61] | Retrospective | 10 | 67 (61–76) | MTX, PCB, VCR ± RTX | None | TMZ [6] | 60/80 | 57 63 | 0 |
| Vu 2019 [62] | Retrospective | 13 | 77 (70–86) | MTX, RTX ± TMZ | None | LNL (NR) | 85 | 29.4 31.6 | 0 |
| Schorb 2020 [24] | Pilot trial | 14 | 74 (69–79) | RTX, HD-MTX, ARAC | HDC-ASCT (Bu-TT) | None | 29/85 | NA NA | 0 |
| Clinical Trial for First-Line | Design | Phase | n | Age | Outcomes |
|---|---|---|---|---|---|
| Induction therapy | |||||
| NCT02836158 | RTX, IDA, ARAC, MTX + IT RTX, MTX, ARAC | 2 | 100 | 60–75 | 3-years OS |
| Induction (I) + Consolidation (C) treatment | |||||
| NCT03569995(CREMA) | I: RTX, MTX + C: RTX, ARAC | 2 | 35 | ≥60 | 2-years PFS PFS, OS, FAE, TTF |
| NCT01399372 | I: RTX, MTX, PCB, VCR + C: ARAC or Low dose WBRT | 2 | 91 | ≥18 | PFS OS, Response rate, Quality of life, Neurocognitive function |
| DRKS00011932 | I: RTX, MTX, ARAC + C: HDT-ASCT (RTX, BU, TT) | 2 | 51 | ≥65 | 1-year PFS CR rate, OS, PFS, Neurotoxicity, FAE |
| Induction (I) + Maintenance (M) treatment | |||||
| NCT03495960 (FIORELLA) | (A) I: RTX, MTX, PCB + M: PCB or LNL | 2 | 208 | ≥70 | 2-years PFS TTF, Response Rates, OS, FAE, Relapse rates and patterns, Neurotoxicity |
| (B) I: WBRT- RTX, TMZ + M: TMZ | |||||
| Maintenance treatment | |||||
| NCT02623010 | Ibrutinib | 2 | 30 | 60–85 | PFS OS |
| NCT04627753 (LEMON-C) | RTX-LNL | 2 | 30 | ≥65 | 1-year PFS 2-years PFS, OS, Overall response, Toxicity profiles |
| NCT04022980 | Nivolumab (After HD-MTX based chemotherapy) | 1 | 20 | ≥65 | Dose-limiting toxicity, 2-years PFS PFS, OS, Response Rates, Conversion Rate (Partial to Complete Response) |
| NCT02313389 (BLOCAGE-1) | M: RTX, MTX, TMZ or Observation (After HD-MTX based polychemotherapy) | 3 | 295 | ≥60 | PFS OS, Toxicity, Cognitive functions, Quality of life |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Martinez, A.; Lozano-Sanchez, F.; Duran-Peña, A.; Hoang-Xuan, K.; Houillier, C. Primary Central Nervous System Lymphoma in Elderly Patients: Management and Perspectives. Cancers 2021, 13, 3479. https://doi.org/10.3390/cancers13143479
Morales-Martinez A, Lozano-Sanchez F, Duran-Peña A, Hoang-Xuan K, Houillier C. Primary Central Nervous System Lymphoma in Elderly Patients: Management and Perspectives. Cancers. 2021; 13(14):3479. https://doi.org/10.3390/cancers13143479
Chicago/Turabian StyleMorales-Martinez, Andrea, Fernando Lozano-Sanchez, Alberto Duran-Peña, Khe Hoang-Xuan, and Caroline Houillier. 2021. "Primary Central Nervous System Lymphoma in Elderly Patients: Management and Perspectives" Cancers 13, no. 14: 3479. https://doi.org/10.3390/cancers13143479
APA StyleMorales-Martinez, A., Lozano-Sanchez, F., Duran-Peña, A., Hoang-Xuan, K., & Houillier, C. (2021). Primary Central Nervous System Lymphoma in Elderly Patients: Management and Perspectives. Cancers, 13(14), 3479. https://doi.org/10.3390/cancers13143479






