Head-to-Head Comparison of Neck 18F-FDG PET/MR and PET/CT in the Diagnosis of Differentiated Thyroid Carcinoma Patients after Comprehensive Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
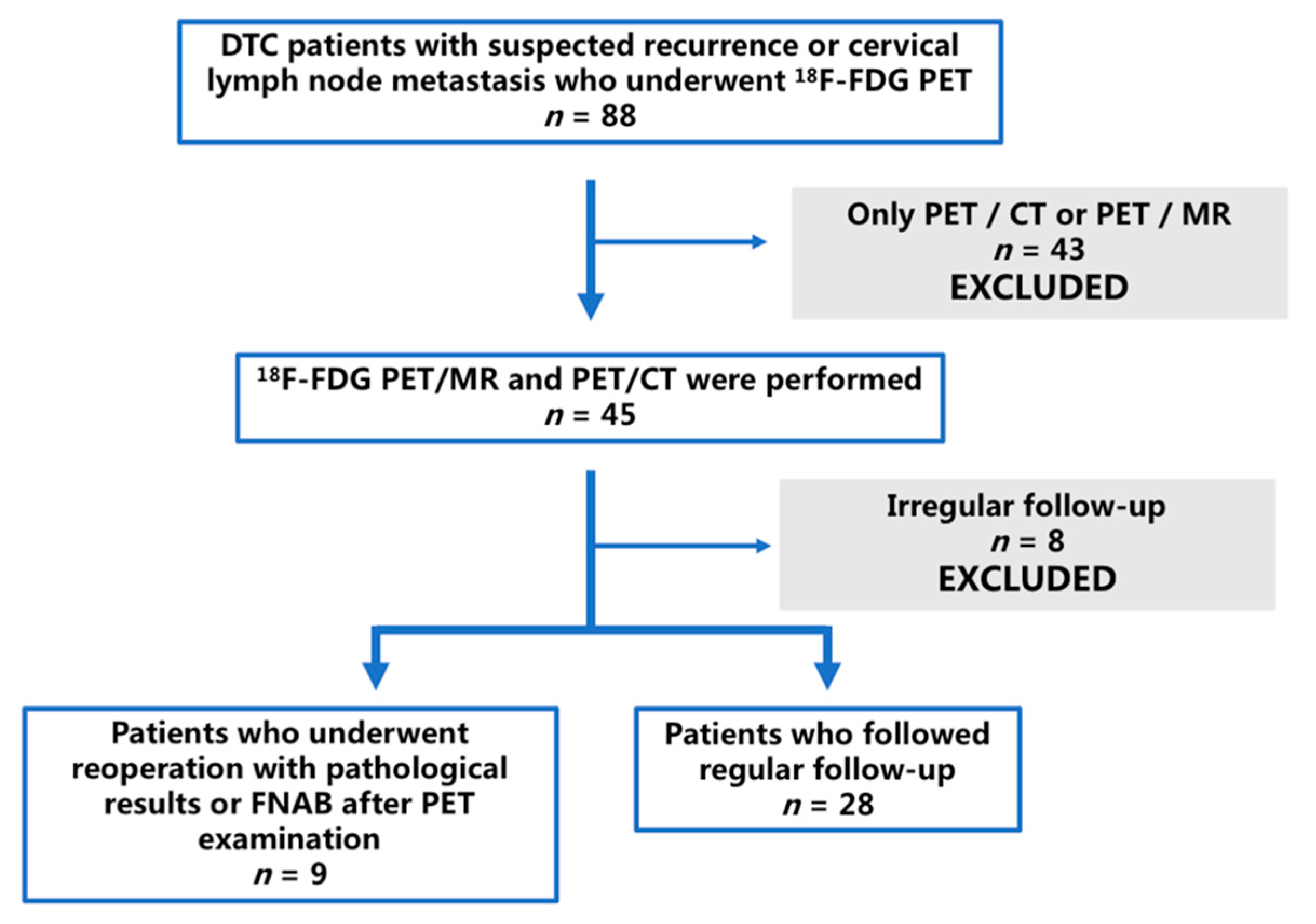
2.1. Patients and Lesions
2.2. 18F-FDG PET/CT Scan
2.3. 18F-FDG PET/MR Scan
2.4. Image Interpretation and Analysis
- (1)
- lesion counts detected on PET/CT and PET/MR, respectively;
- (2)
- lesion conspicuity scoring according to [26] (assessed independently): 1 point for no detection; 2 points for detected suspicious morphological correlation; 3 points for clear morphological correlation;
- (3)
- lesion diagnosis (any difference of opinion resolved by consensus): benign or malignant.
- (1)
- lesion diameters: long and short diameters as referred in RECIST 1.1 [27];
- (2)
- standardized uptake value (SUV) for lesions calculated automatically by the workstation: SUVmax and SUVmean.
2.5. Local Recurrence and Metastatic Lymph Nodes
2.6. Statistical Analyses
3. Results
3.1. Patient-Based Analysis
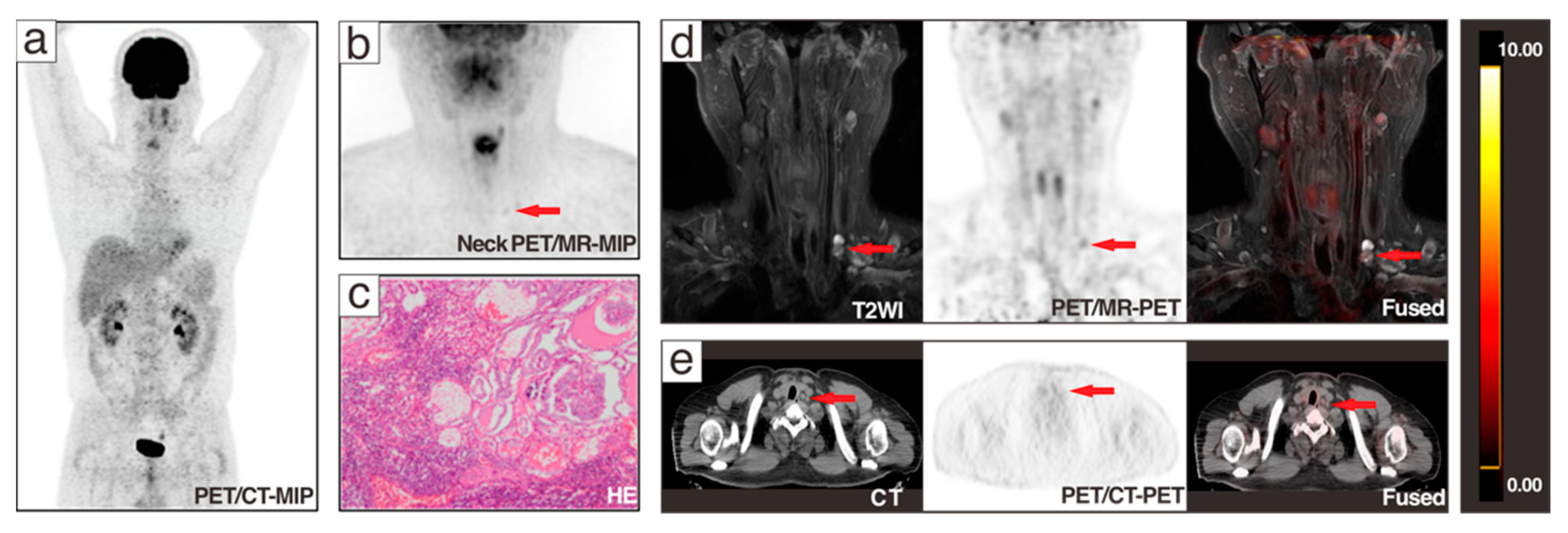
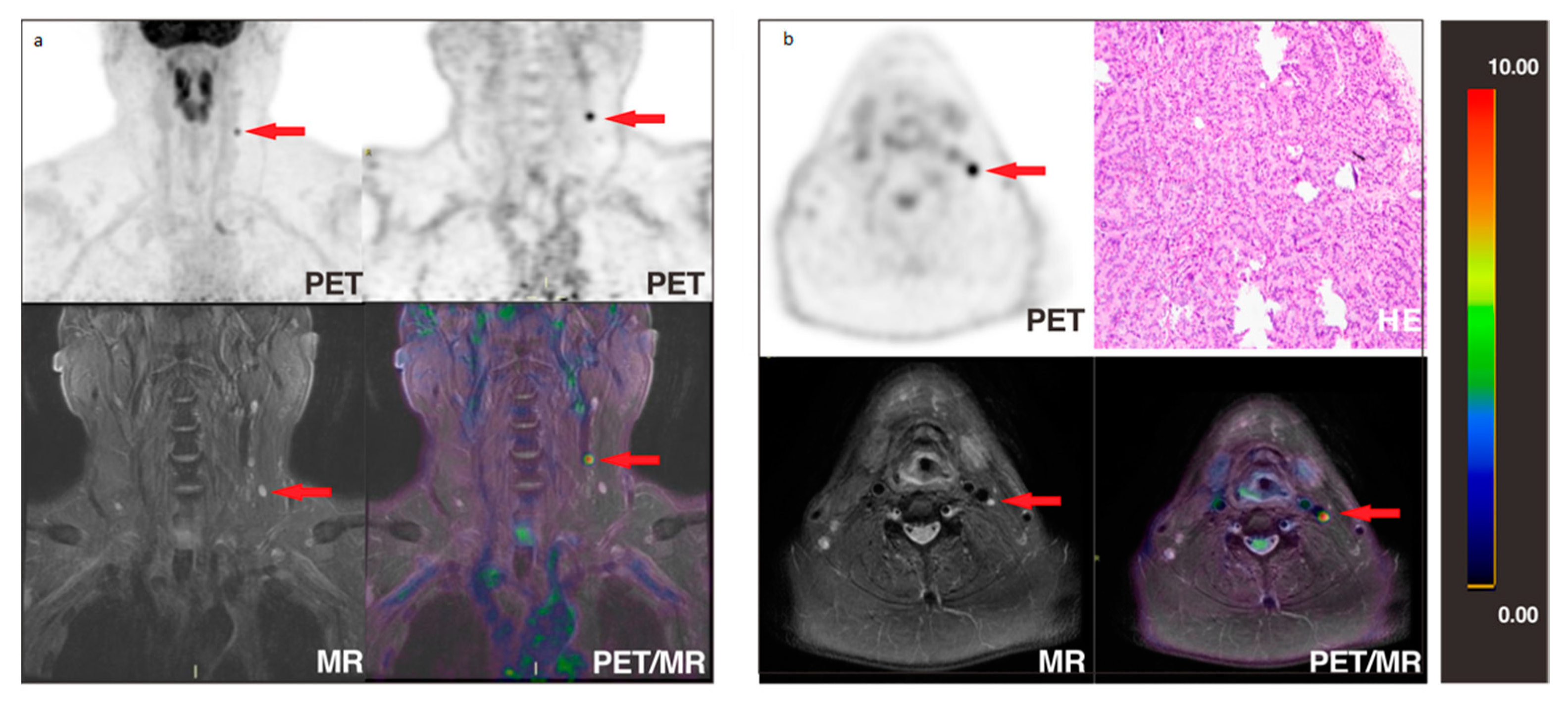
3.2. Lesion-Based Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, H.; Wang, M.; Li, N.; Tian, T.; Wu, Y.; Xu, P.; Yang, S.; Zhai, Z.; Zhou, L.; et al. Global Burden of Thyroid Cancer From 1990 to 2017. JAMA Netw. Open 2020, 3, e208759. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, E.; Moslem, A.; Feizhadad, H.; Jarrahi, A.; Adineh, H.; Sohrabivafa, M.; Khazaei, Z. Epidemiology, incidence and mortality of thyroid cancer and their relationship with the human development index in the world: An ecology study in 2018. Adv. Hum. Biol. 2019, 9, 162–167. [Google Scholar]
- Sherman, S.I. Thyroid carcinoma. Lancet 2003, 361, 501–511. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, Y.; Liang, Z.; Zhang, L.; Mao, X.; Yang, X.; Wang, Y.; Xu, J.; Liu, R.; Zhu, S.; et al. Cervical soft tissue recurrence of differentiated thyroid carcinoma after thyroidectomy indicates a poor prognosis. Int. J. Surg. 2017, 48, 254–259. [Google Scholar] [CrossRef]
- Han, J.M.; Bae, J.C.; Kim, H.I.; Kwon, S.; Jeon, M.J.; Kim, W.G.; Kim, T.Y.; Shong, Y.K.; Kim, W.B. Clinical Outcomes of Differentiated Thyroid Cancer Patients with Local Recurrence or Distant Metastasis Detected in Old Age. Endocrinol. Metab. 2018, 33, 459–465. [Google Scholar] [CrossRef]
- Liu, F.H.; Kuo, S.F.; Hsueh, C.; Chao, T.C.; Lin, J.D. Postoperative recurrence of papillary thyroid carcinoma with lymph node metastasis. J. Surg. Oncol. 2015, 112, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Thyroid Carcinoma. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (accessed on 9 April 2021).
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Bertagna, F.; Bosio, G.; Biasiotto, G.; Rodella, C.; Puta, E.; Gabanelli, S.; Lucchini, S.; Merli, G.; Savelli, G.; Giubbini, R.; et al. F-18 FDG-PET/CT evaluation of patients with differentiated thyroid cancer with negative I-131 total body scan and high thyroglobulin level. Clin. Nucl. Med. 2009, 34, 756–761. [Google Scholar] [CrossRef]
- Lu, C.Z.; Cao, S.S.; Wang, W.; Liu, J.; Fu, N.; Lu, F. Usefulness of PET/CT in the diagnosis of recurrent or metastasized differentiated thyroid carcinoma. Oncol. Lett. 2016, 11, 2420–2423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chao, M. Management of Differentiated Thyroid Cancer with Rising Thyroglobulin and Negative Diagnostic Radioiodine Whole Body Scan. Clin. Oncol. 2010, 22, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Linee Guida SIE-AIMN-AIFM per il Trattamento e Follow-Up del Carcinoma Differenziato della Tiroide. Available online: https://www.aimn.it/pubblicazioni/LG/LG_ca_tiroide.pdf (accessed on 1 February 2019).
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Thyroid Cancer, Guidelines Working Committee of Chinese Society of Clinical Oncology. Guidelines of Chinese So-ciety of Clinical Oncology (CSCO): Persistent/Recurrent and Metastatic Differentiated Thyroid Cancer-2019. J. Cancer Control. Treat. 2019, 32, 1051–1080. [Google Scholar]
- Hempel, J.M.; Kloeckner, R.; Krick, S.; Pinto Dos Santos, D.; Schadmand-Fischer, S.; Boeßert, P.; Bisdas, S.; Weber, M.M.; Fottner, C.; Musholt, T.J.; et al. Impact of combined FDG-PET/CT and MRI on the detection of local recurrence and nodal metastases in thyroid cancer. Cancer Imaging 2016, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Haslerud, T.; Brauckhoff, K.; Reisæter, L.; Küfner Lein, R.; Heinecke, A.; Varhaug, J.E.; Biermann, M. F18-FDG-PET for recurrent differentiated thyroid cancer: A systematic meta-analysis. Acta Radiol. 2016, 57, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Takashima, S.; Takayama, F.; Kawakami, S.; Saito, A.; Matsushita, T.; Matsuba, H.; Kobayashi, S. Tracheal invasion by thyroid carcinoma: Prediction using MR imaging. AJR Am. J. Roentgenol. 2001, 177, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Khizer, A.T.; Raza, S.; Slehria, A.U. Diffusion-weighted MR Imaging and ADC Mapping in Differentiating Benign from Malignant Thyroid Nodules. J. Coll. Physicians Surg. Pak. 2015, 25, 785–788. [Google Scholar]
- Shi, R.; Yao, Q.; Wu, L.; Zhou, Q.; Lu, Q.; Gao, R.; Hu, J.; Kao, L.; Bains, A.; Yan, Z.; et al. T2* mapping at 3.0T MRI for differentiation of papillary thyroid carcinoma from benign thyroid nodules. J. Magn. Reson. Imaging 2016, 43, 956–961. [Google Scholar] [CrossRef]
- Park, J.; Pak, K.; Yun, T.J.; Lee, E.K.; Ryoo, I.; Lee, J.Y.; Hwang, I.; Yoo, R.E.; Kang, K.M.; Choi, S.H.; et al. Diagnostic Accuracy and Confidence of [18F] FDG PET/MRI in comparison with PET or MRI alone in Head and Neck Cancer. Sci. Rep. 2020, 10, 9490. [Google Scholar] [CrossRef]
- Kim, S.G.; Friedman, K.; Patel, S.; Hagiwara, M. Potential Role of PET/MRI for Imaging Metastatic Lymph Nodes in Head and Neck Cancer. AJR Am. J. Roentgenol. 2016, 207, 248–256. [Google Scholar] [CrossRef]
- Byeon, Y.H.; Choi, J.E.; Park, J.Y.; Song, J.H.; Yeo, K.J.; Kong, E.J.; Kang, S.H.; Lee, S.J. Diagnostic Accuracy of PET/MR for Evaluating Central Lymph Node Status in Patients with Papillary Thyroid Carcinoma. J. Endocr. Surg. 2017, 17, 122–130. [Google Scholar] [CrossRef]
- Platzek, I.; Beuthien-Baumann, B.; Schneider, M.; Gudziol, V.; Kitzler, H.H.; Maus, J.; Schramm, G.; Popp, M.; Laniado, M.; Kotzerke, J.; et al. FDG PET/MR for lymph node staging in head and neck cancer. Eur. J. Radiol. 2014, 83, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Schlittenbauer, T.; Zeilinger, M.; Nkenke, E.; Kreißel, S.; Wurm, M.C.; Lell, M.; Kuwert, T.; Beck, M. Positron emission tomography-computed tomography versus positron emission tomography-magnetic resonance imaging for diagnosis of oral squamous cell carcinoma: A pilot study. J. Craniomaxillofac. Surg. 2015, 43, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Vrachimis, A.; Burg, M.C.; Wenning, C.; Allkemper, T.; Weckesser, M.; Schäfers, M.; Stegger, L. [(18)F]FDG PET/CT outperforms [(18)F]FDG PET/MRI in differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, P.; Wernecke, K.; Roos, N.; Peters, P.E. Differentiation of benign from malignant superficial lymphadenopathy: The role of high-resolution US. Radiology 1992, 183, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Rosário, P.W.; de Faria, S.; Bicalho, L.; Alves, M.F.; Borges, M.A.; Purisch, S.; Padrão, E.L.; Rezende, L.L.; Barroso, A.L. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J. Ultrasound Med. 2005, 24, 1385–1389. [Google Scholar] [CrossRef]
- Kuna, S.K.; Bracic, I.; Tesic, V.; Kuna, K.; Herceg, G.H.; Dodig, D. Ultrasonographic differentiation of benign from malignant neck lymphadenopathy in thyroid cancer. J. Ultrasound Med. 2006, 25, 1531–1537; quiz 1538–1540. [Google Scholar] [CrossRef] [PubMed]
- Sadigh, G.; Carlos, R.C.; Neal, C.H.; Dwamena, B.A. Ultrasonographic differentiation of malignant from benign breast lesions: A meta-analytic comparison of elasticity and BIRADS scoring. Breast Cancer Res. Treat. 2012, 133, 23–35. [Google Scholar] [CrossRef]
- Sakai, O.; Curtin, H.D.; Romo, L.V.; Som, P.M. Lymph node pathology. Benign proliferative, lymphoma, and metastatic disease. Radiol. Clin. North. Am. 2000, 38, 979–998. [Google Scholar]
- Kim, E.; Park, J.S.; Son, K.R.; Kim, J.H.; Jeon, S.J.; Na, D.G. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: Comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid 2008, 18, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.L.; Yoon, D.Y.; Baek, S.; Ku, Y.J.; Rho, Y.S.; Chung, E.J.; Koh, S.H. Detection of neck recurrence in patients with differentiated thyroid cancer: Comparison of ultrasound, contrast-enhanced CT and (18)F-FDG PET/CT using surgical pathology as a reference standard: (ultrasound vs. CT vs. (18)F-FDG PET/CT in recurrent thyroid cancer). Eur. Radiol. 2012, 22, 2246–2254. [Google Scholar]
- Becker, M.; Zaidi, H. Imaging in head and neck squamous cell carcinoma: The potential role of PET/MRI. Br. J. Radiol. 2014, 87, 20130677. [Google Scholar] [CrossRef]
- Abouzied, M.M.; Crawford, E.S.; Nabi, H.A. 18F-FDG imaging: Pitfalls and artifacts. J. Nucl. Med. Technol. 2005, 33, 145–155, quiz 162–143. [Google Scholar] [PubMed]
- Owrangi, A.M.; Greer, P.B.; Glide-Hurst, C.K. MRI-only treatment planning: Benefits and challenges. Phys. Med. Biol. 2018, 63, 05tr01. [Google Scholar] [CrossRef]
- Som, P.M.; Brandwein, M.; Lidov, M.; Lawson, W.; Biller, H.F. The varied presentations of papillary thyroid carcinoma cervical nodal disease: CT and MR findings. AJNR Am. J. Neuroradiol. 1994, 15, 1123. [Google Scholar]
- Park, S.-H.; Hahm, M.H.; Bae, B.K.; Chong, G.O.; Jeong, S.Y.; Na, S.; Jeong, S.; Kim, J.-C. Magnetic resonance imaging features of tumor and lymph node to predict clinical outcome in node-positive cervical cancer: A retrospective analysis. Radiat. Oncol. 2020, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Hedgire, S.; Harisinghani, M. Radiologic Assessment of Lymph Nodes in Oncologic Patients. Curr. Radiol. Rep. 2014, 2, 36. [Google Scholar] [CrossRef]
- Binse, I.; Poeppel, T.D.; Ruhlmann, M.; Gomez, B.; Umutlu, L.; Bockisch, A.; Rosenbaum-Krumme, S.J. Imaging with (124)I in differentiated thyroid carcinoma: Is PET/MRI superior to PET/CT? Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1011–1017. [Google Scholar] [CrossRef]
- Chong, V. Cervical lymphadenopathy: What radiologists need to know. Cancer Imaging 2004, 4, 116–120. [Google Scholar] [CrossRef]
- Yang, J.H.; Maciel, R.M.B.; Nakabashi, C.C.D.; Janovsky, C.; Padovani, R.P.; Macellaro, D.; Camacho, C.P.; Osawa, A.; Wagner, J.; Biscolla, R.P.M. Clinical utility of 18F-FDG PET/CT in the follow-up of a large cohort of patients with high-risk differentiated thyroid carcinoma. Arch. Endocrinol. Metab. 2017, 61, 416–425. [Google Scholar] [CrossRef]
- von Falck, C.; Boerner, A.R.; Galanski, M.; Knapp, W.H. Neuroendocrine tumour of the mediastinum: Fusion of 18F-FDG and 68Ga-DOTATOC PET/CT datasets demonstrates different degrees of differentiation. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 812. [Google Scholar] [CrossRef][Green Version]
- Kurokawa, T.; Yoshida, Y.; Kawahara, K.; Tsuchida, T.; Okazawa, H.; Fujibayashi, Y.; Yonekura, Y.; Kotsuji, F. Expression of GLUT-1 glucose transfer, cellular proliferation activity and grade of tumor correlate with [F-18]-fluorodeoxyglucose uptake by positron emission tomography in epithelial tumors of the ovary. Int. J. Cancer 2004, 109, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Subesinghe, M.; Scarsbrook, A.F.; Sourbron, S.; Wilson, D.J.; McDermott, G.; Speight, R.; Roberts, N.; Carey, B.; Forrester, R.; Gopal, S.V.; et al. Alterations in anatomic and functional imaging parameters with repeated FDG PET-CT and MRI during radiotherapy for head and neck cancer: A pilot study. BMC Cancer 2015, 15, 137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loeffelbein, D.J.; Souvatzoglou, M.; Wankerl, V.; Dinges, J.; Ritschl, L.M.; Mücke, T.; Pickhard, A.; Eiber, M.; Schwaiger, M.; Beer, A.J. Diagnostic value of retrospective PET-MRI fusion in head-and-neck cancer. BMC Cancer 2014, 14, 846. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, Y.; Tamai, K.; Saga, T.; Higashi, T.; Hara, T.; Suga, T.; Koyama, T.; Togashi, K. Clinical value of image fusion from MR and PET in patients with head and neck cancer. Mol. Imaging Biol. 2009, 11, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Seiboth, L.; Van Nostrand, D.; Wartofsky, L.; Ousman, Y.; Jonklaas, J.; Butler, C.; Atkins, F.; Burman, K. Utility of PET/neck MRI digital fusion images in the management of recurrent or persistent thyroid cancer. Thyroid 2008, 18, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Friedman, K.; Chandarana, H.; Melsaether, A.; Moy, L.; Ding, Y.S.; Jhaveri, K.; Beltran, L.; Jain, R. Current Status of Hybrid PET/MRI in Oncologic Imaging. AJR Am. J. Roentgenol. 2016, 206, 162–172. [Google Scholar] [CrossRef]

| Characteristics | Number | |
|---|---|---|
| Age | 38.95 ± 12.03 years (range, 12–68 years) | |
| <55 years | 33 (89.2%) | |
| >55 years | 4 (10.8%) | |
| Sex | Male | 12 (32.4%) |
| Female | 25 (67.6%) | |
| Histologic type | PTC 1 | 29 (78.4%) |
| PTMC 2 | 4 (10.8%) | |
| PTC 1 + PTMC 2 | 3 (8.1%) | |
| FTC 3 | 1 (2.7%) | |
| Stage of disease | I | 32 (86.5%) |
| II | 2 (5.4%) | |
| IV | 3 (8.1%) | |
| Clinical indication for PET Imaging | Positive Tg 4 and negative 131I-WBS 5 | 25 (67.6%) |
| Rising TgAb 6 after radioactive iodine ablation | 4 (10.8%) | |
| Suspected metastasis detection | 8 (21.6%) | |
| Gold standard source | Pathology after reoperation | 7 (18.9%) |
| Fine-needle aspiration | 2 (5.4%) | |
| Regular follow-up | 28 (75.7%) | |
| Golden Standard | PET/MR | PET/CT | ||
|---|---|---|---|---|
| Malignant | Thyroid nodules | 3 | 2 | 1 |
| Lymph nodes | 74 | 64 | 53 | |
| Benign | Thyroid nodules | 6 | 6 | 5 |
| Lymph nodes | 47 | 47 | 46 | |
| Total | 130 | 119 | 105 | |
| All Lesions | Malignant Thyroid Nodules | Metastatic Nodes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PET/MR | MR | PET/CT | PET/MR | MR | PET/CT | PET/MR | MR | PET/CT | |
| True-positive | 62 | 60 | 47 | 2 | 2 | 1 | 60 | 58 | 46 |
| True-negative | 45 | 44 | 43 | 4 | 4 | 4 | 41 | 40 | 39 |
| False-positive | 8 | 9 | 10 | 2 | 2 | 2 | 6 | 7 | 8 |
| False-negative | 15 | 17 | 30 | 1 | 1 | 2 | 14 | 16 | 28 |
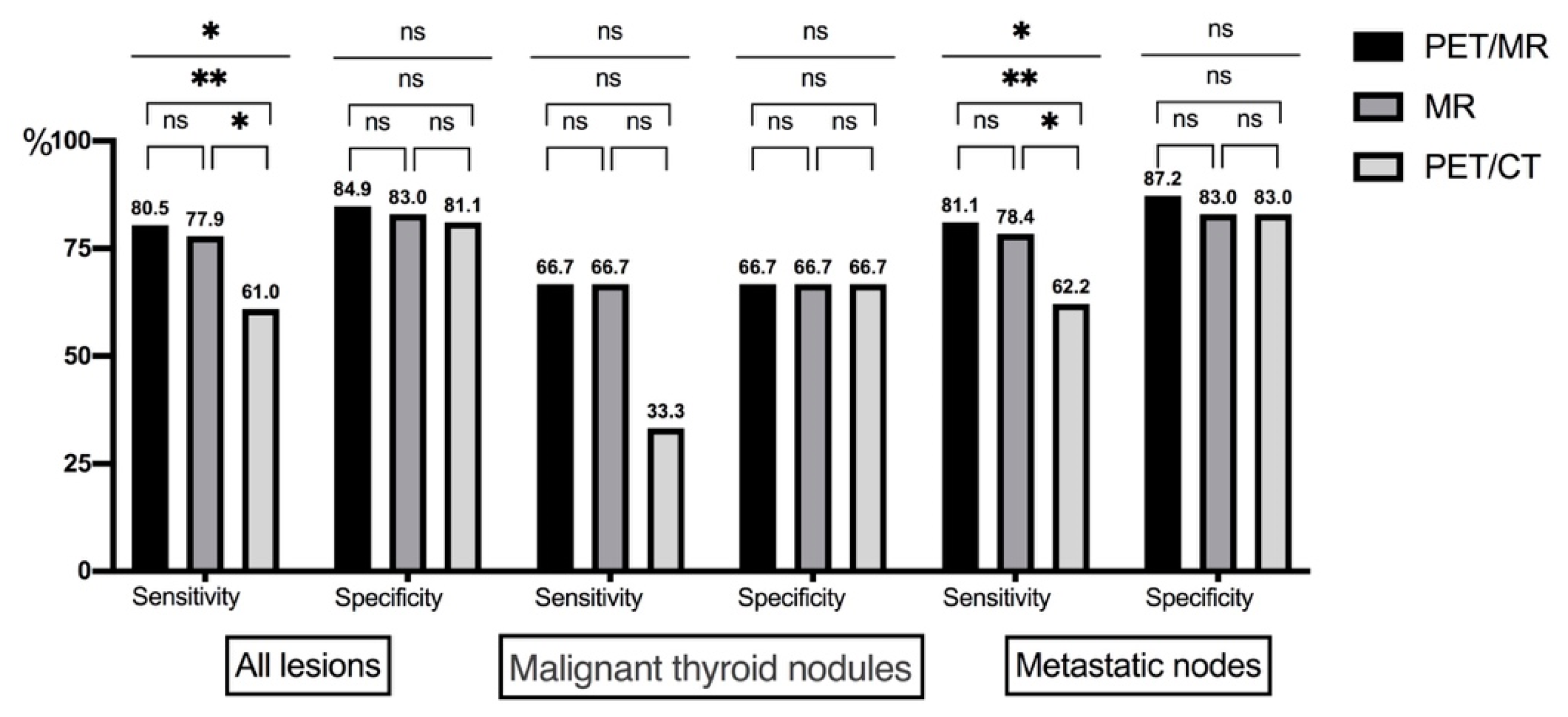
| Sensitivity (%) | 80.5% | 77.9% | 61.0% | 66.7% | 66.7% | 33.3% | 81.1% | 78.4% | 62.2% |
| Specificity (%) | 84.9% | 83.0% | 81.1% | 66.7% | 66.7% | 66.7% | 87.2% | 85.1% | 83.0% |
| PPV (%) | 88.6% | 87.0% | 82.5% | 50.0% | 50.0% | 33.3% | 90.9% | 89.2% | 85.2% |
| NPV (%) | 75.0% | 72.1% | 58.9% | 80.0% | 80.0% | 66.7% | 74.5% | 71.4% | 58.2% |
| Accuracy (%) | 82.3% | 80.0% | 69.2% | 66.7% | 66.7% | 55.6% | 83.5% | 81.0% | 70.2% |
| All Lesions | Malignant | Benign | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | PET/MR | 18F-PET/CT | p | No. | PET/MR | PET/CT | p | No. | PET/MR | 1PET/CT | p | ||
| Thyroid area | 9 | 2.56 ± 0.73 | 2.00 ± 0.707 | 0.027 | 3 | 2.00 ± 0.894 | 2.00 ± 0.894 | 1 | 6 | 2.83 ± 0.39 | 2.00 ± 0.60 | 0.008 | |
| Lymph nodes | II | 53 (43.8%) | 2.92 ± 0.33 | 1.91 ± 0.45 | <0.001 | 24 (45.3%) | 2.83 ± 0.48 | 1.79 ± 0.58 | <0.001 | 29 (54.7%) | 3.00 ± 0.00 | 2.02 ± 0.25 | <0.001 |
| III | 16 (13.2%) | 2.38 ± 0.79 | 1.84 ± 0.52 | <0.001 | 13 (81.3%) | 2.31 ± 0.84 | 1.81 ± 0.57 | 0.003 | 3 (18.8%) | 2.67 ± 0.52 | 2.00 ± 0.00 | 0.46 | |
| IV | 18 (14.9%) | 2.67 ± 0.68 | 1.97 ± 0.56 | <0.001 | 11 (61.1%) | 2.45 ± 0.82 | 1.82 ± 0.60 | 0.035 | 7 (38.9%) | 3.00 ± 0.00 | 2.14 ± 0.36 | 0.001 | |
| V | 19 (15.7%) | 2.74 ± 0.64 | 1.92 ± 0.54 | <0.001 | 13 (68.4%) | 2.73 ± 0.67 | 1.85 ± 0.46 | <0.001 | 6 (31.6%) | 2.75 ± 0.62 | 2.08 ± 0.67 | 0.11 | |
| VI | 15 (12.4%) | 2.50 ± 0.76 | 1.73 ± 0.45 | 0.002 | 13 (86.7%) | 2.54 ± 0.74 | 1.82 ± 0.55 | 0.002 | 2 (13.3%) | 3.00 ± 0.00 | 2.50 ± 0.71 | <0.001 | |
| Total | 121 | 2.74 ± 0.60 | 1.9 ± 0.50 | <0.001 | 74 | 2.61 ± 0.72 | 1.8 ± 0.57 | <0.001 | 47 | 2.96 ± 0.20 | 2.04 ± 0.29 | <0.001 | |
| Kappa = 0.981, p < 0.001 | |||||||||||||
| PET/MR | PET/CT | ||
|---|---|---|---|
| All detected lymph nodes n = 99 | SUVmax | 2.3 (2.0–2.8) 1 | 1.9 (1.5–2.2) |
| SUVmean | 1.8 (1.5–2.2) | 1.4 (1.1–1.8) | |
| Long axis | 9.4 (7.2–11.7) | 7.3 (5.3–9.5) | |
| Short axis | 5.0 (4.0–6.2) | 4.1 (3.0–5.3) | |
| Malignant n = 54 | SUVmax | 2.6 (2.0–3.5) | 2.0 (1.6–2.3) |
| SUVmean | 1.9 (1.5–2.5) | 1.4 (1.2–1.9) | |
| Long axis | 9.7 (7.3–11.4) | 7.3 (5.0–9.4) | |
| Short axis | 4.7 (3.8–6.9) | 3.7 (3.0–5.0) | |
| Benign n = 45 | SUVmax | 2.2 (1.9–2.6) | 1.8 (1.5–2.0) |
| SUVmean | 1.7 (1.5–2.0) | 1.4 (1.0–1.8) | |
| Long axis | 8.9 (7.0–12.7) | 7.3 (5.5–9.6) | |
| Short axis | 5.3 (4.5–6.1) | 4.4 (3.3–5.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Liu, F.; Ruan, W.; Hu, F.; Younis, M.H.; Gao, Z.; Ming, J.; Huang, T.; Cai, W.; Lan, X. Head-to-Head Comparison of Neck 18F-FDG PET/MR and PET/CT in the Diagnosis of Differentiated Thyroid Carcinoma Patients after Comprehensive Treatment. Cancers 2021, 13, 3436. https://doi.org/10.3390/cancers13143436
Song Y, Liu F, Ruan W, Hu F, Younis MH, Gao Z, Ming J, Huang T, Cai W, Lan X. Head-to-Head Comparison of Neck 18F-FDG PET/MR and PET/CT in the Diagnosis of Differentiated Thyroid Carcinoma Patients after Comprehensive Treatment. Cancers. 2021; 13(14):3436. https://doi.org/10.3390/cancers13143436
Chicago/Turabian StyleSong, Yangmeihui, Fang Liu, Weiwei Ruan, Fan Hu, Muhsin H. Younis, Zairong Gao, Jie Ming, Tao Huang, Weibo Cai, and Xiaoli Lan. 2021. "Head-to-Head Comparison of Neck 18F-FDG PET/MR and PET/CT in the Diagnosis of Differentiated Thyroid Carcinoma Patients after Comprehensive Treatment" Cancers 13, no. 14: 3436. https://doi.org/10.3390/cancers13143436
APA StyleSong, Y., Liu, F., Ruan, W., Hu, F., Younis, M. H., Gao, Z., Ming, J., Huang, T., Cai, W., & Lan, X. (2021). Head-to-Head Comparison of Neck 18F-FDG PET/MR and PET/CT in the Diagnosis of Differentiated Thyroid Carcinoma Patients after Comprehensive Treatment. Cancers, 13(14), 3436. https://doi.org/10.3390/cancers13143436








