Association of Physical Activity with the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Physical Activity
2.3. Clinical Evaluation and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
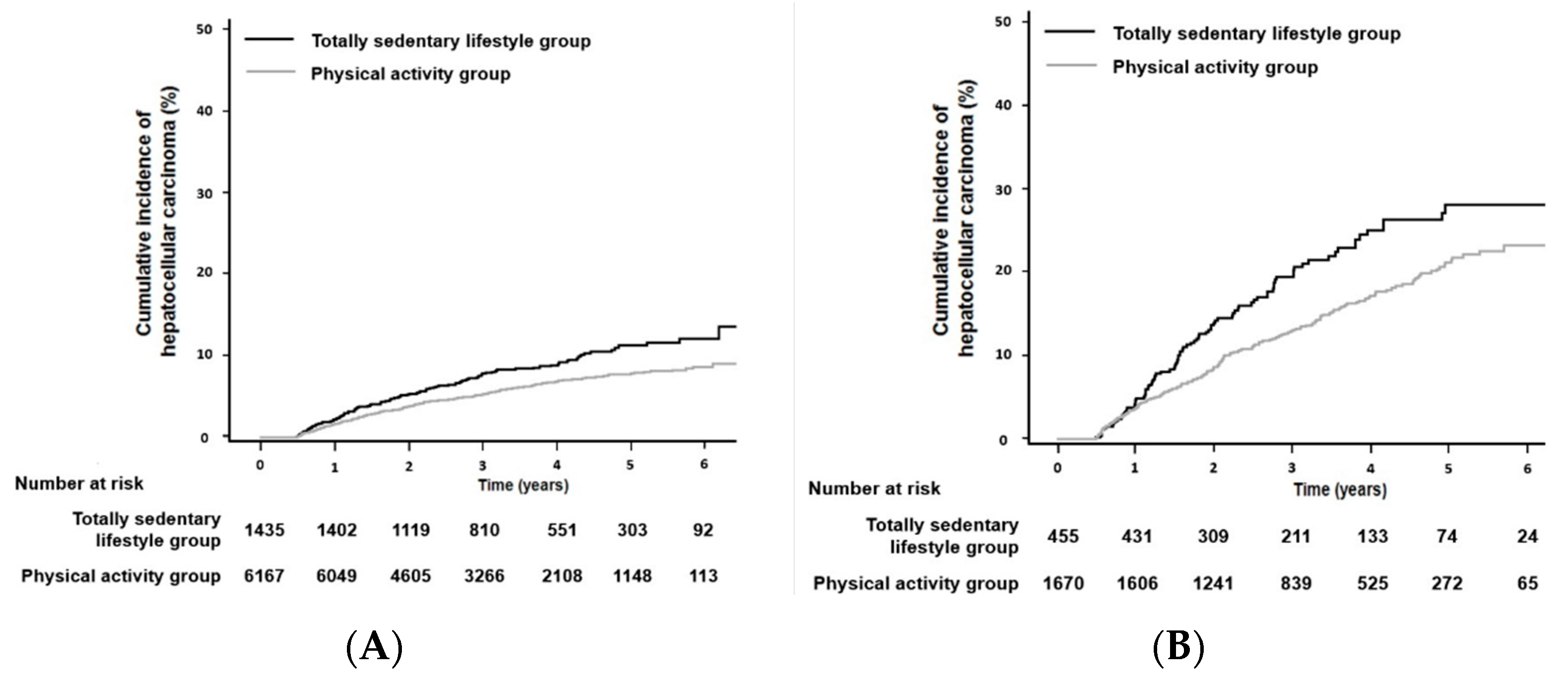
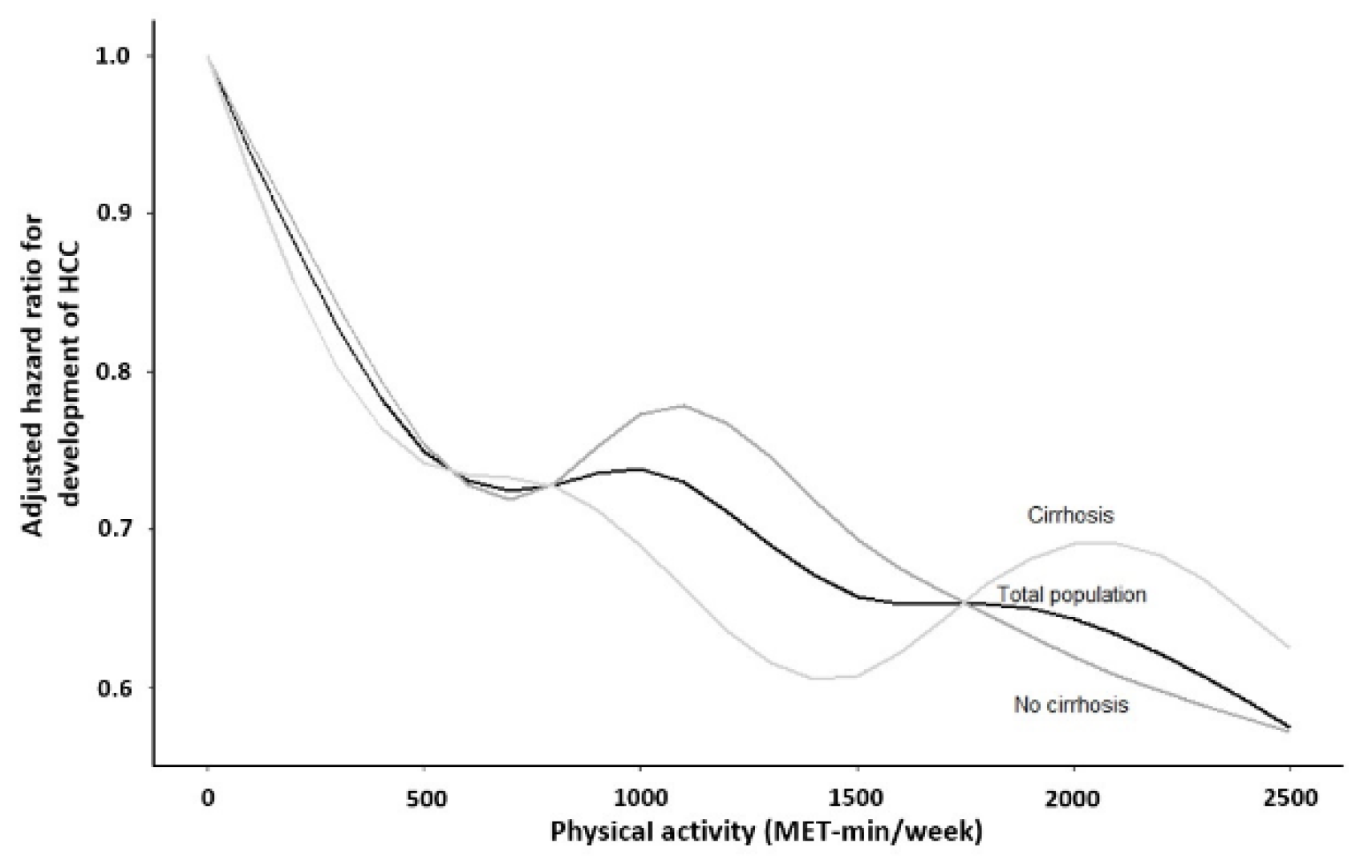
3.2. Association between Physical Activity and the Development of HCC
3.3. Association of Physical Activity with HCC Development According to Subgroups of Age, Sex, Diabetes, and BMI at Baseline
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Papatheodoridis, G.V.; Dalekos, G.N.; Idilman, R.; Sypsa, V.; Van Boemmel, F.; Buti, M.; Calleja, J.L.; Goulis, J.; Manolakopoulos, S.; Loglio, A.; et al. Similar risk of hepatocellular carcinoma during long-term entecavir or tenofovir therapy in Caucasian patients with chronic hepatitis B. J. Hepatol. 2020, 73, 1037–1045. [Google Scholar] [CrossRef]
- Yim, H.J.; Kim, J.H.; Park, J.Y.; Yoon, E.L.; Park, H.; Kwon, J.H.; Sinn, D.H.; Lee, S.H.; Lee, J.H.; Lee, H.W. Comparison of clinical practice guidelines for the management of chronic hepatitis B: When to start, when to change, and when to stop. Clin. Mol. Hepatol. 2020, 26, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.Y.; Wong, G.L. Unmet need in chronic hepatitis B management. Clin. Mol. Hepatol. 2019, 25, 172–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H. Old hepatitis B virus never dies: It just hides itself within the host genome. Clin. Mol. Hepatol. 2021, 27, 107–109. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Kim, B.K.; Lee, J.S.; Lee, H.W.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H.; Kim, S.U. Negligible risks of hepatocellular carcinoma during biomarker-defined immune-tolerant phase for patients with chronic hepatitis B. Clin. Mol. Hepatol. 2021, 27, 295–304. [Google Scholar] [CrossRef]
- Yip, T.C.; Wong, G.L.; Wong, V.W. Negligible risk of hepatocellular carcinoma in chronic hepatitis B patients in immune-tolerant phase: Myth or fact. Clin. Mol. Hepatol. 2021, 27, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.C. Another oral antiviral treatment, but still far away from hepatitis B virus cure. Clin. Mol. Hepatol. 2021, 27, 281–282. [Google Scholar] [CrossRef]
- Wong, G.L.; Wong, V.W.; Yuen, B.W.; Tse, Y.K.; Luk, H.W.; Yip, T.C.; Hui, V.W.; Liang, L.Y.; Lui, G.C.; Chan, H.L. An Aging Population of Chronic Hepatitis B with Increasing Comorbidities: A Territory-Wide Study from 2000 to 2017. Hepatology 2020, 71, 444–455. [Google Scholar] [CrossRef]
- Yu, J.H.; Lee, J.W. How does hepatic steatosis affect the outcome of patients with chronic hepatitis B? Clin. Mol. Hepatol. 2019, 25, 280–282. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.S.; Jeon, M.Y.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H.; Kim, S.U. Influence of hepatic steatosis on the outcomes of patients with chronic hepatitis B treated with entecavir and tenofovir. Clin. Mol. Hepatol. 2019, 25, 283–293. [Google Scholar] [CrossRef]
- Banitalebi, E.; Faramarzi, M.; Nasiri, S.; Mardaniyan, M.; Rabiee, V. Effects of different exercise modalities on novel hepatic steatosis indices in overweight women with type 2 diabetes. Clin. Mol. Hepatol. 2019, 25, 294–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, N. Metabolic disease as a risk of hepatocellular carcinoma. Clin. Mol. Hepatol. 2021, 27, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Jagdish, R.K. Sarcopenia: Ammonia metabolism and hepatic encephalopathy. Clin. Mol. Hepatol. 2019, 25, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Plank, L.D.; Suk, K.T.; Park, Y.E.; Lee, J.; Choi, J.H.; Heo, N.Y.; Park, J.; Kim, T.O.; Moon, Y.S.; et al. Trends in the prevalence of chronic liver disease in the Korean adult population, 1998–2017. Clin. Mol. Hepatol. 2020, 26, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Arfianti, A.; Pok, S.; Barn, V.; Haigh, W.G.; Yeh, M.M.; Ioannou, G.N.; Teoh, N.C.; Farrell, G.C. Exercise retards hepatocarcinogenesis in obese mice independently of weight control. J. Hepatol. 2020, 73, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Saran, U.; Guarino, M.; Rodriguez, S.; Simillion, C.; Montani, M.; Foti, M.; Humar, B.; St-Pierre, M.V.; Dufour, J.F. Anti-tumoral effects of exercise on hepatocellular carcinoma growth. Hepatol. Commun. 2018, 2, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Piguet, A.C.; Saran, U.; Simillion, C.; Keller, I.; Terracciano, L.; Reeves, H.L.; Dufour, J.F. Regular exercise decreases liver tumors development in hepatocyte-specific PTEN-deficient mice independently of steatosis. J. Hepatol. 2015, 62, 1296–1303. [Google Scholar] [CrossRef] [Green Version]
- Seong, S.C.; Kim, Y.Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.J.; Do, C.H.; Song, J.S.; Lee, E.J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef] [Green Version]
- Cheol Seong, S.; Kim, Y.Y.; Khang, Y.H.; Heon Park, J.; Kang, H.J.; Lee, H.; Do, C.H.; Song, J.S.; Hyon Bang, J.; Ha, S.; et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef] [Green Version]
- Spence, M.M.; Makarem, A.F.; Reyes, S.L.; Rosa, L.L.; Nguyen, C.; Oyekan, E.A.; Kiyohara, A.T. Evaluation of an outpatient pharmacy clinical services program on adherence and clinical outcomes among patients with diabetes and/or coronary artery disease. J. Manag. Care Spec. Pharm. 2014, 20, 1036–1045. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.B.; Jeong, J.; Park, J.H.; Jung, S.W.; Jeong, I.D.; Bang, S.J.; Shin, J.W.; Park, B.R.; Park, E.J.; Park, N.H. Low-level viremia and cirrhotic complications in patients with chronic hepatitis B according to adherence to entecavir. Clin. Mol. Hepatol. 2020, 26, 364–375. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, B.K. How does low-level viremia affect the prognosis of patients with chronic hepatitis B? Clin. Mol. Hepatol. 2020, 26, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Korean Association for the Study of the Liver. KASL clinical practice guidelines for management of chronic hepatitis B. Clin. Mol. Hepatol. 2019, 25, 93–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, M.S.; Kim, D.; Chung, G.E.; Kim, W.; Kim, J.S. The preventive effect of sustained physical activity on incident nonalcoholic fatty liver disease. Liver Int. 2017, 37, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.S.; Kim, D.; Chung, G.E.; Kim, W.; Kim, Y.J.; Yoon, J.H. Role of physical activity in nonalcoholic fatty liver disease in terms of visceral obesity and insulin resistance. Liver Int. 2015, 35, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Huisman, M.H.; Seelen, M.; de Jong, S.W.; Dorresteijn, K.R.; van Doormaal, P.T.; van der Kooi, A.J.; de Visser, M.; Schelhaas, H.J.; van den Berg, L.H.; Veldink, J.H. Lifetime physical activity and the risk of amyotrophic lateral sclerosis. J. Neurol Neurosurg. Psychiatry 2013, 84, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kistler, K.D.; Brunt, E.M.; Clark, J.M.; Diehl, A.M.; Sallis, J.F.; Schwimmer, J.B.; Group, N.C.R. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2011, 106, 460–468, quiz 469. [Google Scholar] [CrossRef] [Green Version]
- Tanasescu, M.; Leitzmann, M.F.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Exercise type and intensity in relation to coronary heart disease in men. JAMA 2002, 288, 1994–2000. [Google Scholar] [CrossRef]
- Jeong, S.W.; Kim, S.H.; Kang, S.H.; Kim, H.J.; Yoon, C.H.; Youn, T.J.; Chae, I.H. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur. Heart J. 2019, 40, 3547–3555. [Google Scholar] [CrossRef]
- Seo, H.J.; Oh, I.H.; Yoon, S.J. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac. J. Cancer Prev. 2012, 13, 6163–6168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Reinikainen, J.; Adeleke, K.A.; Pieterse, M.E.; Groothuis-Oudshoorn, C.G.M. Time-varying covariates and coefficients in Cox regression models. Ann. Transl. Med. 2018, 6, 121. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.W.; Yip, T.C.; Tse, Y.K.; Wong, G.L.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Chan, H.L.; Ahn, S.H.; et al. Hepatic Decompensation in Cirrhotic Patients Receiving Antiviral Therapy for Chronic Hepatitis B. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Huang, Y.; Joseph, J.; de Boer, W.B.; Cheng, W.; Adams, L.A.; MacQuillan, G.; Garas, G.; Raftopoulos, S.; Jeffrey, G.P. Long-term Liver-related Outcomes of Patients with Chronic Liver Diseases in Australia. Clin. Gastroenterol. Hepatol. 2020, 18, 496–504.e3. [Google Scholar] [CrossRef] [PubMed]
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; Berrington de Gonzalez, A.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O.; et al. Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Intern. Med. 2015, 175, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, V.N.; Foster-Schubert, K.; Chubak, J.; Sorensen, B.; Ulrich, C.M.; Stancyzk, F.Z.; Plymate, S.; Stanford, J.; White, E.; Potter, J.D.; et al. Effect of exercise on serum sex hormones in men: A 12-month randomized clinical trial. Med. Sci. Sports Exerc. 2008, 40, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, T.C.; Wong, G.L.; Chan, H.L.; Tse, Y.K.; Liang, L.Y.; Hui, V.W.; Lee, H.W.; Lui, G.C.; Kong, A.P.; Wong, V.W. Elevated testosterone increases risk of hepatocellular carcinoma in men with chronic hepatitis B and diabetes mellitus. J. Gastroenterol. Hepatol. 2020, 35, 2210–2219. [Google Scholar] [CrossRef]
- Yeh, S.H.; Chen, P.J. Gender disparity of hepatocellular carcinoma: The roles of sex hormones. Oncology 2010, 78 (Suppl. 1), 172–179. [Google Scholar] [CrossRef]
- Ma, W.L.; Hsu, C.L.; Wu, M.H.; Wu, C.T.; Wu, C.C.; Lai, J.J.; Jou, Y.S.; Chen, C.W.; Yeh, S.; Chang, C. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology 2008, 135, 947–955.e5. [Google Scholar] [CrossRef] [Green Version]
- Han, E.; Lee, Y.H.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Han, K.H.; et al. Sarcopenia is associated with the risk of significant liver fibrosis in metabolically unhealthy subjects with chronic hepatitis B. Aliment. Pharmacol. Ther. 2018, 48, 300–312. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Kim, S.U.; Song, K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Han, K.H. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011). Hepatology 2016, 63, 776–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.; Lee, J. Sarcopenia and blood myokine levels as prognostic biomarkers in patients with liver cirrhosis or hepatocellular carcinoma. Clin. Mol. Hepatol. 2020, 26, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.S.; Pinheiro, M.B.; Fairhall, N.; Walsh, S.; Chesterfield Franks, T.; Kwok, W.; Bauman, A.; Sherrington, C. Evidence on Physical Activity and the Prevention of Frailty and Sarcopenia Among Older People: A Systematic Review to Inform the World Health Organization Physical Activity Guidelines. J. Phys. Act. Health 2020, 17, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Naseeb, M.A.; Volpe, S.L. Protein and exercise in the prevention of sarcopenia and aging. Nutr. Res. 2017, 40, 1–20. [Google Scholar] [CrossRef]
- Fujiwara, N.; Friedman, S.L.; Goossens, N.; Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. [Google Scholar] [CrossRef] [Green Version]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
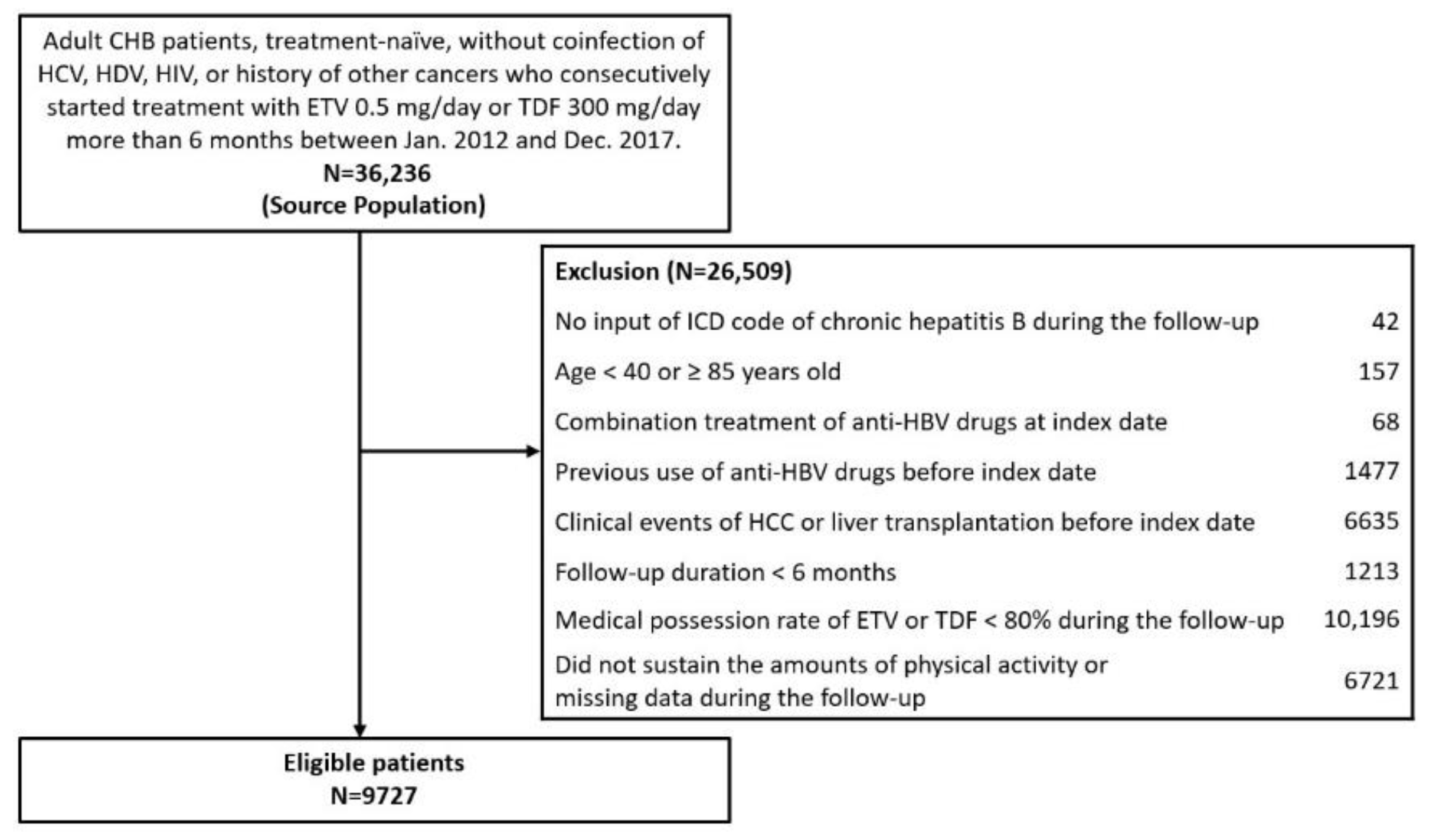
| Variables | Total (n = 9727) | No HCC (n = 8918) | HCC (n = 809) | p-Value a | No Cirrhosis (n = 7602) | Cirrhosis (n = 2125) | p-Value a |
|---|---|---|---|---|---|---|---|
| Age, years | 49 ± 10 | 49 ± 10 | 53 ± 10 | <0.001 | 48 ± 10 | 54 ± 9 | <0.001 |
| Male, n (%) | 6008 (61.8) | 5438 (61.0) | 570 (70.5) | <0.001 | 4665 (61.4) | 1343 (63.2) | 0.13 |
| Cirrhosis, n (%) | 2125 (21.8) | 1788 (20.0) | 337 (41.7) | <0.001 | – | – | – |
| Body mass index, kg/m2 | 23.8 (21.7, 25.9) | 23.7 (21.7, 25.9) | 24.2 (21.9, 26.4) | 0.002 | 23.9 (21.6, 25.9) | 24.1 (22.0, 26.3) | <0.001 |
| Diabetes mellitus, n (%) | 404 (4.2) | 321 (3.6) | 83 (10.3) | <0.001 | 14 (0.2) | 390 (18.4) | <0.001 |
| Hypertension, n (%) | 581 (6.0) | 466 (5.2) | 115 (14.2) | <0.001 | 13 (0.2) | 568 (26.7) | <0.001 |
| Significant alcohol consumption, n (%) | 318 (3.3) | 255 (2.9) | 63 (7.8) | <0.001 | 239 (3.1) | 79 (3.7) | 0.21 |
| Smoking | <0.001 | 0.31 | |||||
| Never, n (%) | 5462 (56.2) | 5077 (56.9) | 385 (47.6) | 4295 (56.5) | 1167 (54.9) | ||
| Previous, n (%) | 1988 (20.4) | 1819 (20.4) | 169 (20.9) | 1526 (20.1) | 462 (21.7) | ||
| Current, n (%) | 2275 (23.4) | 2020 (22.7) | 255 (31.5) | 1779 (23.4) | 496 (23.3) | ||
| Unknown, n (%) | 2 (0.0) | 2 (0.0) | 0 (0.0) | 2 (0.0) | 0 (0.0) | ||
| Laboratory findings | |||||||
| AST, IU/L | 51 (35, 83) | 50 (34, 84) | 54 (39, 76) | 0.04 | 53 (36, 89) | 45 (33, 64) | <0.001 |
| ALT, IU/L | 61 (37, 110) | 62 (37, 113) | 53 (35, 83) | <0.001 | 69 (40, 125) | 44 (30, 67) | <0.001 |
| GGT, IU/L | 42 (25, 79) | 40 (24, 76) | 62 (35, 123) | <0.001 | 40 (24, 76) | 48 (28, 91) | <0.001 |
| Serum creatinine, mg/dL | 0.9 (0.7, 1.0) | 0.9 (0.7, 1.0) | 0.9 (0.7, 1.0) | 0.17 | 0.9 (0.7, 1.0) | 0.9 (0.7, 1.0) | 0.013 |
| GFR, ml/min/1.73 m2 | 89 (78, 103) | 89 (78, 103) | 89 (78, 104) | 0.91 | 89 (78, 103) | 89 (77, 104) | 0.35 |
| Fasting blood glucose, mg/dL | 93 (86, 102) | 93 (85, 102) | 95 (87, 106) | <0.001 | 92 (85, 101) | 95 (87, 105) | <0.001 |
| Total cholesterol, mg/dL | 185 (163, 209) | 186 (164, 209) | 179 (157, 200) | <0.001 | 187 (165, 210) | 179 (157, 201) | <0.001 |
| HDL cholesterol, mg/dL | 56 (47, 67) | 56 (47, 67) | 56 (47, 67) | 0.67 | 56 (47, 67) | 56 (46, 67) | 0.06 |
| LDL cholesterol, mg/dL | 108 (88, 128) | 108 (89, 129) | 101 (83, 121) | <0.001 | 109 (89, 130) | 103 (84, 122) | <0.001 |
| Medication use | |||||||
| Aspirin, n (%) | 355 (3.6) | 334 (3.7) | 21 (2.6) | 0.12 | 271 (3.6) | 84 (4.0) | 0.44 |
| Statin, n (%) | 962 (9.9) | 908 (10.2) | 54 (6.7) | 0.002 | 795 (10.5) | 167 (7.9) | <0.001 |
| Leisure-time physical activity, MET-min/week | 454 (174, 828) | 480 (174, 834) | 360 (0, 729) | <0.001 | 468 (174, 834) | 435 (140, 801) | 0.07 |
| Totally sedentary, n (%) | 1890 (19.4) | 1676 (18.8) | 214 (26.5) | <0.001 | 1435 (18.9) | 455 (21.4) | 0.10 |
| Duration of antiviral treatment, months | 37.7 (22.1, 53.4) | 37.8 (23.7, 41.9) | 19.8 (9.6, 30.1) | <0.001 | 38.4 (23.9, 52.8) | 34.4 (20.2, 48.6) | <0.001 |
| Amount of Leisure-Time Physical Activity | HCC per 100 Person-Years | Multivariable-Adjusted a | |
|---|---|---|---|
| HR (95% CI) | p-Value | ||
| Total population | |||
| Totally sedentary | 3.35 | Reference | |
| <500 MET-min/week | 2.46 | 0.82 (0.68–0.99) | 0.04 |
| 500–1000 MET-min/week | 2.29 | 0.74 (0.61–0.89) | 0.002 |
| 1000–1500 MET-min/week | 1.96 | 0.64 (0.50–0.84) | 0.001 |
| ≥1500 MET-min/week | 2.40 | 0.70 (0.51–0.97) | 0.03 |
| No liver cirrhosis | |||
| Totally sedentary | 2.43 | Reference | |
| <500 MET-min/week | 1.84 | 0.83 (0.65–1.06) | 0.13 |
| 500–1000 MET-min/week | 1.67 | 0.72 (0.56–0.92) | 0.009 |
| 1000–1500 MET-min/week | 1.56 | 0.66 (0.47–0.92) | 0.02 |
| ≥1500 MET-min/week | 1.61 | 0.68 (0.44–1.06) | 0.09 |
| Liver cirrhosis | |||
| Totally sedentary | 6.61 | Reference | |
| <500 MET-min/week | 4.84 | 0.81 (0.60–1.08) | 0.81 |
| 500–1000 MET-min/week | 4.65 | 0.77 (0.57–1.03) | 0.77 |
| 1000–1500 MET-min/week | 3.45 | 0.61 (0.40–0.93) | 0.02 |
| ≥1500 MET-min/week | 5.16 | 0.76 (0.47–1.23) | 0.76 |
| Amount of Leisure-Time Physical Activity | HCC per 100 Person-Years | Multivariable-Adjusted a | HCC per 100 Person-Years | Multivariable-Adjusted a | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |||
| Age | <60 years old | ≥60 years old | ||||
| Totally sedentary | 2.68 | Reference | 6.45 | Reference | ||
| <500 MET-min/week | 2.10 | 0.81 (0.65–1.01) | 0.06 | 4.99 | 0.81 (0.57–1.17) | 0.26 |
| 500–1000 MET-min/week | 2.00 | 0.75 (0.60–0.94) | 0.01 | 4.14 | 0.68 (0.47–0.99) | 0.04 |
| 1000–1500 MET-min/week | 1.68 | 0.65 (0.48–0.88) | 0.005 | 3.92 | 0.64 (0.39–1.05) | 0.08 |
| ≥1500 MET-min/week | 2.00 | 0.71 (0.47–1.06) | 0.09 | 4.22 | 0.64 (0.36–1.13) | 0.13 |
| Sex | Male | Female | ||||
| Totally sedentary | 4.21 | Reference | 2.33 | Reference | ||
| <500 MET-min/week | 2.72 | 0.74 (0.59–0.93) | 0.01 | 2.04 | 1.01 (0.73–1.39) | 0.96 |
| 500–1000 MET-min/week | 2.61 | 0.70 (0.56–0.88) | 0.002 | 1.73 | 0.83 (0.59–1.18) | 0.30 |
| 1000–1500 MET-min/week | 2.24 | 0.63 (0.46–0.85) | 0.003 | 1.41 | 0.70 (0.42–1.15) | 0.16 |
| ≥ 1500 MET-min/week | 2.74 | 0.64 (0.44–0.93) | 0.02 | 1.69 | 0.84 (0.43–1.62) | 0.60 |
| Diabetes mellitus | No diabetes | Diabetes | ||||
| Totally sedentary | 3.15 | Reference | 7.46 | Reference | ||
| <500 MET-min/week | 2.34 | 0.81 (0.67–0.99) | 0.03 | 5.99 | 0.89 (0.48–1.63) | 0.70 |
| 500–1000 MET-min/week | 2.07 | 0.70 (0.57–0.85) | 0.001 | 8.38 | 1.21 (0.69–2.13) | 0.51 |
| 1000–1500 MET-min/week | 1.86 | 0.65 (0.50–0.86) | 0.002 | 4.07 | 0.59 (0.25–1.37) | 0.22 |
| ≥1500 MET-min/week | 2.17 | 0.70 (0.50–0.98) | 0.04 | 7.38 | 1.01 (0.42–2.40) | 0.98 |
| Body mass index (BMI) | BMI ≥ 25 kg/m2 | BMI < 25 kg/m2 | ||||
| Totally sedentary | 4.02 | Reference | 3.00 | Reference | ||
| <500 MET-min/week | 2.89 | 0.78 (0.58–1.05) | 0.10 | 2.24 | 0.84 (0.66–1.07) | 0.16 |
| 500–1000 MET-min/week | 2.45 | 0.66 (0.48–0.90) | 0.009 | 2.20 | 0.78 (0.61–1.00) | 0.049 |
| 1000–1500 MET-min/week | 2.42 | 0.64 (0.42–0.97) | 0.03 | 1.72 | 0.66 (0.47–0.92) | 0.01 |
| ≥1500 MET-min/week | 2.47 | 0.64 (0.38–1.06) | 0.08 | 2.36 | 0.72 (0.47–1.10) | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, H.S.; Park, S.; Lee, M.; Cho, Y.; Kim, H.S.; Choe, A.R.; Kim, H.Y.; Yoo, K.; Kim, T.H. Association of Physical Activity with the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B. Cancers 2021, 13, 3424. https://doi.org/10.3390/cancers13143424
Chun HS, Park S, Lee M, Cho Y, Kim HS, Choe AR, Kim HY, Yoo K, Kim TH. Association of Physical Activity with the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B. Cancers. 2021; 13(14):3424. https://doi.org/10.3390/cancers13143424
Chicago/Turabian StyleChun, Ho Soo, Sojeong Park, Minjong Lee, Yuri Cho, Ha Sung Kim, A Reum Choe, Hwi Young Kim, Kwon Yoo, and Tae Hun Kim. 2021. "Association of Physical Activity with the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B" Cancers 13, no. 14: 3424. https://doi.org/10.3390/cancers13143424
APA StyleChun, H. S., Park, S., Lee, M., Cho, Y., Kim, H. S., Choe, A. R., Kim, H. Y., Yoo, K., & Kim, T. H. (2021). Association of Physical Activity with the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B. Cancers, 13(14), 3424. https://doi.org/10.3390/cancers13143424






