Maintaining Weight Loss in Obese Men with Prostate Cancer Following a Supervised Exercise and Nutrition Program—A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Home-Based Program
2.3. Measurements
2.3.1. Body Composition
2.3.2. Muscle Strength and Cardiorespiratory Fitness
2.3.3. Resting Metabolic Rate
2.3.4. Physical Activity Monitoring
2.3.5. Nutrition Monitoring
2.4. Statistical Analysis
3. Results
3.1. Nutrition and Physical Activity
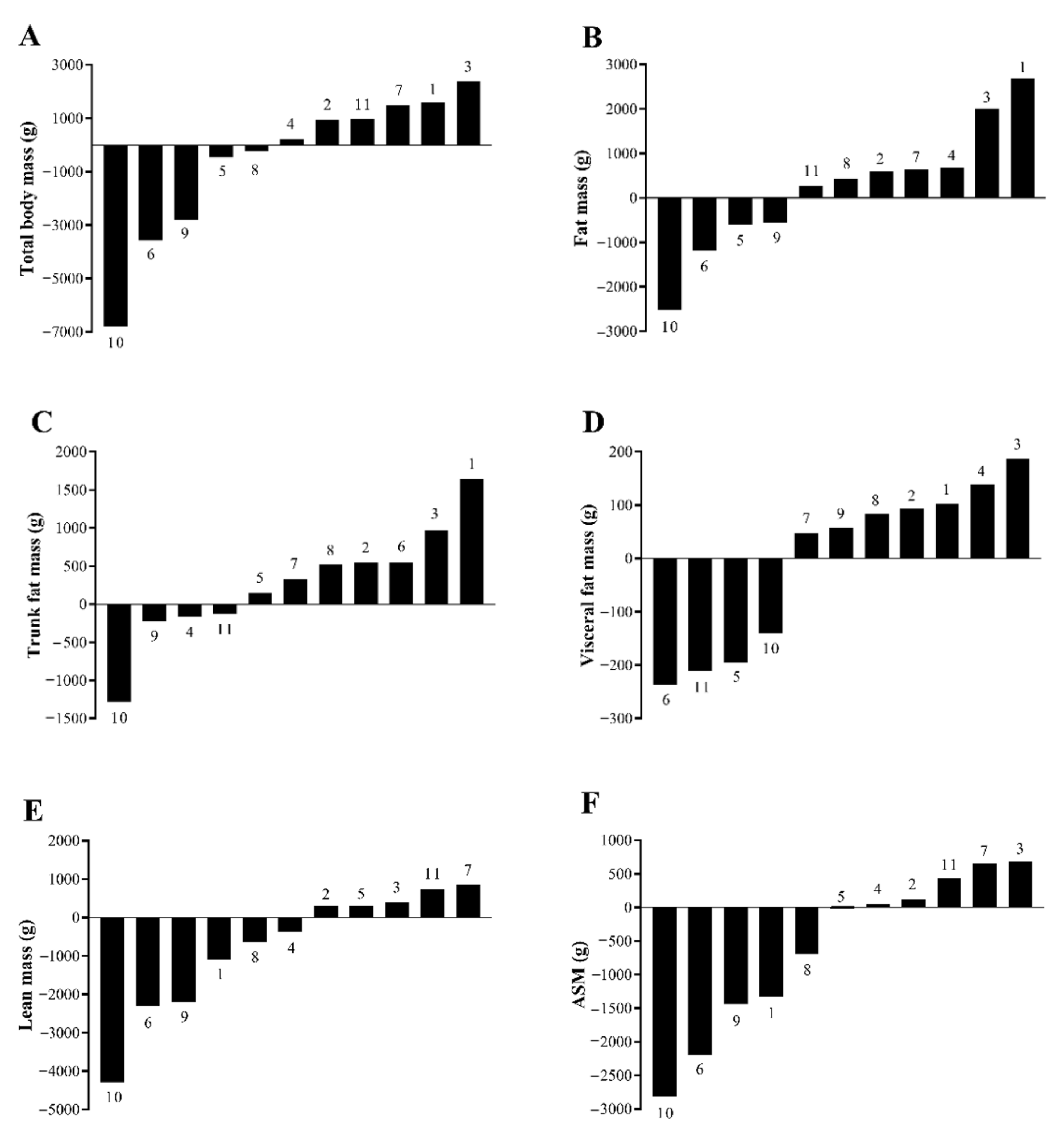
3.2. Body Composition
3.3. Muscle Strength, Cardiorespiratory Fitness, and Resting Metabolic Rate
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moyad:, M.A.; Newton, R.U.; Tunn, U.W.; Gruca, D. Integrating diet and exercise into care of prostate cancer patients on androgen deprivation therapy. Res. Rep. Urol. 2016, 8, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Neal, D.E.; Metcalfe, C.; Donovan, J.L.; Lane, J.A.; Davis, M.; Young, G.J.; Dutton, S.J.; Walsh, E.I.; Martin, R.M.; Peters, T.J.; et al. Ten-year mortality, disease progression, and treatment-related side effects in men with localised prostate cancer from the ProtecT randomised controlled trial according to treatment received. Eur. Urol. 2020, 77, 320–330. [Google Scholar] [CrossRef]
- Shahinian, V.B.; Kuo, Y.F.; Freeman, J.L.; Orihuela, E.; Goodwin, J.S. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer Am. Cancer Soc. 2005, 103, 1615–1624. [Google Scholar] [CrossRef]
- Narayan, V.; Harrison, M.; Cheng, H.; Kenfield, S.; Aggarwal, R.; Kwon, D.; McKay, R.; Hauger, R.; Hart, N.; Conzen, S.; et al. Improving research for prostate cancer survivorship: A statement from the Survivorship Research in Prostate Cancer (SuRECaP) working group. Urol. Oncol. 2020, 38, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Gunter, J.H.; Heathcote, P.; Ho, K.; Stricker, P.; Corcoran, N.M.; Nelson, C.C. Adverse effects of androgen-deprivation therapy in prostate cancer and their management. BJU Int. 2015, 115, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Galvao, D.A.; Taaffe, D.R.; Spry, N.; Joseph, D.; Newton, R.U. Cardiovascular and metabolic complications during androgen deprivation: Exercise as a potential countermeasure. Prostate Cancer Prostatic Dis 2009, 12, 233–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsuzuka, K.; Arai, Y. Metabolic changes in patients with prostate cancer during androgen deprivation therapy. Int. J. Urol. 2018, 25, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Keto, C.J.; Aronson, W.J.; Terris, M.K.; Presti, J.C.; Kane, C.J.; Amling, C.L.; Freedland, S.J. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: esults from the SEARCH database. BJU Int. 2012, 110, 492–498. [Google Scholar] [CrossRef] [Green Version]
- Dickerman, B.A.; Torfadottir, J.E.; Valdimarsdottir, U.A.; Giovannucci, E.; Wilson, K.M.; Aspelund, T.; Tryggvadottir, L.; Sigurdardottir, L.G.; Harris, T.B.; Launer, L.J.; et al. Body fat distribution on computed tomography imaging and prostate cancer risk and mortality in the AGES-Reykjavik study. Cancer 2019, 125, 2877–2885. [Google Scholar] [CrossRef]
- Keating, N.L.; O’Malley, A.J.; Freedland, S.J.; Smith, M.R. Diabetes and cardiovascular disease during androgen deprivation therapy: Observational study of veterans with prostate cancer. J. Natl. Cancer Inst. 2010, 102, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Galvão, D.A.; Taaffe, D.R.; Spry, N.; Newton, R.U. Exercise can prevent and even reverse adverse effects of androgen suppression treatment in men with prostate cancer. Prostate Cancer Prostatic Dis. 2007, 10, 340–346. [Google Scholar] [CrossRef]
- Hayes, S.C.; Newton, R.U.; Spence, R.R.; Galvao, D.A. The Exercise and Sports Science Australia position statement: Exercise medicine in cancer management. J. Sci. Med. Sport 2019, 22, 1175–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storer, T.W.; Miciek, R.; Travison, T.G. Muscle function, physical performance and body composition changes in men with prostate cancer undergoing androgen deprivation therapy. Asian J. Androl. 2012, 14, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Morey, M.C.; Snyder, D.C.; Sloane, R.; Cohen, H.J.; Peterson, B.; Hartman, T.J.; Miller, P.; Mitchell, D.C.; Demark-Wahnefried, W. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: A randomized controlled trial. JAMA 2009, 301, 1883–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlin, S.S.; Caplan, L.; Stone, R.; Stewart, J. A review of home-based physical activity interventions for breast cancer survivors. Curr. Cancer Rep. 2019, 1, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Bourke, L.; Doll, H.; Crank, H.; Daley, A.; Rosario, D.; Saxton, J.M. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: A feasibility study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 647–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourke, L.; Gilbert, S.; Hooper, R.; Steed, L.A.; Joshi, M.; Catto, J.W.; Saxton, J.M.; Rosario, D.J. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: A randomised controlled trial. Eur. Urol. 2014, 65, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L.; Newton, R.U.; Taaffe, D.R.; Hart, N.H.; Lyons-Wall, P.; Galvão, D.A. Weight loss for obese prostate cancer patients on androgen deprivation therapy. Med. Sci. Sports Exerc. 2021, 53, 470–478. [Google Scholar] [CrossRef]
- Alamuddin, N.; Bakizada, Z.; Wadden, T.A. Management of obesity. J. Clin. Oncol. 2016, 34, 4295–4305. [Google Scholar] [CrossRef]
- National Health Medical Research Council (2013). Australian Dietary Guidelines. Available online: https://www.nhmrc.gov.au/adg (accessed on 20 April 2020).
- National Health and Medical Research Council, Australian Government Department of Health and Ageing, New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand; National Health and Medical Research Council: Canberra, Australia, 2006. [Google Scholar]
- Heymsfield, S.B.; Smith, R.; Aulet, M.; Bensen, B.; Lichtman, S.; Wang, J.; Pierson, R.N., Jr. Appendicular skeletal muscle mass: Measurement by dual-photon absorptiometry. Am. J. Clin. Nutr. 1990, 52, 214–218. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription—Ninth Edition; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Taaffe, D.R.; Duret, C.; Wheeler, S.; Marcus, R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J. Am. Geriatr. Soc. 1999, 47, 1208–1214. [Google Scholar] [CrossRef]
- Simonsick, E.M.; Fan, E.; Fleg, J.L. Estimating cardiorespiratory fitness in well-functioning older adults: Treadmill validation of the long distance corridor walk. J. Am. Geriatr. Soc. 2006, 54, 127–132. [Google Scholar] [CrossRef]
- Vandarakis, D.; Salacinski, A.J.; Broeder, C.E. A comparison of COSMED metabolic systems for the determination of resting metabolic rate. Res. Sports Med. 2013, 21, 187–194. [Google Scholar] [CrossRef]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of accelerometer wear and nonwear time classification algorithm. Med. Sci. Sports Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Matthews, C.E.; Chen, K.Y.; Freedson, P.S.; Buchowski, M.S.; Beech, B.M.; Pate, R.R.; Troiano, R.P. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am. J. Epidemiol. 2008, 167, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Peddle-McIntyre, C.J.; Cavalheri, V.; Boyle, T.; McVeigh, J.A.; Jeffery, E.; Lynch, B.M.; Vallance, J.K. A review of accelerometer-based activity monitoring in cancer survivorship research. Med. Sci. Sports Exerc. 2018, 50, 1790–1801. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R., Jr.; Ainsworth, B.E.; Hartman, T.J.; Leon, A.S. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med. Sci. Sports Exerc. 1993, 25, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Howard, L.; Allen, J.; Smith, J.; Stout, J.; Aronson, W.; Inman, B.A.; Armstrong, A.J.; George, D.; Westman, E.; et al. A lifestyle intervention of weight loss via a low-carbohydrate diet plus walking to reduce metabolic disturbances caused by androgen deprivation therapy among prostate cancer patients: Carbohydrate and prostate study 1 (CAPS1) randomized controlled trial. Prostate Cancer Prostatic Dis. 2019, 22, 428–437. [Google Scholar] [CrossRef]
- Galvão, D.A.; Spry, N.A.; Taaffe, D.R.; Newton, R.U.; Stanley, J.; Shannon, T.; Rowling, C.; Prince, R. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008, 102, 44–47. [Google Scholar] [CrossRef]
- Ziaran, S.; Goncalves, F.M.; Sn, J.B. Complex metabolic and skeletal changes in men taking long-term androgen deprivation therapy. Clin. Genitourin. Cancer 2013, 11, 33–38. [Google Scholar] [CrossRef]
- Pratley, R.; Nicklas, B.; Rubin, M.; Miller, J.; Smith, A.; Smith, M.; Hurley, B.; Goldberg, A. Strength training increases resting metabolic rate and norepinephrine levels in healthy 50- to 65-yr-old men. J. Appl. Physiol. (1985) 1994, 76, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Hills, A.P.; Mokhtar, N.; Byrne, N.M. Assessment of physical activity and energy expenditure: An overview of objective measures. Front. Nutr. 2014, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.S.S.; Machado, A.F.; Micheletti, J.K.; de Almeida, A.C.; Cavina, A.P.; Pastre, C.M. Effects of training with elastic resistance versus conventional resistance on muscular strength: A systematic review and meta-analysis. SAGE Open Med. 2019, 7, 2050312119831116. [Google Scholar] [CrossRef] [Green Version]
- Winters-Stone, K.M.; Dobek, J.C.; Bennett, J.A.; Dieckmann, N.F.; Maddalozzo, G.F.; Ryan, C.W.; Beer, T.M. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: Evidence from a randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, A.M.; Smith, K.C.; Coa, K.I.; Helzlsouer, K.J.; Caulfield, L.E.; Peairs, K.S.; Shockney, L.D.; Klassen, A.C. Clinical care providers’ perspectives on body size and weight management among long-term cancer survivors. Integr. Cancer Ther. 2015, 14, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Newton, R.U.; Galvão, D.A.; Spry, N.; Joseph, D.; Chambers, S.K.; Gardiner, R.A.; Wall, B.A.; Bolam, K.A.; Taaffe, D.R. Exercise mode specificity for preserving spine and hip BMD in prostate cancer patients. Med. Sci. Sports Exerc. 2019, 51, 607–614. [Google Scholar] [CrossRef]
| Variable | Patients (n = 11) |
|---|---|
| Age (years), mean ± SD | 74 ± 5 |
| Body mass index (kg/m2), mean ± SD | 33.1 ± 5.3 |
| Post-secondary education, n (%) | 8 (72.7) |
| Married, n (%) | 11 (100) |
| Employed, n (%) | 1 (9.1) |
| Current smoker, n | 0 |
| Number of medications/supplements, mean ± SD | 4.5 ± 2.9 |
| Number of comorbidities, mean ± SD a | 3.4 ± 1.4 |
| Years since prostate cancer diagnosis, median [IQR] | 3.9 [1.5–9.7] |
| Gleason score, n (%) | |
| Gleason 7 | 3 (27.3) |
| Gleason 8 | 1 (9.1) |
| Gleason 9 | 6 (54.5) |
| Gleason 10 | 1 (9.1) |
| Contained within prostate, n (%) | 5 (45.5) |
| Lymph node metastasis, n (%) | 4 (36.4) |
| Organ metastasis, n (%) b | 2 (18.2) |
| Androgen deprivation therapy, n (%) | |
| LHRH agonist + antiandrogen | 7 (63.6) |
| LHRH agonist only | 4 (36.4) |
| Months on ADT, median [IQR] | 16 [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] |
| Other prostate cancer-related treatment, n (%) | |
| Surgery | 4 (36.4) |
| Radiation therapy | 10 (90.9) |
| Chemotherapy | 2 (18.2) |
| Variable | Post-Supervised Intervention | Post-Home-Based Program | Mean Change | p-Value |
|---|---|---|---|---|
| Nutrition intake | ||||
| Energy intake (kJ/d) | 6759 [4994–8980] | 7972 [6353–8535] | - | 0.041 |
| Protein (g/d) | 85.9 ± 24.3 | 93.8 ± 21.1 | 8.0 ± 20.3 | 0.222 |
| Protein (% total energy) | 21.2 ± 4.0 | 20.1 ± 2.9 | −1.1 ± 4.5 | 0.433 |
| Fat (g/d) | 60.7 ± 21.8 | 67.7 ± 20.9 | 7.0 ± 24.1 | 0.360 |
| Fat (% total energy) | 31.5 ± 4.8 | 31.0 ± 4.8 | −0.5 ± 6.7 | 0.823 |
| Carbohydrate (g/d) | 179.8 ± 68.0 | 206.1 ± 67.1 | 26.2 ± 29.9 | 0.016 |
| Carbohydrate (% total energy) | 40.3 ± 4.3 | 40.7 ± 6.1 | 0.4 ± 5.9 | 0.848 |
| Alcohol (% total energy) | 0.0 [0.0–3.0] | 2.1 [0.0–6.8] | - | 0.310 |
| Physical activity | ||||
| Average time in SB (h/d) | 9.6 ± 1.9 | 9.5 ± 2.0 | −0.1 ± 2.0 | 0.871 |
| Time spent in SB (% wake hours) | 65.9 ± 6.2 | 70.2 ± 8.8 | 4.3 ± 3.8 | 0.003 |
| Average time in LPA (h/d) | 4.8 ± 1.2 | 4.0 ± 1.6 | −0.9 ± 0.9 | 0.011 |
| Time spent in LPA (% wake hours) | 33.3 ± 6.3 | 28.6 ± 8.1 | −4.8 ± 3.1 | <0.001 |
| Average time in MVPA (min/d) | 6.9 ± 5.4 | 10.7 ± 10.1 | 3.8 ± 11.2 | 0.286 |
| Time spent in MVPA (% wake hours) | 0.6 [0.3–1.1] | 0.7 [0.3–2.2] | - | 0.424 |
| Variable | Post-Supervised Intervention | Post-Home-Based Program | Mean Change | Percent Change (%) | p-Value |
|---|---|---|---|---|---|
| Total body mass (kg) | 95.5 ± 14.1 | 94.9 ± 12.9 | −0.6 ± 2.8 | −0.4 ± 2.7 | 0.508 |
| Total fat mass (kg) | 37.0 ± 9.5 | 37.3 ± 8.7 | 0.2 ± 1.4 | 1.1 ± 4.0 | 0.619 |
| Percent body fat (%) | 38.3 ± 4.6 | 38.9 ± 4.5 | 0.6 ± 0.8 | - | 0.034 |
| Trunk fat (kg) | 18.3 ± 5.4 | 18.5 ± 5.2 | 0.3 ± 0.7 | 2.0 ± 4.5 | 0.271 |
| Visceral fat (g) | 866 ± 333 | 860 ± 277 | −7 ± 156 | −1.6 ± 17.0 | 0.888 |
| Total lean mass (kg) | 55.9 ± 6.2 | 55.1 ± 6.2 | −0.8 ± 1.6 | −1.3 ± 2.7 | 0.146 |
| ASM (kg) | 23.3 ± 3.1 | 22.7 ± 3.1 | −0.6 ± 1.2 | −2.5 ± 4.9 | 0.130 |
| BMC (g) | 2576 ± 291 | 2544 ± 261 | −32 ± 56 | −1.1 ± 2.0 | 0.087 |
| Waist circumference (cm) | 103.9 ± 8.9 | 103.5 ± 8.5 | −0.4 ± 2.6 | −0.3 ± 2.5 | 0.626 |
| Hip circumference (cm) | 109.7 ± 8.1 | 108.7 ± 7.9 | −1.0 ± 2.2 | −0.9 ± 1.9 | 0.141 |
| Variable | Post-Supervised Intervention | Post-Home-Based Program | Mean Change | p-Value |
|---|---|---|---|---|
| Leg press (kg) (n = 7) | 126.3 ± 47.4 | 132.4 ± 53.7 | 6.1 ± 7.9 | 0.086 |
| Chest press (kg) (n = 8) | 51.8 ± 14.1 | 51.0 ± 13.0 | −0.7 ± 6.0 | 0.745 |
| Seated row (kg) (n = 10) | 68.2 ± 7.9 | 68.8 ± 8.9 | 0.6 ± 5.4 | 0.744 |
| Estimated VO2max (mL/min/kg) (n = 9) | 20.6 ± 3.6 | 20.3 ± 3.3 | −0.3 ± 1.6 | 0.602 |
| RMR (kcal/d) (n = 11) | 1516 ± 207 | 1482 ± 186 | −34 ± 143 | 0.450 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, R.L.; Taaffe, D.R.; Newton, R.U.; Hart, N.H.; Lyons-Wall, P.; Galvão, D.A. Maintaining Weight Loss in Obese Men with Prostate Cancer Following a Supervised Exercise and Nutrition Program—A Pilot Study. Cancers 2021, 13, 3411. https://doi.org/10.3390/cancers13143411
Wilson RL, Taaffe DR, Newton RU, Hart NH, Lyons-Wall P, Galvão DA. Maintaining Weight Loss in Obese Men with Prostate Cancer Following a Supervised Exercise and Nutrition Program—A Pilot Study. Cancers. 2021; 13(14):3411. https://doi.org/10.3390/cancers13143411
Chicago/Turabian StyleWilson, Rebekah L., Dennis R. Taaffe, Robert U. Newton, Nicolas H. Hart, Philippa Lyons-Wall, and Daniel A. Galvão. 2021. "Maintaining Weight Loss in Obese Men with Prostate Cancer Following a Supervised Exercise and Nutrition Program—A Pilot Study" Cancers 13, no. 14: 3411. https://doi.org/10.3390/cancers13143411







