COVID-19 Pandemic: Huge Stress Test for Health System Could Be a Great Opportunity to Update the Workflow in a Modern Surgical Pathology
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Operating Procedures and Reorganization of the Working Environment
- (a)
- Gross examination
- (b) Cytology
- (c) Flow cytometry
- (d) Cytogenetics
- (e) DNA extraction
- (f) Digital Pathology
2.3. Safety of Working Environment
2.4. Statistical Methods
3. Results
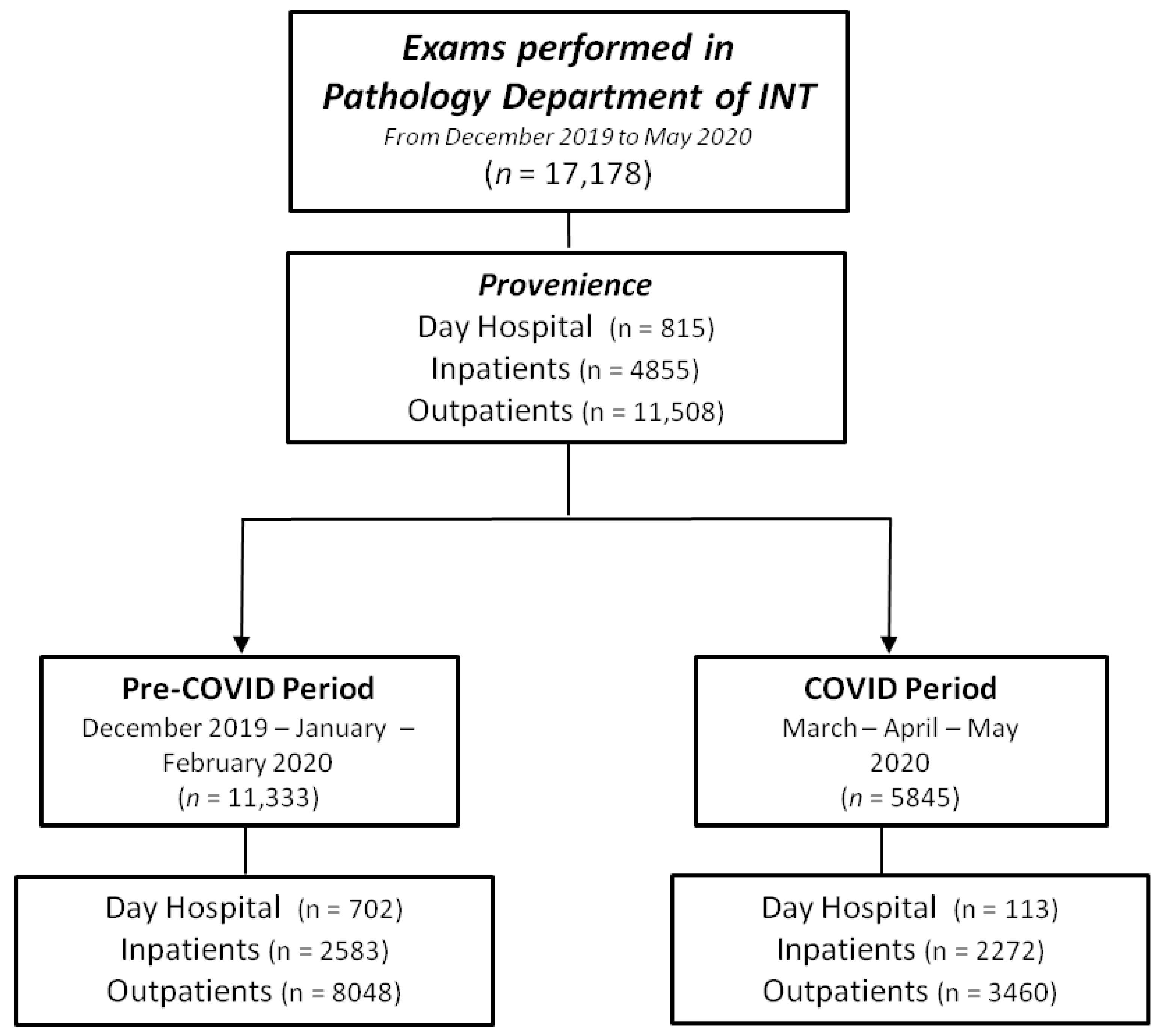
3.1. Exam Volume
3.2. Exam Source
3.3. Turn-Around-Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease (COVID-19) Situation Report. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 25 May 2021).
- Zhang, J.-J.; Dong, X.; Cao, Y.-Y.; Yuan, Y.-D.; Yang, Y.-B.; Yan, Y.-Q.; Akdis, C.A.; Gao, Y.-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea (accessed on 25 May 2021).
- Valenza, F.; Papagni, G.; Marchianò, A.; Daidone, M.G.; DeBraud, F.; Colombo, M.P.; Frignani, A.; Galmozzi, G.; Ladisa, V.; Pruneri, G.; et al. Response of a comprehensive cancer center to the COVID-19 pandemic: The experience of the Fondazione IRCCS-Istituto Nazionale dei Tumori di Milano. Tumori 2020, 300891620923790. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, L.; Deng, Q.; Zhang, G.; Wu, K.; Ni, L.; Yang, Y.; Liu, B.; Wang, W.; Wei, C.; et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020, 92, 833–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/ (accessed on 25 May 2021).
- London, J.W.; Fazio-Eynullayeva, E.; Palchuk, M.B.; Sankey, P.; McNair, C. Effects of the COVID-19 Pandemic on Cancer-Related Patient Encounters. JCO Clin. Cancer Inform. 2020, 4, 657–665. [Google Scholar] [CrossRef]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A.; et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
| Area Involved | Pre-COVID-19 Period | COVID-19 Period | Maintenance Post COVID-19 Period |
|---|---|---|---|
| Department access | INT personnel admitted | Authorized operators only | Yes |
| Personnel | 100% onsite | 50% clinicians onsite | To be considered |
| 50% biologists onsite | |||
| 85% technicians onsite | |||
| Gross Reduction Lab | Standard PPE for operators | Advanced PPE for operators | To be considered |
| Fresh sampling | 24–72 h fixation before sampling | No | |
| Daily intra-operative instrument decontamination | Decontamination after every intra-operative exam | Yes | |
| Cytology Lab | Standard PPE for operators | Advanced PPE for operators | To be considered |
| Instruments on bench | Instruments under class I biocabinet | Yes | |
| Flow Cytometry Lab | Standard PPE for operators | Advanced PPE for operators | To be considered |
| Daily instrument decontamination | Instrument decontamination between samples | Yes | |
| Cytogenetics Lab | Standard PPE for operators | Advanced PPE for operators | To be considered |
| Sample processed under laminal hood | Sample processed under laminal hood | Yes | |
| Molecular Lab | Standard PPE for operators | Advanced PPE for operators | To be considered |
| Automatization of nucleic acid extraction | Automatization of nucleic acid extraction under a laminar hood | To be considered | |
| Digital Pathology | For research use only | For diagnostic use | To be considered |
| Exams | Pre-COVID-19 Period (Dec 2019–Feb 2020) N (%) | COVID-19 Period (Mar–Apr 2020) N (%) | Absolute Change (Relative Change) |
|---|---|---|---|
| All exams | 11,333 (100) | 5845 (100) | −5488 (−48.4%) |
| All except pap test | 8149 (71.9) | 5260 (90.0) | −2889 (−35.5%) |
| Inpatients | 2583 (31.7) | 2272 (43.2) | −311 (−12.0%) |
| Day hospital | 702 (8.6) | 113 (2.1) | −589 (−83.9%) |
| Outpatients | 4864 (59.7) | 2875 (54.7) | −1989 (−40.9%) |
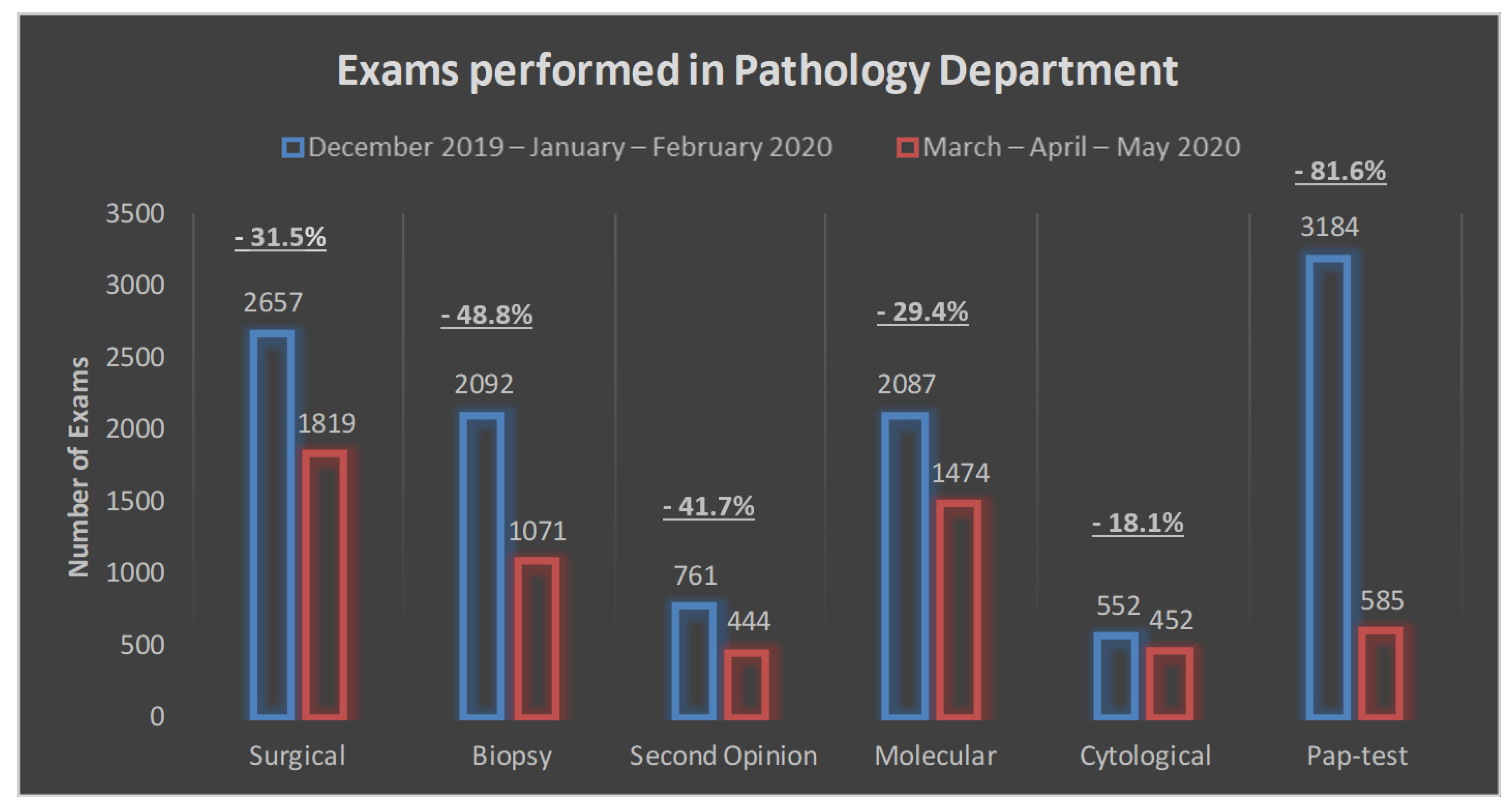
| Surgical | 2657 (23.4) | 1819 (31.1) | −838 (−31.5%) |
| Inpatients | 1214 (45.7) | 1169 (64.3) | −45 (−3.7%) |
| Day hospital | 511 (19.2) | 63 (3.5) | −448 (−88.7%) |
| Outpatients | 932 (35.1) | 587 (32.3) | −345 (−63.0%) |
| Biopsy | 2092 (18.5) | 1071 (18.3) | −1021 (−48.8%) |
| Inpatients | 268 (12.8) | 188 (17.6) | −80 (−29.9%) |
| Day hospital | 143 (6.8) | 18 (1.7) | −125 (−87.4%) |
| Outpatients | 1681 (80.4) | 865 (80.8) | −816 (−51.5%) |
| Second opinion | 761 (6.7) | 444 (7.6) | −317 (−41.7%) |
| Inpatients | 289 (38.0) | 198 (44.6) | −91 (−31.5%) |
| Day hospital | 2 (0.3) | 0 (0.0) | −2 (−100%) |
| Outpatients | 470 (61.7) | 246 (55.4) | −224 (−47.7%) |
| Molecular | 2087 (18.4) | 1474 (25.2) | −613 (−29.4%) |
| Inpatients | 543 (26.0) | 443 (30.0) | −100 (−18.4%) |
| Day hospital | 20 (1.0) | 16 (1.1) | −4 (−20.0%) |
| Outpatients | 1524 (73.0) | 1015 (68.9) | −509 (−33.4%) |
| Cytological | 552 (4.9) | 452 (7.7) | −100 (−18.1%) |
| Inpatients | 269 (48.7) | 274 (60.6) | +5 (+1.9%) |
| Day hospital | 26 (4.7) | 16 (3.5) | −10 (−38.5%) |
| Outpatients | 257 (46.6) | 162 (35.9) | −95 (−37.0%) |
| Pap test | 3184 (28.1) | 585 (10.1) | −2599 (−81.6%) |
| Exams | Pre-COVID-19 Period (Dec 2019–Feb 2020) N (%) in Time | COVID-19 Period (Mar–Apr 2020) N (%) in Time | p-Value * |
|---|---|---|---|
| Surgical | 2549 (95.9) | 1786 (98.2) | <0.0001 |
| Biopsies | 2013 (96.2) | 1027 (95.9) | 0.69 |
| Second Opinion | 650 (85.4) | 415 (93.5) | <0.0001 |
| Molecular | 1916 (91.8) | 1412 (95.8) | <0.0001 |
| Cytological | 541 (98.0) | 441 (97.6) | 0.66 |
| PAP Test | 3184 (100) | 585 (100) | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belfiore, A.; Centonze, G.; Maisonneuve, P.; Riva, C.; Morelli, D.; Mangogna, A.; Sabella, G.; Pruneri, G.; Milione, M. COVID-19 Pandemic: Huge Stress Test for Health System Could Be a Great Opportunity to Update the Workflow in a Modern Surgical Pathology. Cancers 2021, 13, 3283. https://doi.org/10.3390/cancers13133283
Belfiore A, Centonze G, Maisonneuve P, Riva C, Morelli D, Mangogna A, Sabella G, Pruneri G, Milione M. COVID-19 Pandemic: Huge Stress Test for Health System Could Be a Great Opportunity to Update the Workflow in a Modern Surgical Pathology. Cancers. 2021; 13(13):3283. https://doi.org/10.3390/cancers13133283
Chicago/Turabian StyleBelfiore, Antonino, Giovanni Centonze, Patrick Maisonneuve, Carla Riva, Daniele Morelli, Alessandro Mangogna, Giovanna Sabella, Giancarlo Pruneri, and Massimo Milione. 2021. "COVID-19 Pandemic: Huge Stress Test for Health System Could Be a Great Opportunity to Update the Workflow in a Modern Surgical Pathology" Cancers 13, no. 13: 3283. https://doi.org/10.3390/cancers13133283
APA StyleBelfiore, A., Centonze, G., Maisonneuve, P., Riva, C., Morelli, D., Mangogna, A., Sabella, G., Pruneri, G., & Milione, M. (2021). COVID-19 Pandemic: Huge Stress Test for Health System Could Be a Great Opportunity to Update the Workflow in a Modern Surgical Pathology. Cancers, 13(13), 3283. https://doi.org/10.3390/cancers13133283







