Cost-Effectiveness of Nutrient Supplementation in Cancer Survivors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Population Characteristics
3.2. Health and Economic Outcomes
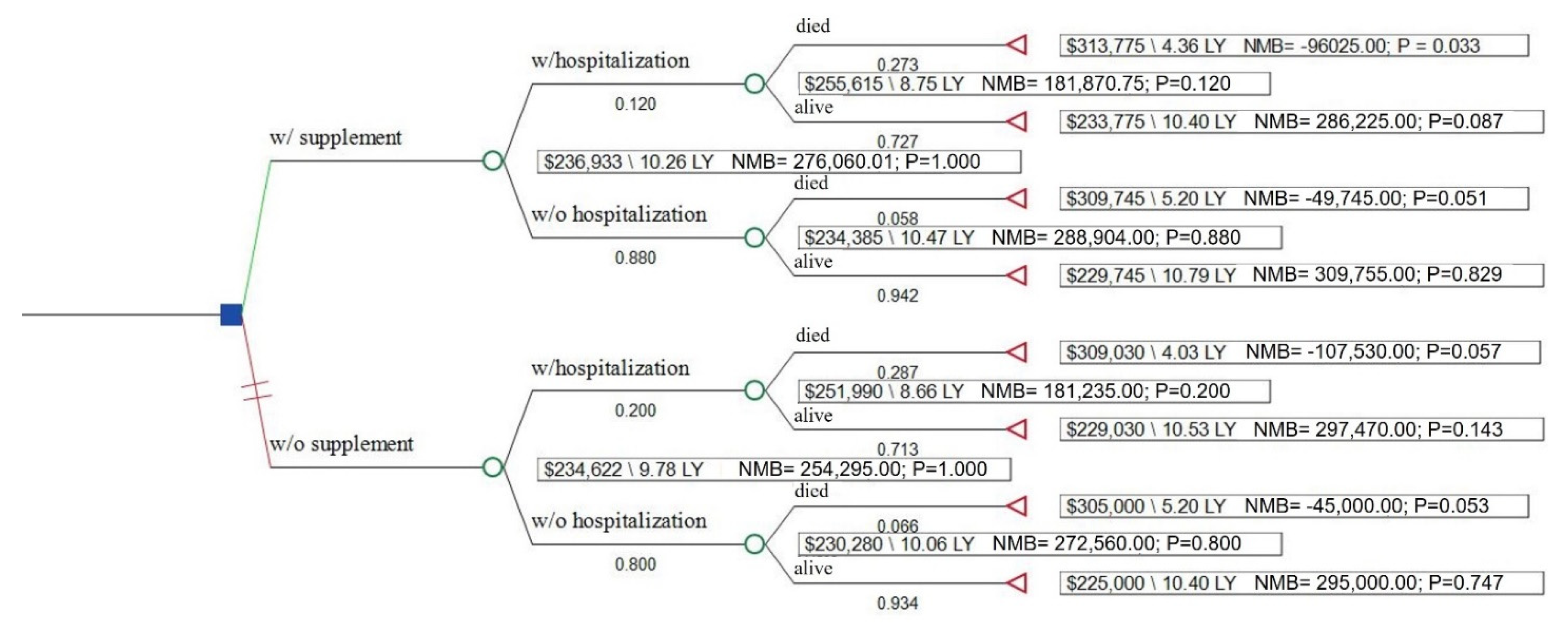
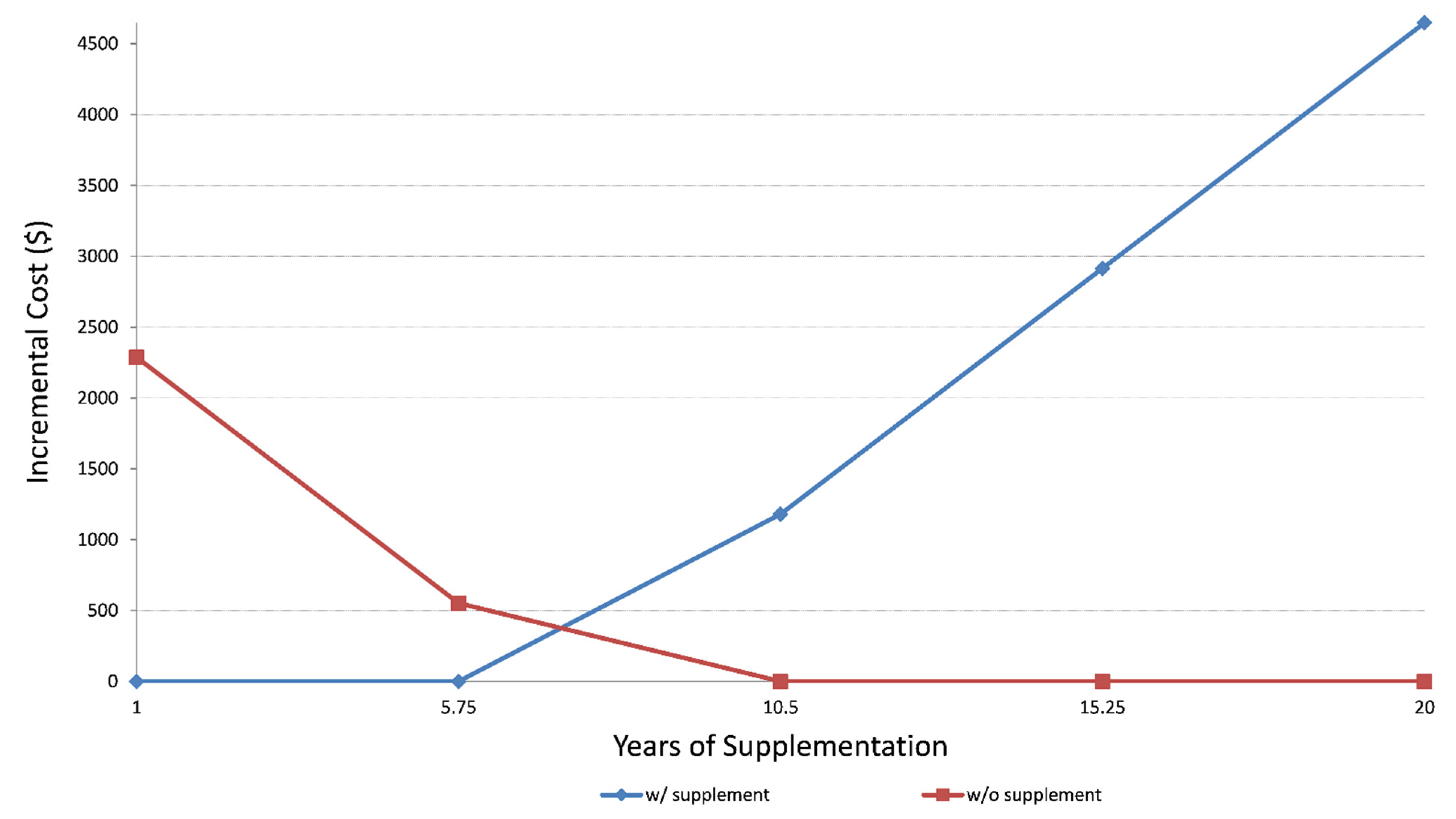
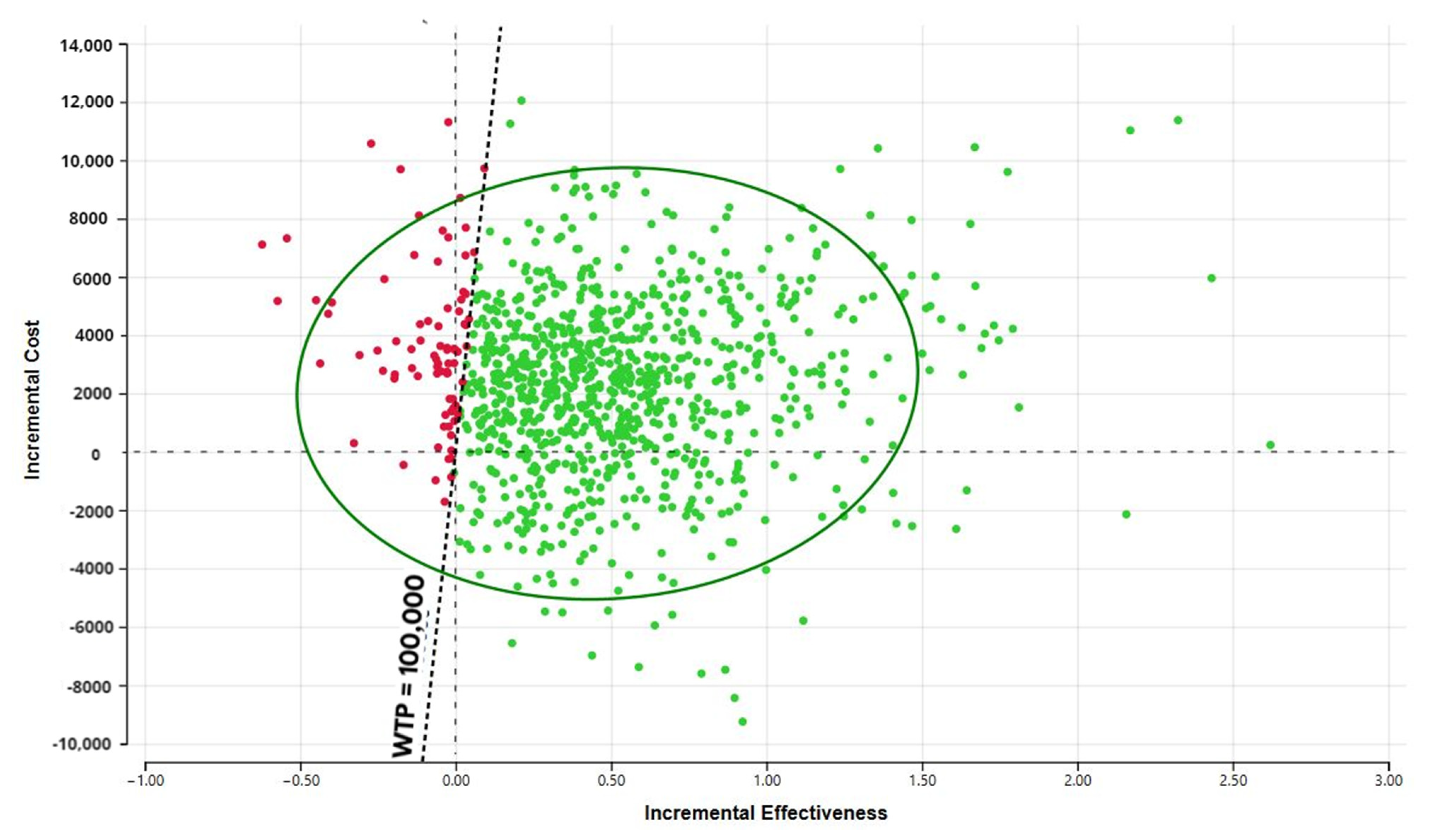
3.3. Cost-Effectiveness and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alderman, H.; Behrman, J.R.; Hoddinott, J. Economic and nutritional analyses offer substantial synergies for understanding human nutrition. J. Nutr. 2007, 137, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Bowen, D.J.; Beresford, S.A. Dietary interventions to prevent disease. Annu. Rev. Public Health 2002, 23, 255–286. [Google Scholar] [CrossRef] [PubMed]
- Nahin, R.L.; Barnes, P.M.; Stussman, B.J. Expenditures on Complementary Health Approaches: United States, 2012; National Health Statistics Reports; 2016; pp. 1–11. Available online: https://www.cdc.gov/nchs/data/nhsr/nhsr095.pdf (accessed on 20 September 2020).
- PDQ Supportive Palliative Care Editorial Board. Nutrition in Cancer Care (PDQ®): Patient Version. In PDQ Cancer Information Summaries [Internet]; PDQ Supportive Palliative Care Editorial Board: Washington, DC, USA, 2002. [Google Scholar]
- Velicer, C.M.; Ulrich, C.M. Vitamin and mineral supplement use among US adults after cancer diagnosis: A systematic review. J. Clin. Oncol. 2008, 26, 665–673. [Google Scholar] [CrossRef] [PubMed]
- John, G.M.; Hershman, D.L.; Falci, L.; Shi, Z.; Tsai, W.-Y.; Greenlee, H. Complementary and alternative medicine use among US cancer survivors. J. Cancer Surviv. 2016, 10, 850–864. [Google Scholar] [CrossRef]
- Du, M.; Luo, H.; Blumberg, J.B.; Rogers, G.; Chen, F.; Ruan, M.; Shan, Z.; Biever, E.; Zhang, F.F. Dietary Supplement Use among Adult Cancer Survivors in the United States. J. Nutr. 2020, 150, 1499–1508. [Google Scholar] [CrossRef]
- Pouchieu, C.; Fassier, P.; Druesne-Pecollo, N.; Zelek, L.; Bachmann, P.; Touillaud, M.; Bairati, I.; Hercberg, S.; Galan, P.; Cohen, P.; et al. Dietary supplement use among cancer survivors of the NutriNet-Santé cohort study. Br. J. Nutr. 2015, 113, 1319–1329. [Google Scholar] [CrossRef] [Green Version]
- Whitney, R.L.; Bell, J.F.; Tancredi, D.J.; Romano, P.S.; Bold, R.J.; Wun, T.; Joseph, J.G. Unplanned Hospitalization Among Individuals With Cancer in the Year After Diagnosis. J. Oncol. Pract. 2019, 15, e20–e29. [Google Scholar] [CrossRef]
- CMS. Oncology Care Model. Available online: https://innovation.cms.gov/innovation-models/map#model=oncology-care-model (accessed on 6 July 2020).
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Risks and Side Effects of Dietary Supplements. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/complementary-and-alternative-medicine/dietary-supplements/risks-and-side-effects.html (accessed on 14 July 2020).
- Weinstein, M.C.; O’Brien, B.; Hornberger, J.; Jackson, J.; Johannesson, M.; McCabe, C.; Luce, B.R. Principles of good practice for decision analytic modeling in health-care evaluation: Report of the ISPOR Task Force on Good Research Practices—Modeling Studies. Value Health 2003, 6, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. Consolidated health economic evaluation reporting standards (CHEERS) statement. Int. J. Technol. Assess. Health Care 2013, 29, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.W.; Cohen, S.B.; Banthin, J.S. The medical expenditure panel survey: A national information resource to support healthcare cost research and inform policy and practice. Med. Care 2009, 47, S44–S50. [Google Scholar] [CrossRef]
- CDC. 2011-2012 Data Documentation, Codebook, and Frequencies: Dietary Supplement Use 30-Day—Total Dietary Supplements (DSQTOT_G). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/DSQTOT_G.htm (accessed on 6 July 2020).
- CDC. 2015-2016 Data Documentation, Codebook, and Frequencies: Dietary Supplement Use 30-Day—Total Dietary Supplements (DSQTOT_I). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/DSQTOT_I.htm (accessed on 6 July 2020).
- NCHS. The Linkage of National Center for Health. Statistics Survey Data to the National Death Index–2015 Linked Mortality File (LMF): Methodology Overview and Analytic Considerations; NCHS: Hyattsville, MD, USA, 2018. Available online: https://www.cdc.gov/nchs/data/datalinkage/LMF2015_Methodology_Analytic_Considerations.pdf (accessed on 8 July 2020).
- Arias, E. United States Life Tables, 2011. Natl. Vital Stat. Rep. 2015, 64, 1–63. [Google Scholar]
- Syriopoulou, E.; Bower, H.; Andersson, T.M.; Lambert, P.C.; Rutherford, M.J. Estimating the impact of a cancer diagnosis on life expectancy by socio-economic group for a range of cancer types in England. Br. J. Cancer 2017, 117, 1419–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capocaccia, R.; Gatta, G.; Dal Maso, L. Life expectancy of colon, breast, and testicular cancer patients: An analysis of US-SEER population-based data. Ann. Oncol. 2015, 26, 1263–1268. [Google Scholar] [CrossRef]
- Botta, L.; Dal Maso, L.; Guzzinati, S.; Panato, C.; Gatta, G.; Trama, A.; Rugge, M.; Tagliabue, G.; Casella, C.; Caruso, B.; et al. Changes in life expectancy for cancer patients over time since diagnosis. J. Adv. Res. 2019, 20, 153–159. [Google Scholar] [CrossRef]
- NICE. Guide to the Methods of Technology Appraisal; National Institute for Health and Clinical Excellence (NICE): London, UK, 2013. [Google Scholar]
- Sanders, G.D.; Neumann, P.J.; Basu, A.; Brock, D.W.; Feeny, D.; Krahn, M.; Kuntz, K.M.; Meltzer, D.O.; Owens, D.K.; Prosser, L.A.; et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016, 316, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Reynolds, M.R. Interpreting the results of cost-effectiveness studies. J. Am. Coll. Cardiol. 2008, 52, 2119–2126. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Lubetkin, E.I. Estimating EuroQol EQ-5D scores from Population Healthy Days data. Med. Decis. Making 2008, 28, 491–499. [Google Scholar] [CrossRef]
- Yabroff, K.R.; Lund, J.; Kepka, D.; Mariotto, A. Economic burden of cancer in the United States: Estimates, projections, and future research. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 2006–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisu, M.; Henrikson, N.B.; Banegas, M.P.; Yabroff, K.R. Costs of cancer along the care continuum: What we can expect based on recent literature. Cancer 2018, 124, 4181–4191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellison, A. 10 Largest Retail Pharmacies in America. Available online: https://www.beckershospitalreview.com/lists/10-largest-retail-pharmacies-in-america.html (accessed on 1 June 2020).
- Ranard, K.M.; Jeon, S.; Mohn, E.S.; Griffiths, J.C.; Johnson, E.J.; Erdman, J.W., Jr. Dietary guidance for lutein: Consideration for intake recommendations is scientifically supported. Eur. J. Nutr. 2017, 56, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- drugs.com. Lycopene. Available online: https://www.drugs.com/npp/lycopene.html (accessed on 30 May 2020).
- NIH. Omega-3 Fatty Acids–Fact Sheet for Health Professionals; NIH: Bethesda, MD, USA, 2019. [Google Scholar]
- Patterson, R.E.; Neuhouser, M.L.; Hedderson, M.M.; Schwartz, S.M.; Standish, L.J.; Bowen, D.J. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J. Am. Diet. Assoc. 2003, 103, 323–328. [Google Scholar] [CrossRef]
- Greenlee, H.; Kwan, M.L.; Ergas, I.J.; Strizich, G.; Roh, J.M.; Wilson, A.T.; Lee, M.; Sherman, K.J.; Ambrosone, C.B.; Hershman, D.L.; et al. Changes in vitamin and mineral supplement use after breast cancer diagnosis in the Pathways Study: A prospective cohort study. BMC Cancer 2014, 14, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bours, M.J.; Beijer, S.; Winkels, R.M.; van Duijnhoven, F.J.; Mols, F.; Breedveld-Peters, J.J.; Kampman, E.; Weijenberg, M.P.; van de Poll-Franse, L.V. Dietary changes and dietary supplement use, and underlying motives for these habits reported by colorectal cancer survivors of the Patient Reported Outcomes Following Initial Treatment and Long-Term Evaluation of Survivorship (PROFILES) registry. Br. J. Nutr. 2015, 114, 286–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef] [Green Version]
- Daly, J.M.; Redmond, H.P.; Gallagher, H. Perioperative nutrition in cancer patients. JPEN J. Parenter. Enter. Nutr. 1992, 16, 100s–105s. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Abalo, R.; Bornstein, J.C.; Nurgali, K. Chemotherapy-Induced Constipation and Diarrhea: Pathophysiology, Current and Emerging Treatments. Front. Pharmacol. 2016, 7, 414. [Google Scholar] [CrossRef] [Green Version]
- Bouabdallah, I.; D’Journo, X.B. Risk factors of post-esophagectomy-induced malnutrition. J. Thorac. Dis. 2019, 11, S1357–S1359. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef]
- Inoue-Choi, M.; Greenlee, H.; Oppeneer, S.J.; Robien, K. The association between postdiagnosis dietary supplement use and total mortality differs by diet quality among older female cancer survivors. Cancer Epidemiol. Biomark. Prev. 2014, 23, 865–875. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, S.; De Curtis, A.; Di Castelnuovo, A.; Persichillo, M.; Bonaccio, M.; Pounis, G.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Serum vitamin D deficiency and risk of hospitalization for heart failure: Prospective results from the Moli-sani study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Wassertheil-Smoller, S.; Thomson, C.; Aragaki, A.; Anderson, G.L.; Manson, J.E.; Patterson, R.E.; Rohan, T.E.; van Horn, L.; Shikany, J.M.; et al. Multivitamin Use and Risk of Cancer and Cardiovascular Disease in the Women’s Health Initiative Cohorts. Arch. Intern. Med. 2009, 169, 294–304. [Google Scholar] [CrossRef]
- Marian, M.J. Dietary Supplements Commonly Used by Cancer Survivors: Are There Any Benefits? Nutr. Clin. Pract. 2017, 32, 607–627. [Google Scholar] [CrossRef]
- Paur, I.; Lilleby, W.; Bøhn, S.K.; Hulander, E.; Klein, W.; Vlatkovic, L.; Axcrona, K.; Bolstad, N.; Bjøro, T.; Laake, P.; et al. Tomato-based randomized controlled trial in prostate cancer patients: Effect on PSA. Clin. Nutr. 2017, 36, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Peters, U.; Foster, C.B.; Chatterjee, N.; Schatzkin, A.; Reding, D.; Andriole, G.L.; Crawford, E.D.; Sturup, S.; Chanock, S.J.; Hayes, R.B. Serum selenium and risk of prostate cancer-a nested case-control study. Am. J. Clin. Nutr. 2007, 85, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Debniak, T.; Falco, M.; Kladny, J.; et al. Serum selenium levels predict survival after breast cancer. Breast Cancer Res. Treat. 2018, 167, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Orsini, N.; Wolk, A. Vitamin C and survival among women with breast cancer: A meta-analysis. Eur. J. Cancer 2014, 50, 1223–1231. [Google Scholar] [CrossRef]
- American Cancer Society. American Cancer Society Guideline for Diet and Physical Activity. Available online: https://www.cancer.org/healthy/eat-healthy-get-active/acs-guidelines-nutrition-physical-activity-cancer-prevention/guidelines.html (accessed on 23 September 2020).
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schloss, J. Cancer treatment and nutritional deficiencies. In Nutritional Deficiency; InTech: Vienna, Austria, 2016; pp. 173–196. [Google Scholar]
- Ströhle, A.; Zänker, K.; Hahn, A. Nutrition in oncology: The case of micronutrients (review). Oncol. Rep. 2010, 24, 815–828. [Google Scholar] [CrossRef] [Green Version]
- Dreizen, S.; McCredie, K.B.; Keating, M.J.; Andersson, B.S. Nutritional deficiencies in patients receiving cancer chemotherapy. Postgrad. Med. 1990, 87, 163–167, 170. [Google Scholar] [CrossRef]
- DiMartino, L.D.; Birken, S.A.; Mayer, D.K. The Relationship Between Cancer Survivors’ Socioeconomic Status and Reports of Follow-up Care Discussions with Providers. J. Cancer Educ. 2017, 32, 749–755. [Google Scholar] [CrossRef]
- ACL Administration for Community Living. Profile of Older Americans. Available online: https://acl.gov/aging-and-disability-in-america/data-and-research/profile-older-americans (accessed on 22 April 2021).
- CDC. NHANES Survey Methods and Analytic Guidelines; Retrieved January; CDC: Atlanta, GA, USA, 2019; Volume 23.
- CDC. NHANES Dietary Data 2011–2012. Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Dietary&CycleBeginYear=2011 (accessed on 20 September 2020).
- AHRQ. Medical Expenditure Panel Survey. 2012. Available online: https://meps.ahrq.gov/mepsweb/data_stats/download_data_files_detail.jsp?cboPufNumber=HC-155 (accessed on 20 September 2020).
- AHRQ. Medical Expenditure Panel Survey. 2011. Available online: https://meps.ahrq.gov/mepsweb/data_stats/download_data_files_detail.jsp?cboPufNumber=HC-147 (accessed on 20 September 2020).
| Characteristic | No Supplement Use n = 7,097,880 | Supplement Use n = 7,267,101 | p |
|---|---|---|---|
| Age | 60.5 {1.3} | 61.6 {1.3} | 0.52 |
| Sex | |||
| Male | 3,791,028 (53.4) | 3,759,878 (51.7) | 0.81 |
| Female | 3,306,851 (46.6) | 3,507,222 (48.3) | |
| Race | |||
| Mexican American | 137,969 (1.9) | 159,221 (2.2) | 0.97 |
| Other Hispanic | 176,846 (2.5) | 150,702 (2.1) | |
| NH White | 6,090,800 (85.8) | 6,196,220 (85.3) | |
| NH Black | 443,511 (6.2) | 429,859 (5.9) | |
| Other | 248,754 (3.5) | 331,098 (4.6) | |
| Education | |||
| ≤High School Graduate | 2,286,161 (32.2) | 1,982,235 (27.3) | 0.61 |
| Some College | 2,800,442 (39.5) | 2,786,318 (38.3) | |
| ≥ Bachelor’s Degree | 2,011,276 (28.3) | 2,498,547 (34.4) | |
| Income to Poverty Ratio | |||
| <100% FPL | 850,849 (12.0) | 716,112 (9.9) | 0.63 |
| 100 to <200% FPL | 1,516,662 (21.4) | 1,440,526 (19.8) | |
| 200 to <300% FPL | 702,935 (9.9) | 683,737 (9.4) | |
| >=300% FPL | 3,051,271 (43.0) | 3,807,709 (52.4) | |
| Missing | 976,163 (13.8) | 619,017 (8.5) | |
| Comorbidities | |||
| Arthritis | 3,966,058 (55.9) | 3,444,156 (47.4) | 0.26 |
| Diabetes | 1,281,946 (18.1) | 1,586,416 (21.8) | 0.49 |
| CHF | 349,043 (4.9) | 417,474 (5.7) | 0.75 |
| COPD | 1,235,382 (17.4) | 768,467 (10.6) | 0.17 |
| HTN | 3,777,368 (53.2) | 3,737,143 (51.4) | 0.79 |
| Obese | 3,016,614 (42.5) | 2,923,929 (40.2) | 0.77 |
| US Citizen | 6,602,817 (93.0) | 6,857,931 (94.4) | 0.49 |
| Regular Health Care Provider | 6,687,467 (94.2) | 6,774,950 (93.2) | 0.80 |
| Smoker | |||
| Current | 1,846,695 (26.0) | 1,777,151 (24.5) | 0.52 |
| Former | 2,182,592 (30.7) | 2,766,162 (38.1) | |
| Never | 3,068,593 (43.2) | 2,723,787 (37.5) | |
| Insurance | |||
| Private | 2,746,533 (38.7) | 2,892,542 (39.8) | 0.50 |
| Medicare | 2,603,265 (36.7) | 2,634,374 (36.3) | |
| Other | 1,184,830 (16.7) | 1,499,233 (20.6) | |
| None | 563,252 (7.9) | 240,952 (3.3) | |
| Quality of life | |||
| Physical health poor | 6.7 [1.2] | 3.6 [1.1] | 0.09 |
| Mental health poor | 4.9 [1.1] | 3.6 [1.1] | 0.36 |
| EQ5D | 0.8 [0.0] | 0.9 [0.0] | 0.01 |
| Variable Name | Distribution Type | Parameters Mean (St. Dev) | ||
|---|---|---|---|---|
| Probability Hospitalization | ||||
| With supplement use | Beta | 0.12 (0.05) | ||
| No supplement use | Beta | 0.20 (0.05) | ||
| Probability mortality | ||||
| Supplement Use | Hospitalized | |||
| X | X | Beta | 0.273 (0.05) | |
| X | Beta | 0.058 (0.01) | ||
| X | Beta | 0.287 (0.05) | ||
| Beta | 0.066 (0.01) | |||
| Years survival | Gamma | 13 (5) | ||
| Cost of hospitalization | Gamma | 4030 (2000) | ||
| Cost of health care | ||||
| Initial year | Gamma | 60,000 (20,000) | ||
| Continuing years | Gamma | 15,000 (5000) | ||
| Last year of life | Gamma | 80,000 (30,000) | ||
| EQ5D | ||||
| Supplement use | Hospitalized | Died | ||
| X | X | Beta | 0.80 (0.02) | |
| X | X | X | Beta | 0.67 (0.02) |
| X | Beta | 0.83 (0.02) | ||
| X | X | Beta | 0.80 (0.02) | |
| X | Beta | 0.81 (0.02) | ||
| X | X | Beta | 0.62 (0.02) | |
| Beta | 0.80 (0.02) | |||
| X | Beta | 0.80 (0.02) | ||
| Measure (Average Per Patient) | No Supplement Use | Supplement Use |
|---|---|---|
| Health outcome | ||
| QALYs | 9.78 | 10.26 |
| ICER | ||
| Base case costs | $234,839 | $236,933 |
| ΔCost/ΔQALY | 4362 | |
| Simulation: 1 year life expectancy | $54,839 | $52,553 |
| ΔCost/ΔQALY | dominant | |
| Simulation: 20 year life expectancy | $339,839 | $344,488 |
| ΔCost/ΔQALY | 6348 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaver, A.L.; Tufuor, T.A.; Nie, J.; Ekimura, S.; Marshall, K.; Mitmesser, S.H.; Noyes, K. Cost-Effectiveness of Nutrient Supplementation in Cancer Survivors. Cancers 2021, 13, 6276. https://doi.org/10.3390/cancers13246276
Shaver AL, Tufuor TA, Nie J, Ekimura S, Marshall K, Mitmesser SH, Noyes K. Cost-Effectiveness of Nutrient Supplementation in Cancer Survivors. Cancers. 2021; 13(24):6276. https://doi.org/10.3390/cancers13246276
Chicago/Turabian StyleShaver, Amy L., Theresa A. Tufuor, Jing Nie, Shauna Ekimura, Keri Marshall, Susan Hazels Mitmesser, and Katia Noyes. 2021. "Cost-Effectiveness of Nutrient Supplementation in Cancer Survivors" Cancers 13, no. 24: 6276. https://doi.org/10.3390/cancers13246276
APA StyleShaver, A. L., Tufuor, T. A., Nie, J., Ekimura, S., Marshall, K., Mitmesser, S. H., & Noyes, K. (2021). Cost-Effectiveness of Nutrient Supplementation in Cancer Survivors. Cancers, 13(24), 6276. https://doi.org/10.3390/cancers13246276






