Fibroblast Activation Protein Expressing Mesenchymal Cells Promote Glioblastoma Angiogenesis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients, Survival Analysis
2.2. Cell Lines and Cell Culture
2.3. Derivation of Primary Cell Cultures
2.4. Preparation of a Conditioned Medium
2.5. Comparative Genomic Hybridisation/Single-Nucleotide Polymorphism Analysis
2.6. Tumourigenicity Assay
2.7. Transwell Migration Assay
2.8. Cell Growth Assays
2.9. Immunohistochemistry and Immunocytochemistry, Quantification of FAP Expression in Glioblastoma
2.10. Proteome Profiler Human Angiogenesis Array
2.11. Chicken Chorioallantoic Membrane Assay (CAM Assay)
2.12. 3D Angiogenic Sprouting Assay
2.13. Statistical Analyses
3. Results
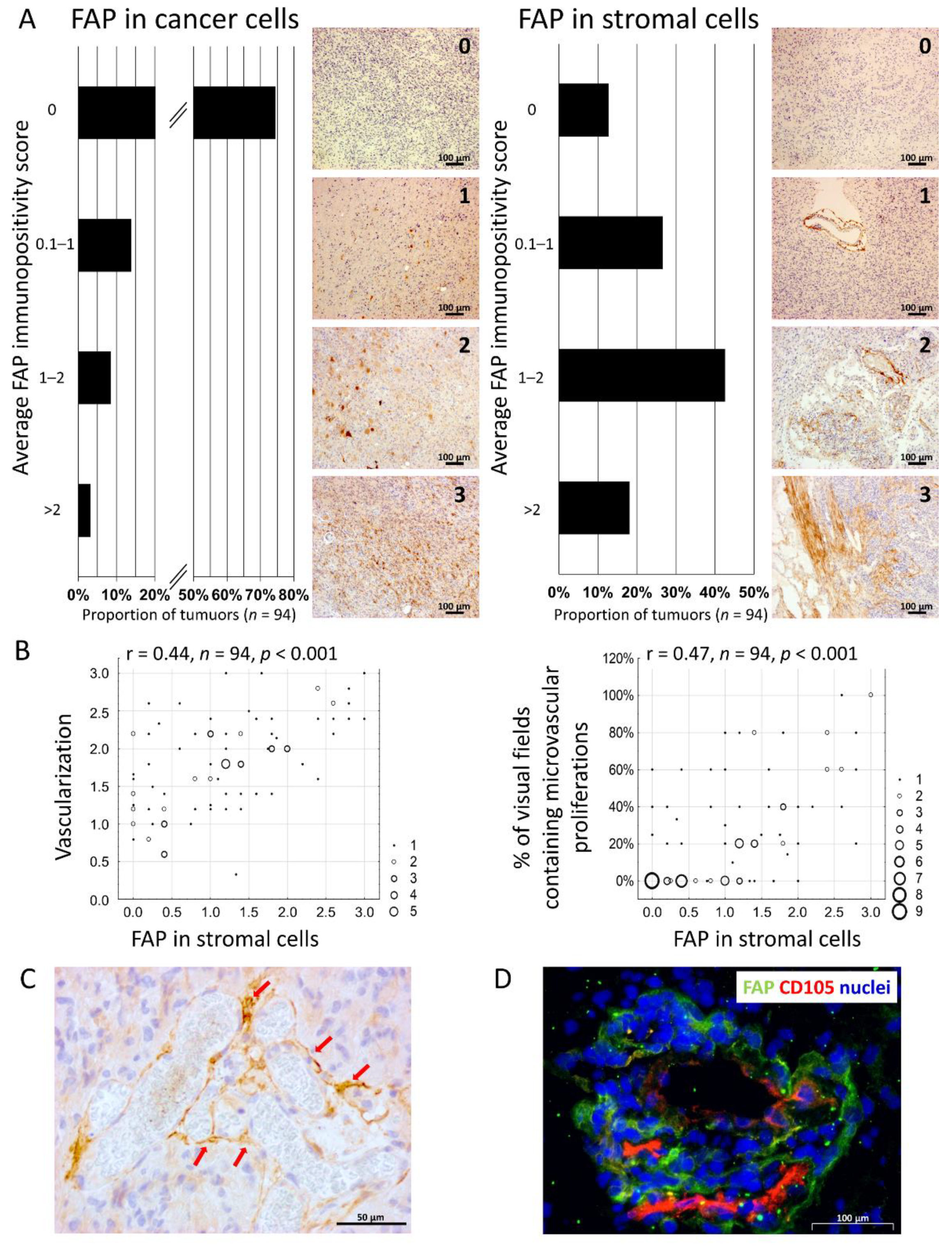
3.1. The Presence of FAP+ Stromal Cells Is Associated with Neovascularisation in Glioblastoma
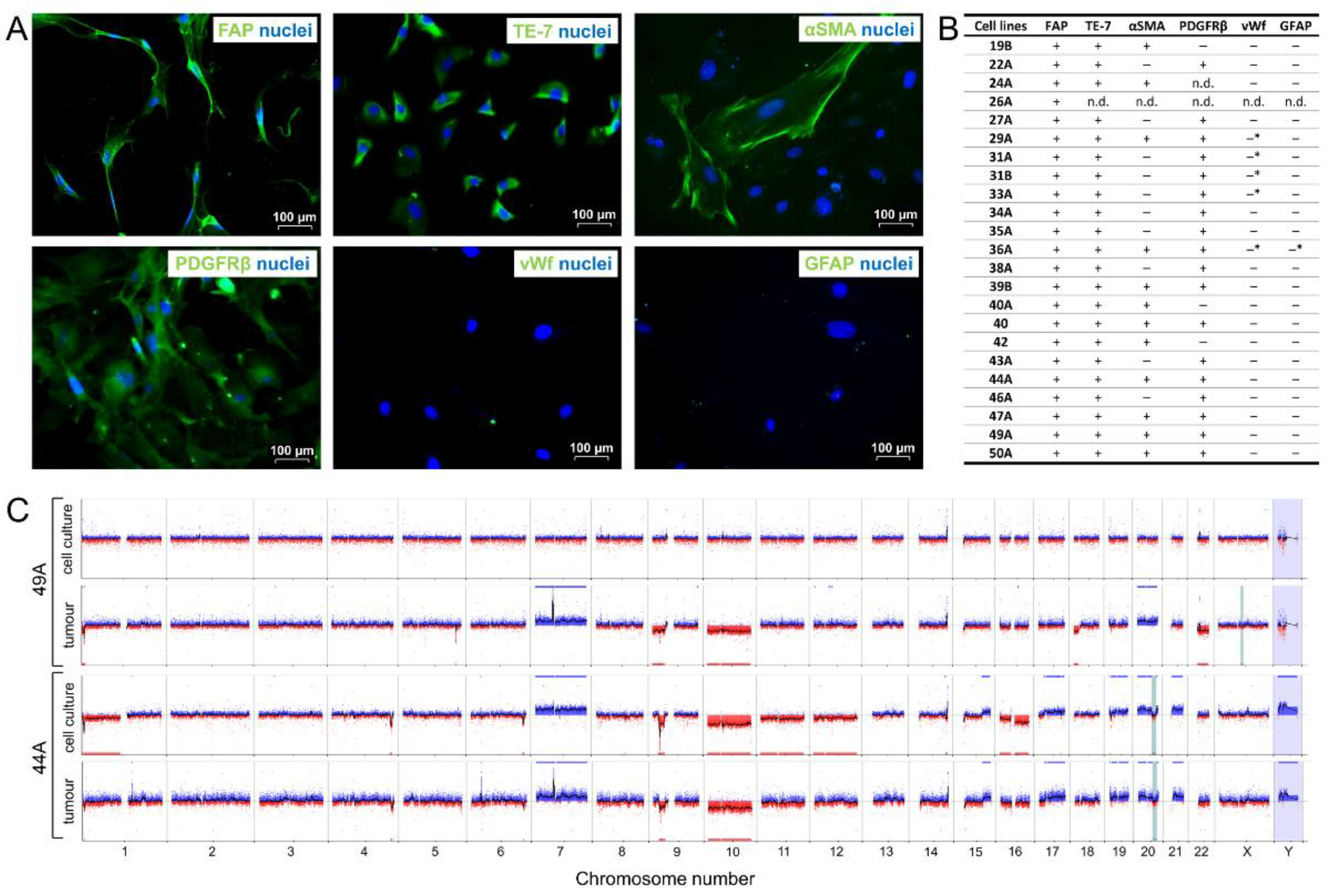
3.2. FAP+ Stromal Cells from Human Glioblastomas Express Mesenchymal Markers, Are Non-Tumorigenic, and Mostly Lack Aberrations Characteristic of Glioma Cells
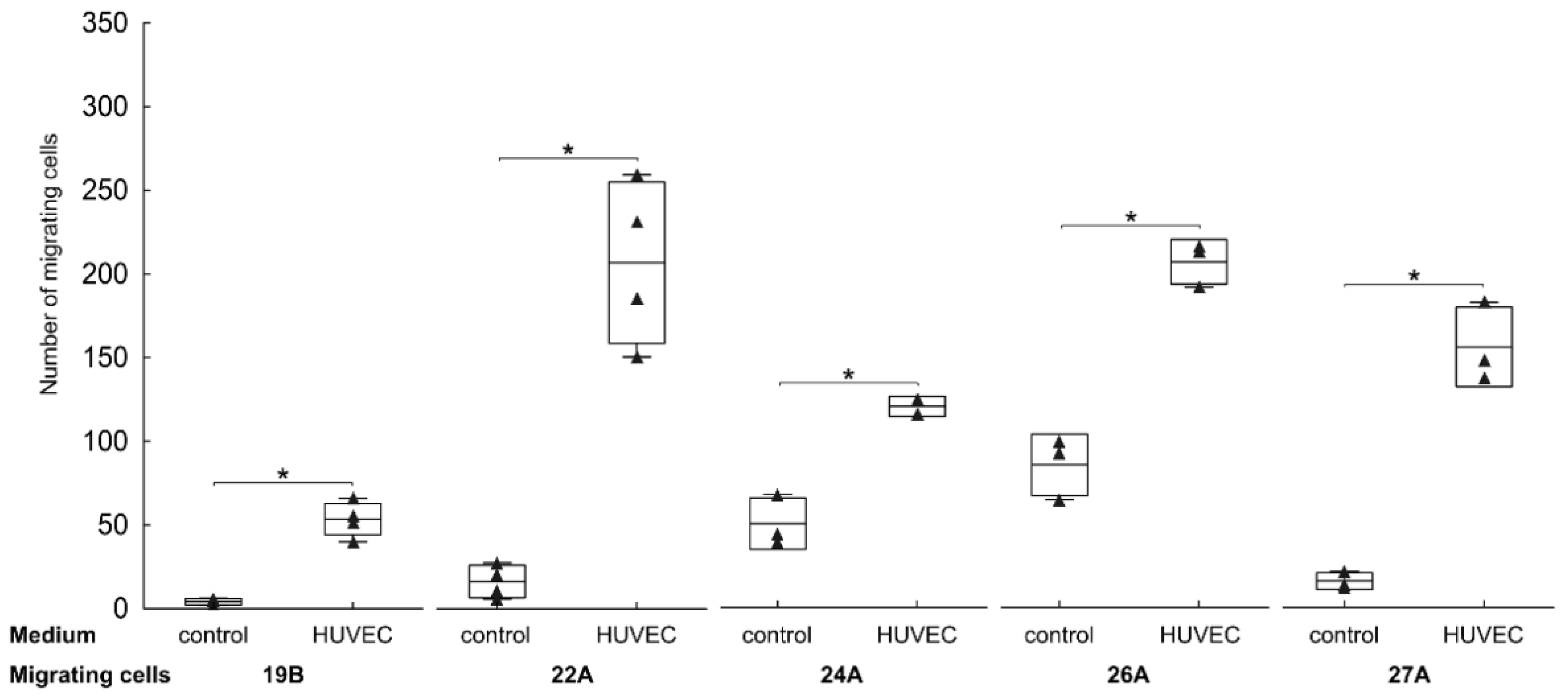
3.3. Endothelial Cells Attract FAP+ Mesenchymal Cells by Soluble Factors
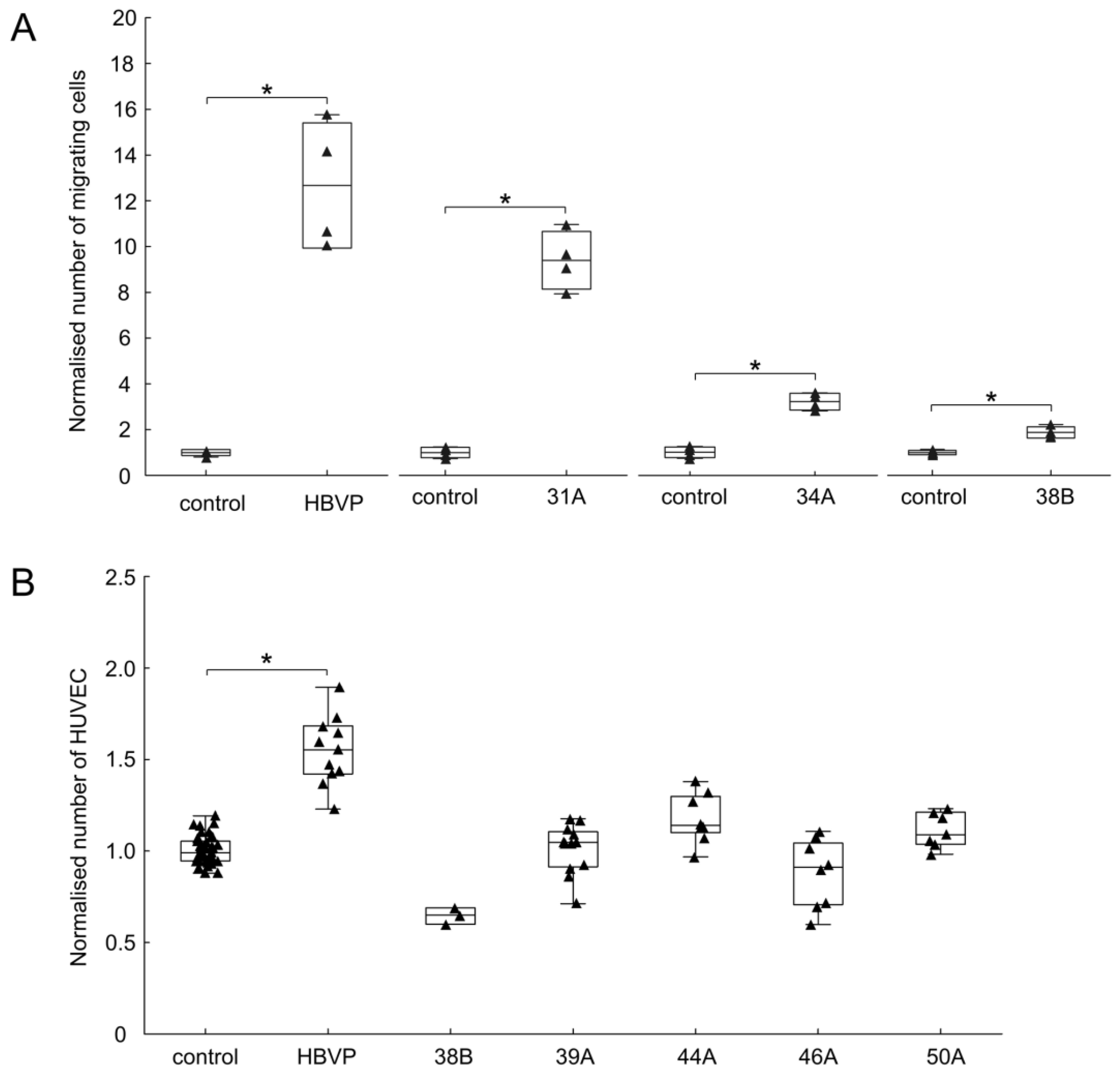
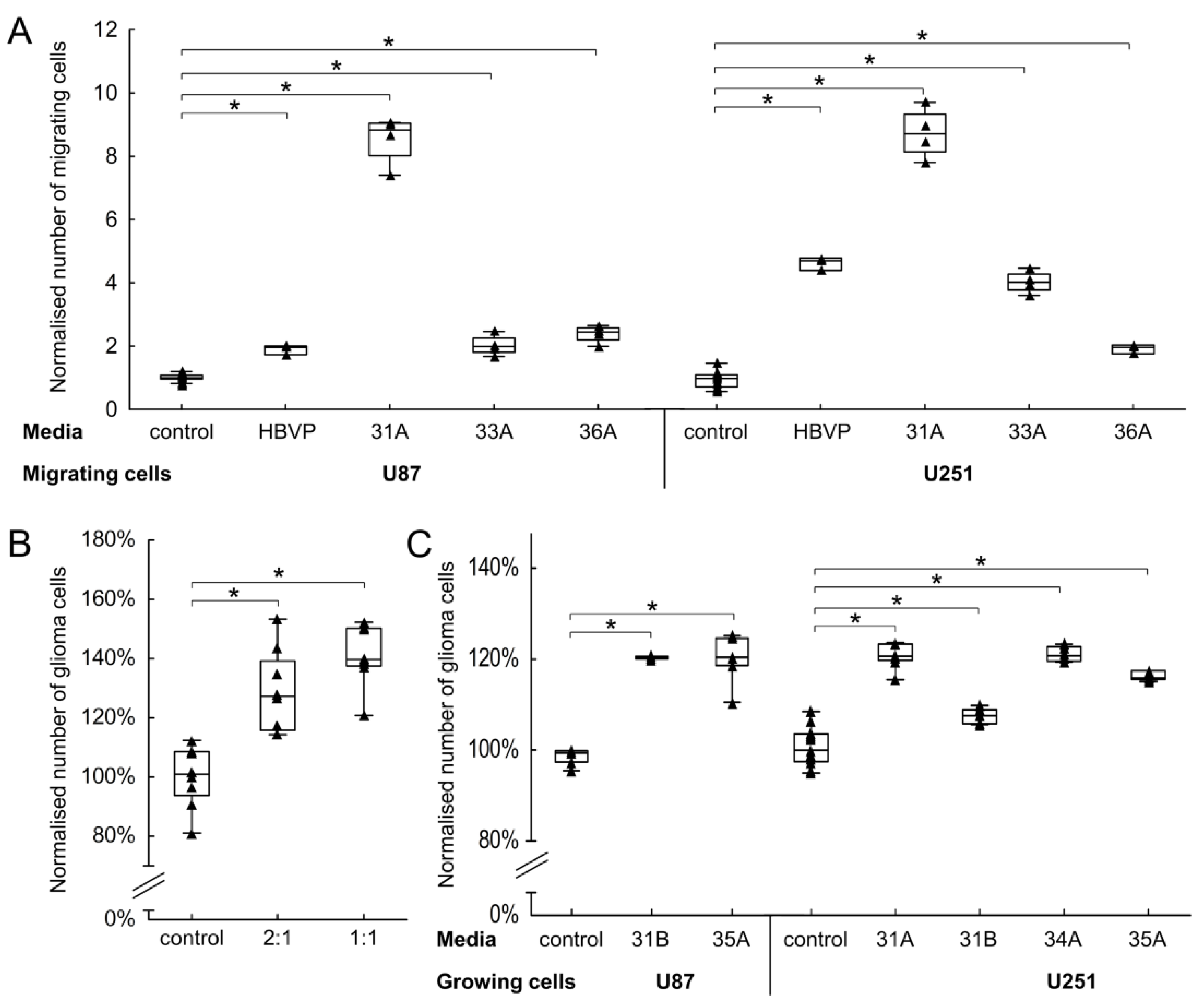
3.4. The Effect of FAP+ Mesenchymal Cells on the Migration and Growth of Endothelial and Glioma Cells
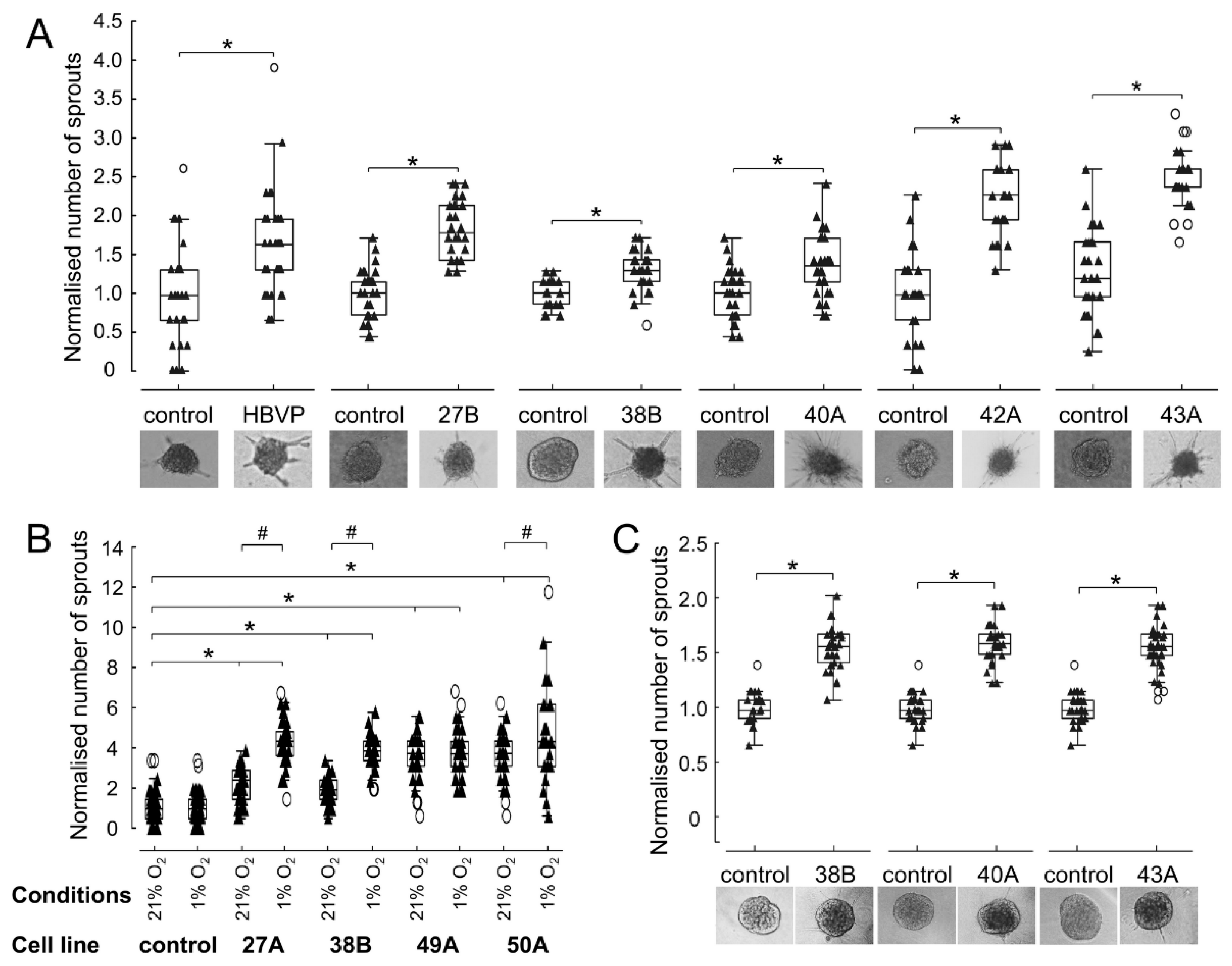
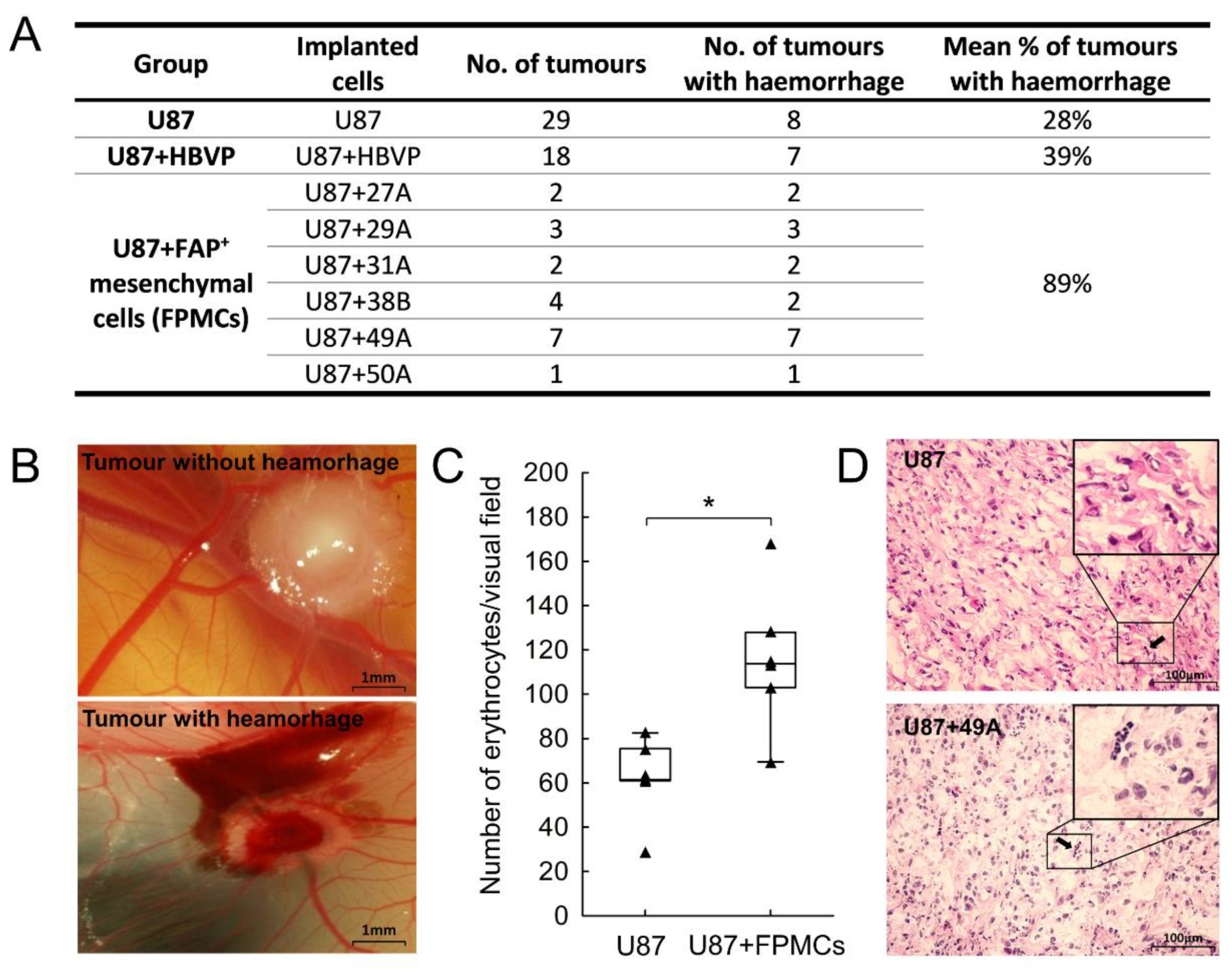
3.5. FAP+ Mesenchymal Cells Promote Endothelial Sprouting and Contribute to Vascular Destabilisation
3.6. Changed Proportion of Pro-Angiogenic, Anti-Angiogenic and Blood Vessel-Stabilising Mediators in FAP+ Mesenchymal Cells
3.7. Presence of FAP+ Stromal Cells Is Associated with Tumour Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Brat, D.J.; Van Meir, E.G. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab. Investig. 2004, 84, 397–405. [Google Scholar] [CrossRef]
- Scharpfenecker, M.; Fiedler, U.; Reiss, Y.; Augustin, H.G. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J. Cell Sci. 2005, 118, 771–780. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, N.; Park, J.W.; Katsaros, D.; Fracchioli, S.; Cao, G.; O’Brien-Jenkins, A.; Randall, T.C.; Rubin, S.C.; Coukos, G. Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res. 2003, 63, 3403–3412. [Google Scholar]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Broekman, M.L.; Maas, S.L.N.; Abels, E.R.; Mempel, T.R.; Krichevsky, A.M.; Breakefield, X.O. Multidimensional communication in the microenvirons of glioblastoma. Nat. Rev. Neurol. 2018, 14, 482–495. [Google Scholar] [CrossRef]
- Mikheeva, S.A.; Mikheev, A.M.; Petit, A.; Beyer, R.; Oxford, R.G.; Khorasani, L.; Maxwell, J.P.; Glackin, C.A.; Wakimoto, H.; Gonzalez-Herrero, I.; et al. TWIST1 promotes invasion through mesenchymal change in human glioblastoma. Mol. Cancer 2010, 9, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busek, P.; Balaziova, E.; Matrasova, I.; Hilser, M.; Tomas, R.; Syrucek, M.; Zemanova, Z.; Krepela, E.; Belacek, J.; Sedo, A. Fibroblast activation protein alpha is expressed by transformed and stromal cells and is associated with mesenchymal features in glioblastoma. Tumour Biol. 2016, 37, 13961–13971. [Google Scholar] [CrossRef] [PubMed]
- Mentlein, R.; Hattermann, K.; Hemion, C.; Jungbluth, A.A.; Held-Feindt, J. Expression and role of the cell surface protease seprase/fibroblast activation protein-alpha (FAP-alpha) in astroglial tumors. Biol. Chem. 2011, 392, 199–207. [Google Scholar] [CrossRef]
- Pure, E.; Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: Back to the basics. Oncogene 2018, 37, 4343–4357. [Google Scholar] [CrossRef]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebert, L.M.; Yu, W.; Gargett, T.; Toubia, J.; Kollis, P.M.; Tea, M.N.; Ebert, B.W.; Bardy, C.; van den Hurk, M.; Bonder, C.S.; et al. Endothelial, pericyte and tumor cell expression in glioblastoma identifies fibroblast activation protein (FAP) as an excellent target for immunotherapy. Clin. Transl. Immunol. 2020, 9, e1191. [Google Scholar] [CrossRef]
- Calabrese, C.; Poppleton, H.; Kocak, M.; Hogg, T.L.; Fuller, C.; Hamner, B.; Oh, E.Y.; Gaber, M.W.; Finklestein, D.; Allen, M.; et al. A perivascular niche for brain tumor stem cells. Cancer Cell 2007, 11, 69–82. [Google Scholar] [CrossRef] [Green Version]
- von Bulow, C.; Hayen, W.; Hartmann, A.; Mueller-Klieser, W.; Allolio, B.; Nehls, V. Endothelial capillaries chemotactically attract tumour cells. J. Pathol. 2001, 193, 367–376. [Google Scholar] [CrossRef]
- Schiffer, D.; Cavalla, P.; Dutto, A.; Borsotti, L. Cell proliferation and invasion in malignant gliomas. Anticancer Res. 1997, 17, 61–69. [Google Scholar]
- Lugassy, C.; Haroun, R.I.; Brem, H.; Tyler, B.M.; Jones, R.V.; Fernandez, P.M.; Patierno, S.R.; Kleinman, H.K.; Barnhill, R.L. Pericytic-like angiotropism of glioma and melanoma cells. Am. J. Dermatopathol. 2002, 24, 473–478. [Google Scholar] [CrossRef]
- Kraman, M.; Bambrough, P.J.; Arnold, J.N.; Roberts, E.W.; Magiera, L.; Jones, J.O.; Gopinathan, A.; Tuveson, D.A.; Fearon, D.T. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010, 330, 827–830. [Google Scholar] [CrossRef] [Green Version]
- Kawase, T.; Yasui, Y.; Nishina, S.; Hara, Y.; Yanatori, I.; Tomiyama, Y.; Nakashima, Y.; Yoshida, K.; Kishi, F.; Nakamura, M.; et al. Fibroblast activation protein-alpha-expressing fibroblasts promote the progression of pancreatic ductal adenocarcinoma. BMC Gastroenterol. 2015, 15, 109. [Google Scholar] [CrossRef] [Green Version]
- Koczorowska, M.M.; Tholen, S.; Bucher, F.; Lutz, L.; Kizhakkedathu, J.N.; De Wever, O.; Wellner, U.F.; Biniossek, M.L.; Stahl, A.; Lassmann, S.; et al. Fibroblast activation protein-alpha, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations. Mol. Oncol. 2016, 10, 40–58. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.; Li, Z.; Wang, C.; Xian, S.; Xu, G.; Peng, F.; Wei, Y.; Lu, Y. Short hairpin RNA targeting of fibroblast activation protein inhibits tumor growth and improves the tumor microenvironment in a mouse model. BMB Rep. 2013, 46, 252–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, A.; Wang, L.S.; Scholler, J.; Monslow, J.; Avery, D.; Newick, K.; O’Brien, S.; Evans, R.A.; Bajor, D.J.; Clendenin, C.; et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015, 75, 2800–2810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Molinaro, A.M.; Butowski, N.; Prados, M. Clinical trial endpoints for patients with gliomas. Neuro-Oncol. Pr. 2017, 4, 201–208. [Google Scholar] [CrossRef]
- Schemper, M.; Kaider, A.; Wakounig, S.; Heinze, G. Estimating the correlation of bivariate failure times under censoring. Stat. Med. 2013, 32, 4781–4790. [Google Scholar] [CrossRef]
- Miebach, S.; Grau, S.; Hummel, V.; Rieckmann, P.; Tonn, J.C.; Goldbrunner, R.H. Isolation and culture of microvascular endothelial cells from gliomas of different WHO grades. J. Neuro-Oncol. 2006, 76, 39–48. [Google Scholar] [CrossRef]
- Busek, P.; Stremenova, J.; Sromova, L.; Hilser, M.; Balaziova, E.; Kosek, D.; Trylcova, J.; Strnad, H.; Krepela, E.; Sedo, A. Dipeptidyl peptidase-IV inhibits glioma cell growth independent of its enzymatic activity. Int. J. Biochem. Cell Biol. 2012, 44, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Heiss, M.; Hellstrom, M.; Kalen, M.; May, T.; Weber, H.; Hecker, M.; Augustin, H.G.; Korff, T. Endothelial cell spheroids as a versatile tool to study angiogenesis in vitro. FASEB J. 2015, 29, 3076–3084. [Google Scholar] [CrossRef]
- Stremenova, J.; Krepela, E.; Mares, V.; Trim, J.; Dbaly, V.; Marek, J.; Vanickova, Z.; Lisa, V.; Yea, C.; Sedo, A. Expression and enzymatic activity of dipeptidyl peptidase-IV in human astrocytic tumours are associated with tumour grade. Int. J. Oncol. 2007, 31, 785–792. [Google Scholar] [CrossRef]
- Guarnaccia, L.; Navone, S.E.; Trombetta, E.; Cordiglieri, C.; Cherubini, A.; Crisa, F.M.; Rampini, P.; Miozzo, M.; Fontana, L.; Caroli, M.; et al. Angiogenesis in human brain tumors: Screening of drug response through a patient-specific cell platform for personalized therapy. Sci. Rep. 2018, 8, 8748. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Chi, Y.; Zhang, Q.; Xu, F.; Yang, Z.; Meng, L.; Yang, S.; Yan, S.; Mao, A.; et al. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis. 2013, 4, e950. [Google Scholar] [CrossRef] [Green Version]
- Fomchenko, E.I.; Dougherty, J.D.; Helmy, K.Y.; Katz, A.M.; Pietras, A.; Brennan, C.; Huse, J.T.; Milosevic, A.; Holland, E.C. Recruited cells can become transformed and overtake PDGF-induced murine gliomas in vivo during tumor progression. PLoS ONE 2011, 6, e20605. [Google Scholar] [CrossRef] [Green Version]
- McGahan, B.G.; Neilsen, B.K.; Kelly, D.L.; McComb, R.D.; Kazmi, S.A.; White, M.L.; Zhang, Y.; Aizenberg, M.R. Assessment of vascularity in glioblastoma and its implications on patient outcomes. J. Neurooncol. 2017, 132, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Huang, Z.; Zhou, W.; Wu, Q.; Donnola, S.; Liu, J.K.; Fang, X.; Sloan, A.E.; Mao, Y.; Lathia, J.D.; et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 2013, 153, 139–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, A.; Gumin, J.; Gao, F.; Figueroa, J.; Shinojima, N.; Takezaki, T.; Priebe, W.; Villarreal, D.; Kang, S.G.; Joyce, C.; et al. Mesenchymal Stem Cells Isolated From Human Gliomas Increase Proliferation and Maintain Stemness of Glioma Stem Cells Through the IL-6/gp130/STAT3 Pathway. Stem Cells 2015, 33, 2400–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaziova, E.; Charles University, Prague, Czech Republic; Vymola, P.; Charles University, Prague, Czech Republic. Personal communication, 2021.
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- Mitsuhashi, N.; Shimizu, H.; Ohtsuka, M.; Wakabayashi, Y.; Ito, H.; Kimura, F.; Yoshidome, H.; Kato, A.; Nukui, Y.; Miyazaki, M. Angiopoietins and Tie-2 expression in angiogenesis and proliferation of human hepatocellular carcinoma. Hepatology 2003, 37, 1105–1113. [Google Scholar] [CrossRef]
- Herrlinger, U.; Schafer, N.; Steinbach, J.P.; Weyerbrock, A.; Hau, P.; Goldbrunner, R.; Friedrich, F.; Rohde, V.; Ringel, F.; Schlegel, U.; et al. Bevacizumab Plus Irinotecan Versus Temozolomide in Newly Diagnosed O6-Methylguanine-DNA Methyltransferase Nonmethylated Glioblastoma: The Randomized GLARIUS Trial. J. Clin. Oncol. 2016, 34, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef]
- Busek, P.; Mateu, R.; Zubal, M.; Kotackova, L.; Sedo, A. Targeting fibroblast activation protein in cancer—Prospects and caveats. Front. Biosci. 2018, 23, 1933–1968. [Google Scholar]
- Huang, Y.; Simms, A.E.; Mazur, A.; Wang, S.; Leon, N.R.; Jones, B.; Aziz, N.; Kelly, T. Fibroblast activation protein-alpha promotes tumor growth and invasion of breast cancer cells through non-enzymatic functions. Clin. Exp. Metastasis 2011, 28, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Clavreul, A.; Etcheverry, A.; Chassevent, A.; Quillien, V.; Avril, T.; Jourdan, M.L.; Michalak, S.; Francois, P.; Carre, J.L.; Mosser, J.; et al. Isolation of a new cell population in the glioblastoma microenvironment. J. Neuro-Oncol. 2012, 106, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Trylcova, J.; Busek, P.; Smetana, K., Jr.; Balaziova, E.; Dvorankova, B.; Mifkova, A.; Sedo, A. Effect of cancer-associated fibroblasts on the migration of glioma cells in vitro. Tumour Biol. 2015, 36, 5873–5879. [Google Scholar] [CrossRef]
- Krepela, E.; Vanickova, Z.; Hrabal, P.; Zubal, M.; Chmielova, B.; Balaziova, E.; Vymola, P.; Matrasova, I.; Busek, P.; Sedo, A. Regulation of Fibroblast Activation Protein by Transforming Growth Factor Beta-1 in Glioblastoma Microenvironment. Int. J. Mol. Sci. 2021, 22, 1046. [Google Scholar] [CrossRef]
- Santos, A.M.; Jung, J.; Aziz, N.; Kissil, J.L.; Pure, E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J. Clin. Investig. 2009, 119, 3613–3625. [Google Scholar] [CrossRef]
- Wang, T.; Shi, W. Expression of fibroblast activation proteins in corneal stromal neovascularization. Curr. Eye Res. 2009, 34, 112–117. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Kelly, T. Seprase promotes rapid tumor growth and increased microvessel density in a mouse model of human breast cancer. Cancer Res. 2004, 64, 2712–2716. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.F.; Gall, M.G.; Bachovchin, W.W.; McCaughan, G.W.; Keane, F.M.; Gorrell, M.D. Neuropeptide Y is a physiological substrate of fibroblast activation protein: Enzyme kinetics in blood plasma and expression of Y2R and Y5R in human liver cirrhosis and hepatocellular carcinoma. Peptides 2016, 75, 80–95. [Google Scholar] [CrossRef]
- Levy, M.T.; McCaughan, G.W.; Abbott, C.A.; Park, J.E.; Cunningham, A.M.; Muller, E.; Rettig, W.J.; Gorrell, M.D. Fibroblast activation protein: A cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology 1999, 29, 1768–1778. [Google Scholar] [CrossRef]
- Lin, N.; Meng, L.; Lin, J.; Chen, S.; Zhang, P.; Chen, Q.; Lin, Y. Activated hepatic stellate cells promote angiogenesis in hepatocellular carcinoma by secreting angiopoietin-1. J. Cell Biochem. 2020, 121, 1441–1451. [Google Scholar] [CrossRef]
- Sanz-Cameno, P.; Martin-Vilchez, S.; Lara-Pezzi, E.; Borque, M.J.; Salmeron, J.; Munoz de Rueda, P.; Solis, J.A.; Lopez-Cabrera, M.; Moreno-Otero, R. Hepatitis B virus promotes angiopoietin-2 expression in liver tissue: Role of HBV x protein. Am. J. Pathol. 2006, 169, 1215–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirri, P.; Chiarugi, P. Cancer associated fibroblasts: The dark side of the coin. Am. J. Cancer Res. 2011, 1, 482–497. [Google Scholar] [PubMed]
- Clavreul, A.; Guette, C.; Faguer, R.; Tetaud, C.; Boissard, A.; Lemaire, L.; Rousseau, A.; Avril, T.; Henry, C.; Coqueret, O.; et al. Glioblastoma-associated stromal cells (GASCs) from histologically normal surgical margins have a myofibroblast phenotype and angiogenic properties. J. Pathol. 2014, 233, 74–88. [Google Scholar] [CrossRef]
- Li, M.; Li, G.; Kiyokawa, J.; Tirmizi, Z.; Richardson, L.G.; Ning, J.; Das, S.; Martuza, R.L.; Stemmer-Rachamimov, A.; Rabkin, S.D.; et al. Characterization and Oncol.ytic virus targeting of FAP-expressing tumor-associated pericytes in glioblastoma. Acta Neuropathol. Commun. 2020, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, P.; Johansson, B.R.; Leveen, P.; Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997, 277, 242–245. [Google Scholar] [CrossRef]
- Sundberg, C.; Ivarsson, M.; Gerdin, B.; Rubin, K. Pericytes as collagen-producing cells in excessive dermal scarring. Lab. Investig. 1996, 74, 452–466. [Google Scholar]
- Hosaka, K.; Yang, Y.; Seki, T.; Fischer, C.; Dubey, O.; Fredlund, E.; Hartman, J.; Religa, P.; Morikawa, H.; Ishii, Y.; et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2016, 113, E5618–E5627. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, H.; Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003, 314, 15–23. [Google Scholar] [CrossRef]
- Dore-Duffy, P.; LaManna, J.C. Physiologic angiodynamics in the brain. Antioxid. Redox Signal. 2007, 9, 1363–1371. [Google Scholar] [CrossRef]
- Nehls, V.; Denzer, K.; Drenckhahn, D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992, 270, 469–474. [Google Scholar] [CrossRef]
- Orlidge, A.; D’Amore, P.A. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J. Cell Biol. 1987, 105, 1455–1462. [Google Scholar] [CrossRef] [Green Version]
- Virgintino, D.; Girolamo, F.; Errede, M.; Capobianco, C.; Robertson, D.; Stallcup, W.B.; Perris, R.; Roncali, L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis 2007, 10, 35–45. [Google Scholar] [CrossRef]
- Hirschi, K.K.; Rohovsky, S.A.; Beck, L.H.; Smith, S.R.; D’Amore, P.A. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ. Res. 1999, 84, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Wakui, S.; Yokoo, K.; Muto, T.; Suzuki, Y.; Takahashi, H.; Furusato, M.; Hano, H.; Endou, H.; Kanai, Y. Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab. Investig. 2006, 86, 1172–1184. [Google Scholar] [CrossRef]
- Suri, C.; Jones, P.F.; Patan, S.; Bartunkova, S.; Maisonpierre, P.C.; Davis, S.; Sato, T.N.; Yancopoulos, G.D. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996, 87, 1171–1180. [Google Scholar] [CrossRef] [Green Version]
- Visconti, R.P.; Richardson, C.D.; Sato, T.N. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF). Proc. Natl. Acad. Sci. USA 2002, 99, 8219–8224. [Google Scholar] [CrossRef] [Green Version]
- Hosono, J.; Morikawa, S.; Ezaki, T.; Kawamata, T.; Okada, Y. Pericytes promote abnormal tumor angiogenesis in a rat RG2 glioma model. Brain Tumor Pathol. 2017, 34, 120–129. [Google Scholar] [CrossRef]
- Mao, P.; Smith, L.; Xie, W.; Wang, M. Dying endothelial cells stimulate proliferation of malignant glioma cells via a caspase 3-mediated pathway. Oncol. Lett. 2013, 5, 1615–1620. [Google Scholar] [CrossRef]
- Clavreul, A.; Etcheverry, A.; Tetaud, C.; Rousseau, A.; Avril, T.; Henry, C.; Mosser, J.; Menei, P. Identification of two glioblastoma-associated stromal cell subtypes with different carcinogenic properties in histologically normal surgical margins. J. Neuro-Oncol. 2015, 122, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Qi, L.; Liu, B.; Liu, J.; Zhang, H.; Che, D.; Cao, J.; Shen, J.; Geng, J.; Bi, Y.; et al. Fibroblast activation protein overexpression and clinical implications in solid tumors: A meta-analysis. PLoS ONE 2015, 10, e0116683. [Google Scholar] [CrossRef] [PubMed]
- Leon, S.P.; Folkerth, R.D.; Black, P.M. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 1996, 77, 362–372. [Google Scholar] [CrossRef]

| Number of Patients | 90 |
|---|---|
| Age–median (range) | 63.2 (28.6–81.5) |
| Sex | 56M/34F |
| Extent of resection | 62 radical, 28 subtotal |
| Preoperative Karnofsky performance status (median, range) | 90 (40–100) |
| Overall survival–median (range) | 13.6 months (3.1–57.9) |
| Progression-free survival–median (range) | 5 months (2–39) |
| Score | FAP in Cancer Cells | FAP in Stromal Cells |
|---|---|---|
| 0 | negative | negative |
| 1 | 1–10% positive cells | perivascular positivity in sporadic blood vessels |
| 2 | 10–20% positive cells | perivascular positivity in over 2/3 of the visual field |
| 3 | more than 20% positive cells | extensive perivascular positivity and FAP+ trabeculae |
| FAP Immunopositivity in Stromal Cells | Odds Ratio | Odds Ratio 95% Confidence Interval |
|---|---|---|
| 1 vs. 0 | 9.9 | 2.0–50.1 |
| 2 vs. 0 | 18.8 | 2.4–145.6 |
| 3 vs. 0 | 27.8 | 2.8–279.8 |
| 2 vs. 1 | 1.9 | 1.2–3.0 |
| 3 vs. 1 | 2.8 | 1.3–6.0 |
| 3 vs. 2 | 1.5 | 1.1–2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaziova, E.; Vymola, P.; Hrabal, P.; Mateu, R.; Zubal, M.; Tomas, R.; Netuka, D.; Kramar, F.; Zemanova, Z.; Svobodova, K.; et al. Fibroblast Activation Protein Expressing Mesenchymal Cells Promote Glioblastoma Angiogenesis. Cancers 2021, 13, 3304. https://doi.org/10.3390/cancers13133304
Balaziova E, Vymola P, Hrabal P, Mateu R, Zubal M, Tomas R, Netuka D, Kramar F, Zemanova Z, Svobodova K, et al. Fibroblast Activation Protein Expressing Mesenchymal Cells Promote Glioblastoma Angiogenesis. Cancers. 2021; 13(13):3304. https://doi.org/10.3390/cancers13133304
Chicago/Turabian StyleBalaziova, Eva, Petr Vymola, Petr Hrabal, Rosana Mateu, Michal Zubal, Robert Tomas, David Netuka, Filip Kramar, Zuzana Zemanova, Karla Svobodova, and et al. 2021. "Fibroblast Activation Protein Expressing Mesenchymal Cells Promote Glioblastoma Angiogenesis" Cancers 13, no. 13: 3304. https://doi.org/10.3390/cancers13133304
APA StyleBalaziova, E., Vymola, P., Hrabal, P., Mateu, R., Zubal, M., Tomas, R., Netuka, D., Kramar, F., Zemanova, Z., Svobodova, K., Brabec, M., Sedo, A., & Busek, P. (2021). Fibroblast Activation Protein Expressing Mesenchymal Cells Promote Glioblastoma Angiogenesis. Cancers, 13(13), 3304. https://doi.org/10.3390/cancers13133304






