A Prospectively Validated Prognostic Model for Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck Based on Radiomics of Computed Tomography Images
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. CT Acquisition Parameters
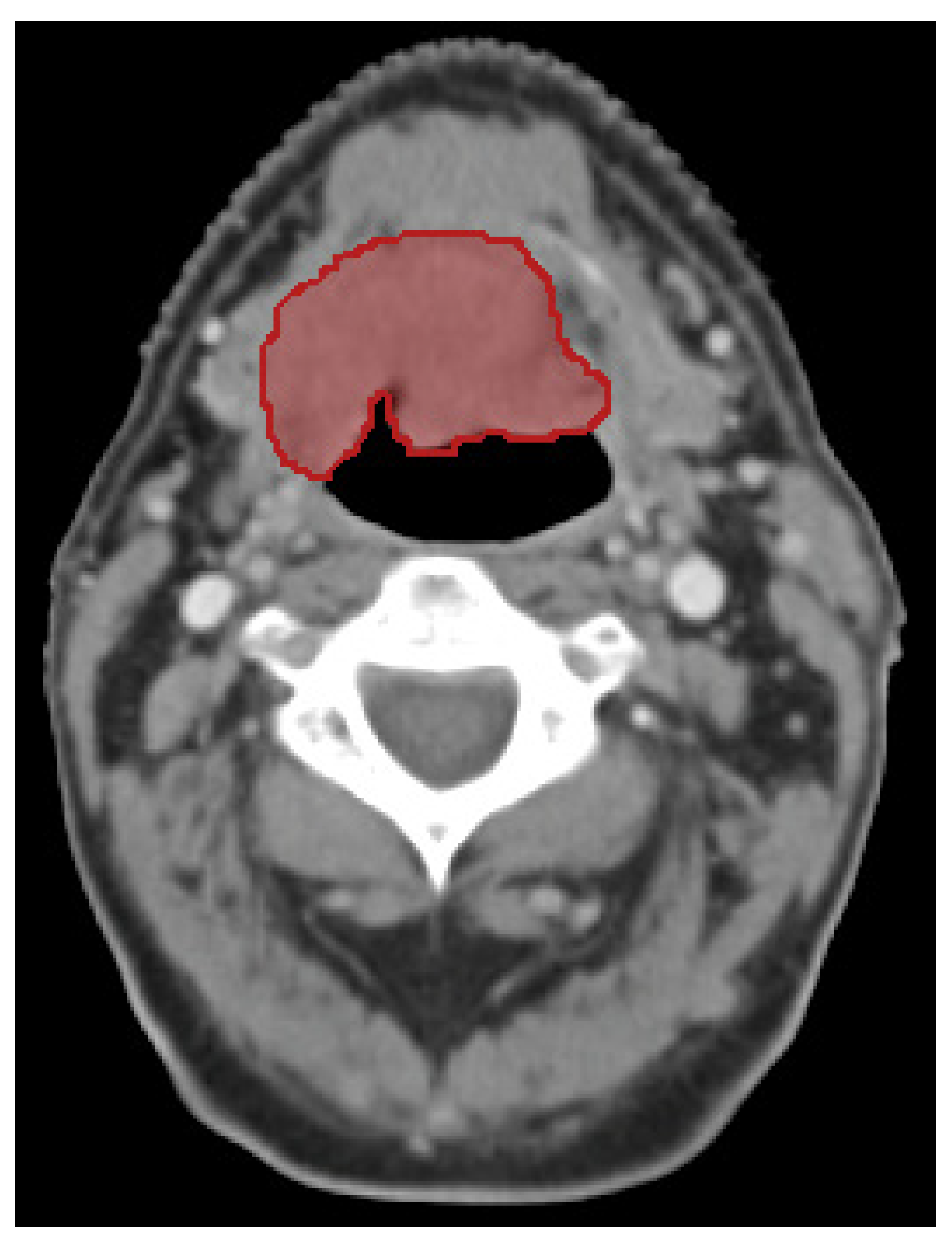
2.3. Feature Extraction
2.4. Feature Selection
2.5. Radiomics Model
2.6. Staging, Volume, and Clinical Models
2.7. Validation of Existing Radiomics Signatures
2.8. Radiomics Quality Score and TRIPOD
3. Results
3.1. Clinical, Biological, and Imaging Characteristics
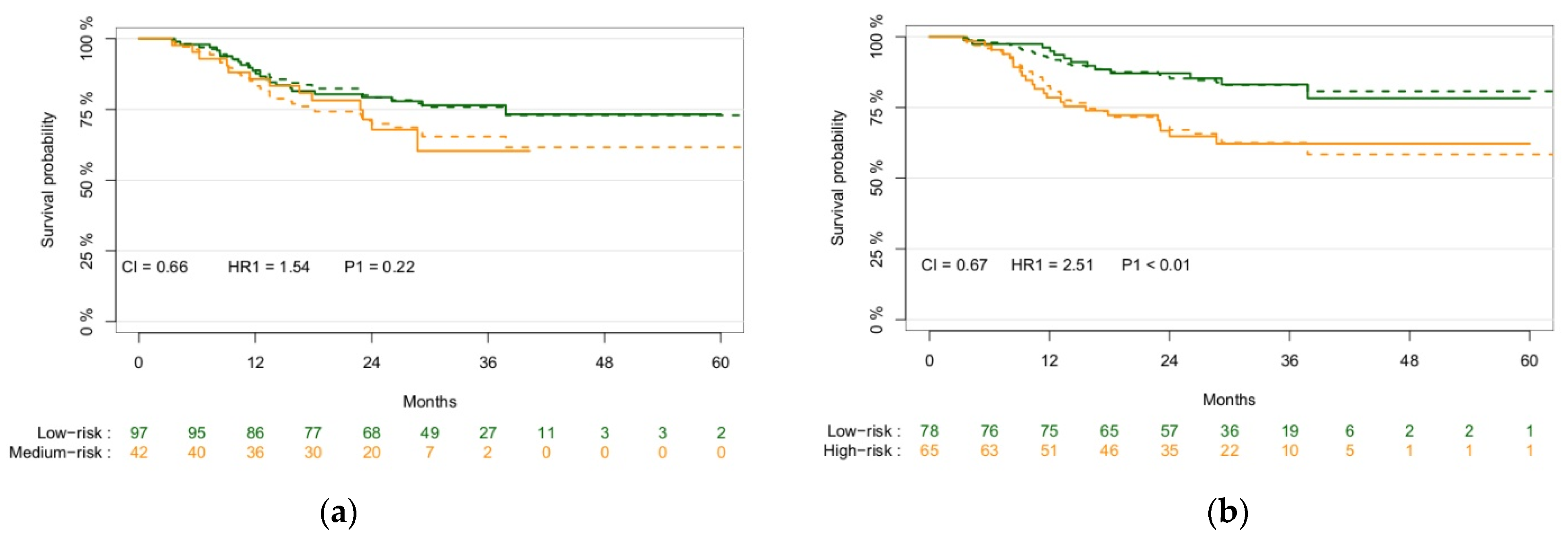
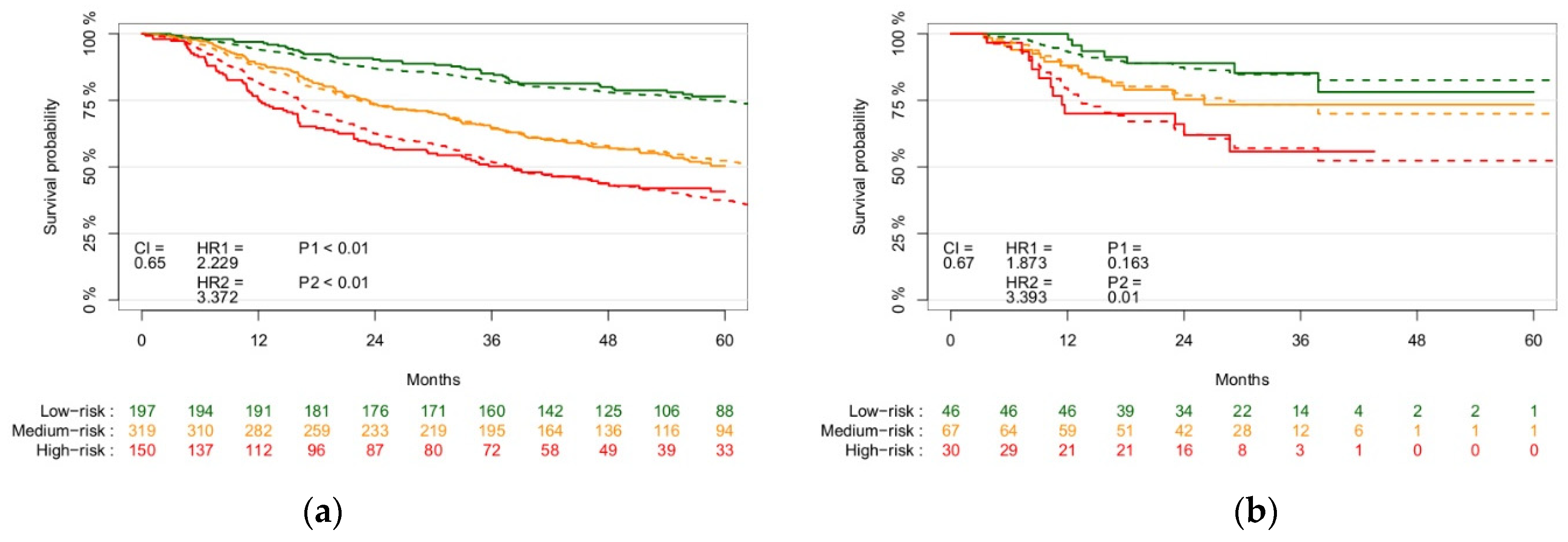
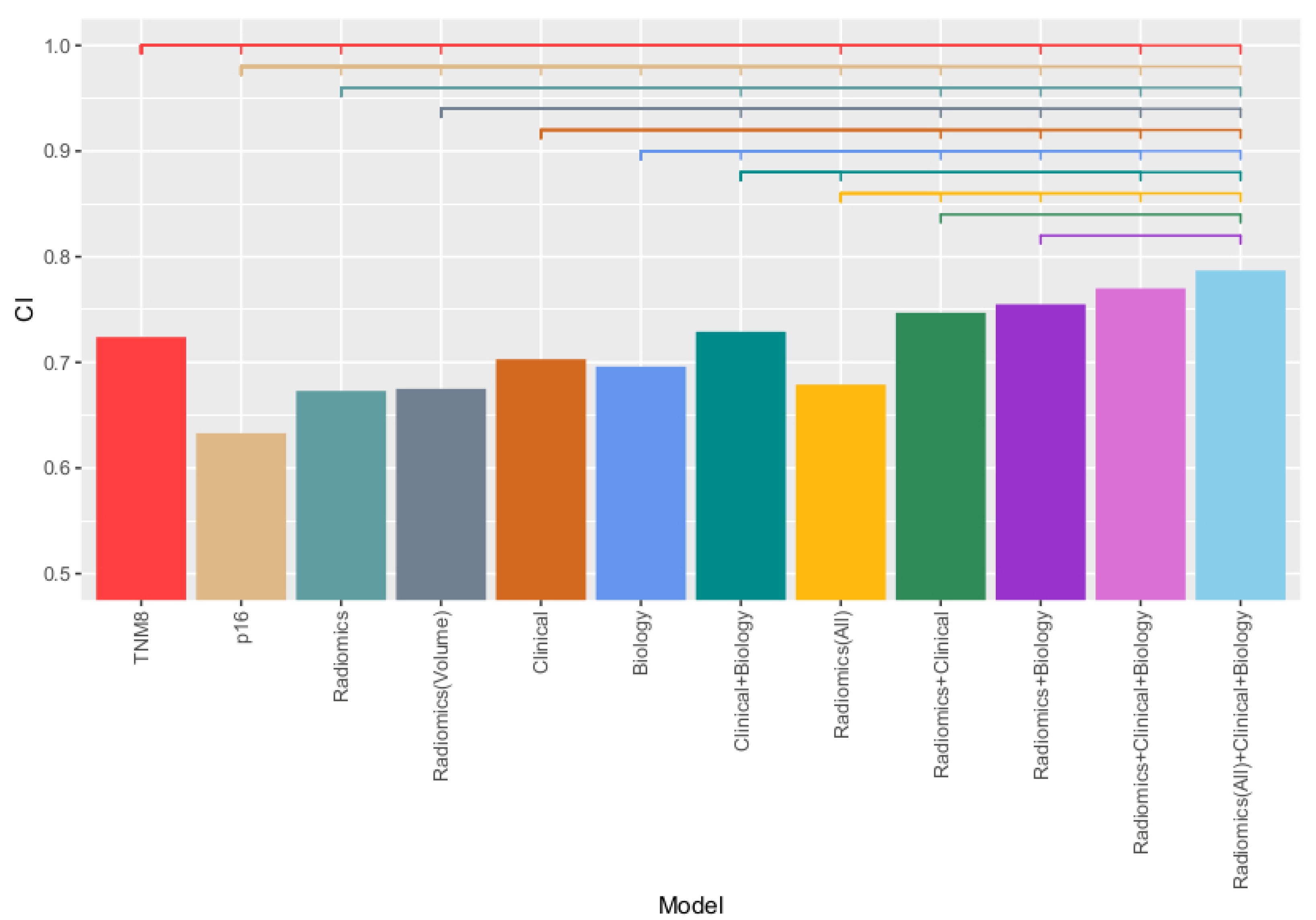
3.2. Model Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Mehra, R.; Ang, K.K.; Burtness, B. Management of human papillomavirus-positive and human papillomavirus-negative head and neck cancer. Semin. Radiat. Oncol. 2012, 22, 194–197. [Google Scholar] [CrossRef]
- Lubin, J.H.; Purdue, M.; Kelsey, K.; Zhang, Z.F.; Winn, D.; Wei, Q.; Talamini, R.; Dabrowska, N.S.; Sturgis, E.M.; Smith, E.; et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: A pooled analysis of case-control studies. Am. J. Epidemiol. 2009, 170, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, W.; O’Sullivan, B.; Patel, S. Major Changes in Head and Neck Staging for 2018. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Qi, Z.; Barrett, T.; Parikh, A.S.; Tirosh, I.; Puram, S.V. Single-cell sequencing and its applications in head and neck cancer. Oral Oncol. 2019, 99, 104441. [Google Scholar] [CrossRef] [PubMed]
- Mroz, E.A.; Rocco, J.W. Intra-tumor heterogeneity in head and neck cancer and its clinical implications. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogowicz, M.; Riesterer, O.; Ikenberg, K.; Stieb, S.; Moch, H.; Studer, G.; Guckenberger, M.; Lang, S.T. Computed Tomography Radiomics Predicts HPV Status and Local Tumor Control After Definitive Radiochemotherapy in Head and Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 921–928. [Google Scholar] [CrossRef]
- Mukherjee, P.; Cintra, M.; Huang, C.; Zhou, M.; Zhu, S.; Colevas, A.D.; Fischbein, N.; Gevaert, O. CT-based Radiomic Signatures for Predicting Histopathologic Features in Head and Neck Squamous Cell Carcinoma. Radiol. Imaging Cancer 2020, 2, e190039. [Google Scholar] [CrossRef]
- Ou, D.; Blanchard, P.; Rosellini, S.; Levy, A.; Nguyen, F.; Leijenaar, R.T.H.; Garberis, I.; Gorphe, P.; Bidault, F.; Ferté, C.; et al. Predictive and prognostic value of CT based radiomics signature in locally advanced head and neck cancers patients treated with concurrent chemoradiotherapy or bioradiotherapy and its added value to Human Papillomavirus status. Oral Oncol. 2017, 71, 150–155. [Google Scholar] [CrossRef]
- Xie, C.; Yang, P.; Zhang, X.; Xu, L.; Wang, X.; Li, X.; Zhang, L.; Xie, R.; Yang, L.; Jing, Z.; et al. Sub-region based radiomics analysis for survival prediction in oesophageal tumours treated by definitive concurrent chemoradiotherapy. EBioMedicine 2019, 44, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Cozzi, L.; Franzese, C.; Fogliata, A.; Franceschini, D.; Navarria, P.; Tomatis, S.; Scorsetti, M. Predicting survival and local control after radiochemotherapy in locally advanced head and neck cancer by means of computed tomography based radiomics. Strahlenther. Onkol. 2019, 195, 805–818. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, Y.; Diao, W.; Cheng, Y.; Jia, Z.; Peng, X. Radiomics-based prediction of survival in patients with head and neck squamous cell carcinoma based on pre- and post-treatment (18)F-PET/CT. Aging 2020, 12, 14593–14619. [Google Scholar] [CrossRef]
- Haider, S.P.; Zeevi, T.; Baumeister, P.; Reichel, C.; Sharaf, K.; Forghani, R.; Kann, B.H.; Judson, B.L.; Prasad, M.L.; Burtness, B.; et al. Potential Added Value of PET/CT Radiomics for Survival Prognostication beyond AJCC 8th Edition Staging in Oropharyngeal Squamous Cell Carcinoma. Cancers 2020, 12, 1778. [Google Scholar] [CrossRef] [PubMed]
- Head, MD Anderson Cancer Center; Neck Quantitative Imaging Working Group. Investigation of radiomic signatures for local recurrence using primary tumor texture analysis in oropharyngeal head and neck cancer patients. Sci. Rep. 2018, 8, 1524. [Google Scholar]
- Li, W.; Wei, D.; Wushouer, A.; Cao, S.; Zhao, T.; Yu, D.; Lei, D. Discovery and Validation of a CT-Based Radiomic Signature for Preoperative Prediction of Early Recurrence in Hypopharyngeal Carcinoma. Biomed. Res. Int. 2020, 2020, 4340521. [Google Scholar]
- Bogowicz, M.; Tanadini-Lang, S.; Guckenberger, M.; Riesterer, O. Combined CT radiomics of primary tumor and metastatic lymph nodes improves prediction of loco-regional control in head and neck cancer. Sci. Rep. 2019, 9, 15198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, T.T.; Langendijk, J.A.; van Dijk, L.V.; Halmos, G.B.; Witjes, M.J.H.; Oosting, S.F.; Noordzij, W.; Sijtsema, N.M.; Steenbakkers, R.J.H.M. The prognostic value of CT-based image-biomarkers for head and neck cancer patients treated with definitive (chemo-)radiation. Oral Oncol. 2019, 95, 178–186. [Google Scholar] [CrossRef]
- Leger, S.; Zwanenburg, A.; Leger, K.; Lohaus, F.; Linge, A.; Schreiber, A.; Kalinauskaite, G.; Tinhofer, I.; Guberina, N.; Guberina, M.; et al. Comprehensive Analysis of Tumour Sub-Volumes for Radiomic Risk Modelling in Locally Advanced HNSCC. Cancers 2020, 12, 3047. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, J.P.; Sinha, S.; Goda, J.S.; Joshi, K.; Mhatre, R.; Kannan, S.; Laskar, S.G.; Gupta, T.; Murthy, V.; Budrukkar, A.; et al. Tumor radiomic features complement clinico-radiological factors in predicting long-term local control and laryngectomy free survival in locally advanced laryngo-pharyngeal cancers. Br. J. Radiol. 2020, 93, 20190857. [Google Scholar] [CrossRef]
- Tang, S.; Ou, J.; Liu, J.; Wu, Y.P.; Wu, C.Q.; Chen, T.W.; Zhang, X.M.; Li, R.; Tang, M.J.; Yang, L.Q.; et al. Application of contrast-enhanced CT radiomics in prediction of early recurrence of locally advanced oesophageal squamous cell carcinoma after trimodal therapy. Cancer Imaging 2021, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ye, J.; Wang, Q.; Luo, J.; Xu, S. CT-Based Radiomics Signature for the Preoperative Discrimination Between Head and Neck Squamous Cell Carcinoma Grades. Front. Oncol. 2019, 9, 821. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.H.; Cao, D.; Ginat, D.T. Radiomic Model Predicts Lymph Node Response to Induction Chemotherapy in Locally Advanced Head and Neck Cancer. Diagnostics 2021, 11, 588. [Google Scholar] [CrossRef]
- Zhai, T.T.; Wesseling, F.; Langendijk, J.A.; Shi, Z.; Kalendralis, P.; van Dijk, L.V.; Hoebers, F.; Steenbakkers, R.J.H.M.; Dekker, A.; Wee, L.; et al. External validation of nodal failure prediction models including radiomics in head and neck cancer. Oral Oncol. 2021, 112, 105083. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Guo, J.; Zhang, L.; Qu, X.; Dai, S.; Peng, R.; Chong, V.F.H.; Xian, J. CT-based radiomics features in the prediction of thyroid cartilage invasion from laryngeal and hypopharyngeal squamous cell carcinoma. Cancer Imaging 2020, 20, 81. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, H.; Huang, S.; Chen, X.; Zhou, H.; Chang, H.; Xia, Y.; Wang, G.; Yang, X. Early prediction of acute xerostomia during radiation therapy for nasopharyngeal cancer based on delta radiomics from CT images. Quant. Imaging Med. Surg. 2019, 9, 1288–1302. [Google Scholar] [CrossRef]
- van Dijk, L.V.; Langendijk, J.A.; Zhai, T.T.; Vedelaar, T.A.; Noordzij, W.; Steenbakkers, R.J.H.M.; Sijtsema, N.M. Delta-radiomics features during radiotherapy improve the prediction of late xerostomia. Sci. Rep. 2019, 9, 12483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, K.; Lee, S.H.; Cheng, Z.; Lakshminarayanan, P.; Peng, L.; Han, P.; McNutt, T.R.; Quon, H.; Lee, J. Predicting acute radiation induced xerostomia in head and neck Cancer using MR and CT Radiomics of parotid and submandibular glands. Radiat. Oncol. 2019, 14, 131. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.; Li, R.; Zeng, R.; Wu, C.Q.; Chen, Y.; Chen, T.W.; Zhang, X.M.; Wu, L.; Jiang, Y.; Yang, J.Q.; et al. CT radiomic features for predicting resectability of oesophageal squamous cell carcinoma as given by feature analysis: A case control study. Cancer Imaging 2019, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Cintra, M.; Brennan, K.; Zhou, M.; Colevas, A.D.; Fischbein, N.; Zhu, S.; Gevaert, O. Development and validation of radiomic signatures of head and neck squamous cell carcinoma molecular features and subtypes. EBioMedicine 2019, 45, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Mohamed, A.S.R.; Lai, S.Y.; Yang, S.; Kanwar, A.; Wei, L.; Kamal, M.; Sengupta, S.; Elhalawani, H.; Skinner, H.; et al. Imaging-Genomic Study of Head and Neck Squamous Cell Carcinoma: Associations Between Radiomic Phenotypes and Genomic Mechanisms via Integration of The Cancer Genome Atlas and The Cancer Imaging Archive. JCO Clin. Cancer Inform. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Cavalieri, S.; De Cecco, L.; Brakenhoff, R.H.; Serafini, M.S.; Canevari, S.; Rossi, S.; Lanfranco, D.; Hoebers, F.J.P.; Wesseling, F.W.R.; Keek, S.; et al. Development of a multiomics database for personalized prognostic forecasting in head and neck cancer: The Big Data to Decide EU Project. Head Neck 2020, 43, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.L.; Hernández, L.; Ottaviano, M.; Martinelli, E.; Poli, T.; Licitra, L.; Arredondo, M.T.; Fico, G. BD2Decide: Big Data and Models for Personalized Head and Neck Cancer Decision Support. In Proceedings of the 2019 IEEE 32nd International Symposium on Computer-Based Medical Systems (CBMS), Cordoba, Spain, 5–7 June 2019. [Google Scholar]
- Ramroth, H.; Schoeps, A.; Rudolph, E.; Dyckhoff, G.; Plinkert, P.; Lippert, B.; Feist, K.; Delank, K.W.; Scheuermann, K.; Baier, G.; et al. Factors predicting survival after diagnosis of laryngeal cancer. Oral Oncol. 2011, 47, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Faye-Lund, H.; Abdelnoor, M. Prognostic factors of survival in a cohort of head and neck cancer patients in Oslo. Eur. J. Cancer B Oral Oncol. 1996, 32B, 83–90. [Google Scholar] [CrossRef]
- Smith, E.M.; Rubenstein, L.M.; Haugen, T.H.; Pawlita, M.; Turek, L.P. Complex etiology underlies risk and survival in head and neck cancer human papillomavirus, tobacco, and alcohol: A case for multifactor disease. J. Oncol. 2012, 2012, 571862. [Google Scholar] [CrossRef]
- Shuster, J.J. Median follow-up in clinical trials. J. Clin. Oncol. 1991, 9, 191–192. [Google Scholar] [CrossRef]
- Breen, S.L.; Publicover, J.; de Silva, S.; Pond, G.; Brock, K.; O’Sullivan, B.; Cummings, B.; Dawson, L.; Keller, A.; Kim, J.; et al. Intraobserver and interobserver variability in GTV delineation on FDG-PET-CT images of head and neck cancers. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 763–770. [Google Scholar] [CrossRef]
- Steenbakkers, R.J.H.M.; Duppen, J.C.; Fitton, I.; Deurloo, K.E.I.; Zijp, L.J.; Comans, E.F.I.; Uitterhoeve, A.L.J.; Rodrigus, P.T.R.; Kramer, G.W.P.; Bussink, J.; et al. Reduction of observer variation using matched CT-PET for lung cancer delineation: A three-dimensional analysis. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 435–448. [Google Scholar] [CrossRef]
- Rasch, C.R.N.; Steenbakkers, R.J.H.M.; Fitton, I.; Duppen, J.C.; Nowak, P.J.C.M.; Pameijer, F.A.; Eisbruch, A.; Kaanders, J.H.A.M.; Paulsen, F.; van Herk, M. Decreased 3D observer variation with matched CT-MRI, for target delineation in Nasopharynx cancer. Radiat. Oncol. 2010, 5, 21. [Google Scholar] [CrossRef]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Tan, R.G.H.B.; Robin, J.C.F.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [Green Version]
- Hatt, M.; Vallieres, M.; Visvikis, D.; Zwanenburg, A. IBSI: An international community radiomics standardization initiative. J. Nucl. Med. 2018, 59, 287. [Google Scholar]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Community, P. Available online: https://pyradiomics.readthedocs.io/en/latest/features.html 2016 (accessed on 13 May 2021).
- Emura, T.; Matsui, S.; Chen, H.Y. compound.Cox: Univariate feature selection and compound covariate for predicting survival. Comput. Methods Programs Biomed. 2019, 168, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. Royal Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Royston, P.; Altman, D.G. External validation of a Cox prognostic model: Principles and methods. BMC Med. Res. Methodol. 2013, 13, 33. [Google Scholar] [CrossRef] [Green Version]
- Welch, M.L.; McIntosh, C.; Kains, B.H.; Milosevic, M.F.; Wee, L.; Dekker, A.; Huang, S.H.; Purdie, T.G.; O’Sullivan, B.; Aerts, H.J.W.L.; et al. Vulnerabilities of radiomic signature development: The need for safeguards. Radiother. Oncol. 2019, 130, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Stekhoven, D.J.; Bühlmann, P. MissForest—Non-parametric missing value imputation for mixed-type data. Bioinformatics 2011, 28, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Kains, B.H.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Sanduleanu, S.; Woodruff, H.C.; de Jong, E.E.C.; van Timmeren, J.E.; Jochems, A.; Dubois, L.; Lambin, P. Tracking tumor biology with radiomics: A systematic review utilizing a radiomics quality score. Radiother. Oncol. 2018, 127, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD). Ann. Intern. Med. 2015, 162, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Vallières, M.; Rivest, E.K.; Perrin, L.J.; Liem, X.; Furstoss, C.; Aerts, H.J.W.L.; Khaouam, N.; Tan, P.F.N.; Wang, C.H.; Sultanem, K.; et al. Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer. Sci. Rep. 2017, 7, 10117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidler, I.J. Critical factors in the biology of human cancer metastasis: Twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990, 50, 6130–6138. [Google Scholar] [PubMed]
- Yokota, J. Tumor progression and metastasis. Carcinogenesis 2000, 21, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Granzier, R.W.Y.; Verbakel, N.M.H.; Ibrahim, A.; van Timmeren, J.E.; van Nijnatten, T.J.A.; Leijenaar, R.T.H.; Lobbes, M.B.I.; Smidt, M.L.; Woodruff, H.C. MRI-based radiomics in breast cancer: Feature robustness with respect to inter-observer segmentation variability. Sci. Rep. 2020, 10, 14163. [Google Scholar] [CrossRef]
- Nikolov, S.; Blackwell, S.; Zverovitch, A.; Mendes, R.; Livne, M.; de Fauw, J.; Patel, Y.; Meyer, C.; Askham, H.; Paredes, B.R.; et al. Deep learning to achieve clinically applicable segmentation of head and neck anatomy for radiotherapy. arXiv 2018, arXiv:1809.04430. [Google Scholar]

| Study | Retrospective (n = 666) | Prospective (n = 143) | p-Value | ||
|---|---|---|---|---|---|
| Sex (% male/n) | 72/482 | 65/93 | p = 0.10 | ||
| Age (Median/range) | 63/ | 64/ | p = 0.17 | ||
| 29–89 | 38–93 | ||||
| HN tumour site (%/n) | -Hypopharynx | 15/96 | 15/21 | p = 0.93 | |
| -Oropharynx | 43/289 | 36/51 | p = 0.11 | ||
| -Oral cavity | 15/100 | 29/42 | p < 0.01 | ||
| -Larynx | 27/181 | 20/29 | p = 0.11 | ||
| p16+ Oropharynx (%/n) | 22/146 | 26/37 | p = 0.36 | ||
| Stage TNM7th edition (%/n) | -III | 31/206 | 28/40 | p = 0.55 | |
| -IVa | 59/390 | 67/96 | p = 0.07 | ||
| -IVb | 10/70 | 5/7 | p = 0.06 | ||
| Stage TNM8th edition(%/n) | p16+ oropharynx | -I | 11/74 | 12/17 | p = 0.90 |
| -II | 6/42 | 9/13 | p = 0.31 | ||
| -III | 5/30 | 5/7 | p = 1 | ||
| Non-oropharynx/p16-oropharynx | -III | 25/169 | 28/40 | p = 0.59 | |
| -IVa | 37/248 | 38/54 | p = 0.98 | ||
| -IVb | 16/103 | 8/12 | p = 0.04 | ||
| Treatment (% of patients received type of treatment/n) | -RT only | 29/191 | 15/22 | p < 0.01 | |
| -Surgery only | 5/34 | 4/5 | p < 0.01 | ||
| -CRT | 37/245 | 36/51 | p = 0.55 | ||
| -Surgery + RT | 15/102 | 24/34 | p = 0.16 | ||
| -Surgery + CH + RT | 14/93 | 12/17 | p = 0.60 | ||
| Order of CH (% of CH patients/n) | -Adjuvant | 15/51 | 12/8 | p = 0.61 | |
| -Concomitant | 81/273 | 84/57 | p = 0.64 | ||
| -Induction | 4/15 | 4/3 | p = 1 | ||
| ACE-27 Comorbidity (%/n) | =0 | 30/204 | 38/52 | p = 0.20 | |
| =1 | 41/272 | 38/52 | p = 0.37 | ||
| =2 | 20/133 | 16/21 | p = 0.18 | ||
| =3 | 9/57 | 8/11 | p = 0.86 | ||
| Smoking (%/n) | -Current | 52/350 | 40/55 | p = 0.01 | |
| -Former | 36/237 | 33/45 | p = 0.44 | ||
| -Never | 12/79 | 27/37 | p < 0.01 | ||
| Pack years (Median/range) | 35/0–174 | 30/0–220 | p = 1 | ||
| Alcohol consumption (%/n) | -Current | 66/445 | 48/67 | p < 0.01 | |
| -Former | 13/84 | 12/17 | p = 1 | ||
| -Never | 21/137 | 40/55 | p < 0.01 | ||
| ECOG PS (%/n) | =0 | 39/262 | 49/68 | p < 0.01 | |
| =1 | 16/106 | 43/59 | p < 0.01 | ||
| =2 | 3/21 | 8/11 | p = 0.22 | ||
| =3 | 1/4 | - | p = - | ||
| =NA | 41/273 | 4/5 | p < 0.01 | ||
| Hb level (Median/range) | 8.8/5.0–15.1 | 8.7/4.8-14.0 | p = 0.27 | ||
| Feature Name | Model Coefficient | Hazard Ratio | p-Value |
|---|---|---|---|
| TNM8 | 0.76 | 2.14 | <0.01 |
| Age | 0.034 | 1.035 | <0.01 |
| ACE-27 comorbidity score | 0.28 | 1.33 | <0.01 |
| Pack years | 0.005 | 1.005 | 0.02 |
| Alcohol at diagnosis | 0.47 | 1.61 | <0.01 |
| P16-status | −1.3 | 0.27 | <0.01 |
| Haemoglobin level | −0.3 | 0.74 | <0.01 |
| Full Patient Cohort | Oropharynx Patient Cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Training | Validation | Training | Validation | |||||||||
| Model | CI (95% CI) | HR 1 vs. 2 (95% CI) | HR 1 vs. 3 (95% CI) | CI (95% CI) | HR 1 vs. 2 (95% CI) | HR 1 vs. 3 (95% CI) | CI (95% CI) | HR 1 vs. 2 (95% CI) | HR 1 vs. 3 (95% CI) | CI (95% CI) | HR 1 vs. 2 (95% CI) | HR 1 vs. 3 (95% CI) |
| Staging TNM8 | 0.65 (0.64–0.65) | 1.82 (1.40–2.35) | 3.12 (2.32–4.21) | 0.74 (0.73–0.75) | 5.01 (2.11–11.85) | 14.03 (5.16–38.17) | 0.71 (0.69–0.72) | 2.50 (1.62–3.87) | 5.16 (3.24–8.23) | 0.86 (0.81–0.87) | 9.12 (1.28–64.90) | 30.15 (4.97–182.90) |
| Radiomics | 0.65 (0.64–0.65) | 2.22 (1.64–3.03) | 3.37 (2.41–4.72) | 0.67 (0.66–0.69) | 1.87 (0.78–4.52) | 3.39 (1.33–8.64) | 0.68 (0.67–0.69) | 2.36 (1.45–3.86) | 3.80 (2.21–6.52) | 0.82 (0.78–0.85) | -* | -* |
| Radiomics + Staging | 0.68 (0.68–0.69) | 2.49 (1.77–3.44) | 4.60 (3.24–6.53) | 0.77 (0.75–0.78) | 8.54 (1.97–37.98) | 29.35 (6.73–127.94) | 0.73 (0.73–0.74) | 3.97 (2.20–7.18) | 7.87 (4.39.27) | 0.90 (0.88–0.92) | -* | -* |
| Radiomics (Volume) | 0.62 (0.62–0.62) | 1.48 (1.08–2.03) | 3.17 (2.16–4.66) | 0.68 (0.66–0.69) | 1.23 (0.54–2.78) | 7.98 (2.85–22.31) | 0.64 (0.63–0.64) | 1.81 (1.10–2.99) | 3.29 (1.82–5.92) | 0.87 (0.84–0.90) | -* | -* |
| Clinical | 0.66 (0.66–0.67) | 2.37 (1.76–3.19) | 3.25 (2.40–4.40) | 0.70 (0.69–0.72) | 3.66 (1.40–9.54) | 5.37 (2.10–13.72) | 0.73 (0.72–0.74) | 3.80 (2.18–6.63) | 8.27 (4.82–14.18) | 0.84 (0.81–0.87) | ||
| Biological | 0.63 (0.63–0.63) | 2.83 (1.95–4.09) | 3.94 (2.71–5.74) | 0.70 (0.68–0.71) | 13.03 (1.74–97.73) | 23.19 (3.08–174.46) | 0.68 (0.68–0.69) | 4.28 (2.79–6.56) | 6.74 (0.91–49.82) | 0.84 (0.80–0.89) | * | * |
| Clinical + Biological | 0.67 (0.66–0.67) | 2.71 (1.95–3.75) | 4.17 (3.00–5.78) | 0.73 (0.72–0.74) | 8.21 (2.37–28.39) | 10.10(2.97–34.36) | 0.74 (0.74–0.75) | 3.82 (2.16–6.76) | 8.66 (5.08–14.76) | 0.88 (0.85–0.90) | -* | -* |
| Radiomics (includes volume) | 0.65 (0.65–0.66) | 1.78 (1.32–2.42) | 3.64 (2.61–5.08) | 0.68 (0.67–0.69) | 2.19 (0.92–5.26) | 3.84 (1.48–9.95) | 0.68 (0.67–0.69) | 2.47 (1.50–4.06) | 3.94 (2.28–6.82) | 0.82 (0.78–0.86) | -* | -* |
| Radiomics + Clinical | 0.69 (0.69–0.70) | 2.94 (2.15–4.03) | 4.79 (3.45–6.67) | 0.74 (0.74–0.76) | 4.65 (1.86–17.16) | 11.38 (3.84–33.74) | 0.73 (0.72–0.74) | 3.80 (2.18–6.64) | 8.27 (4.82–14.18) | 0.84 (0.81–0.87) | * | * |
| Radiomics + Biological | 0.68 (0.68–0.68) | 2.89 (2.04–4.08) | 5.03 (3.52–7.17) | 0.76 (0.74–0.77) | 6.49 (1.91–22.06) | 13.74 (3.96–47.66) | 0.74 (0.74–0.75) | 3.61 (2.13–6.12) | 6.85 (4.12–11.39) | 0.91 (0.90–0.93) | * | * |
| Radiomics + Clinical + Biological | 0.70 (0.70–0.70) | 3.04 (2.17–4.27) | 5.82 (4.10–8.28) | 0.77 (0.77–0.78) | 8.17 (2.36–28.24) | 13.17 (3.86–44.85) | 0.77 (0.77–0.78) | 4.77 (2.65–8.60) | 12.53 (7.03–22.31) | 0.88 (0.85–0.90) | -* | -* |
| Radiomics (includes volume) + Clinical + Biological | 0.71 (0.71–0.71) | 2.91 (2.11–4.01) | 6.21 (4.44–8.68) | 0.79 (0.78–0.80) | 5.21 (1.70–15.98) | 15.26 (5.14–45.32) | 0.77 (0.76–0.77) | 6.11 (3.23–11.53) | 15.40 (8.17–29.03) | 0.87 (0.84–0.89) | -* | -* |
| p16-status | - | - | - | - | - | - | 0.67 (0.67–0.68) | 4.3 (2.81–6.59) | - | 0.82 (0.78–0.85) | 19.8 (2.38–165) | - |
| Aerts. 2014 [50] | 0.61 (0.61–0.61) | 1.65 (1.30–2.09) | - | 0.66 | 1.54 (0.77–3.06) | - | 0.65 (0.64–0.66) | 1.90 (1.3–2.77) | - | 0.68 (0.63–0.73) | -* | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keek, S.A.; Wesseling, F.W.R.; Woodruff, H.C.; van Timmeren, J.E.; Nauta, I.H.; Hoffmann, T.K.; Cavalieri, S.; Calareso, G.; Primakov, S.; Leijenaar, R.T.H.; et al. A Prospectively Validated Prognostic Model for Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck Based on Radiomics of Computed Tomography Images. Cancers 2021, 13, 3271. https://doi.org/10.3390/cancers13133271
Keek SA, Wesseling FWR, Woodruff HC, van Timmeren JE, Nauta IH, Hoffmann TK, Cavalieri S, Calareso G, Primakov S, Leijenaar RTH, et al. A Prospectively Validated Prognostic Model for Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck Based on Radiomics of Computed Tomography Images. Cancers. 2021; 13(13):3271. https://doi.org/10.3390/cancers13133271
Chicago/Turabian StyleKeek, Simon A., Frederik W. R. Wesseling, Henry C. Woodruff, Janita E. van Timmeren, Irene H. Nauta, Thomas K. Hoffmann, Stefano Cavalieri, Giuseppina Calareso, Sergey Primakov, Ralph T. H. Leijenaar, and et al. 2021. "A Prospectively Validated Prognostic Model for Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck Based on Radiomics of Computed Tomography Images" Cancers 13, no. 13: 3271. https://doi.org/10.3390/cancers13133271
APA StyleKeek, S. A., Wesseling, F. W. R., Woodruff, H. C., van Timmeren, J. E., Nauta, I. H., Hoffmann, T. K., Cavalieri, S., Calareso, G., Primakov, S., Leijenaar, R. T. H., Licitra, L., Ravanelli, M., Scheckenbach, K., Poli, T., Lanfranco, D., Vergeer, M. R., Leemans, C. R., Brakenhoff, R. H., Hoebers, F. J. P., & Lambin, P. (2021). A Prospectively Validated Prognostic Model for Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck Based on Radiomics of Computed Tomography Images. Cancers, 13(13), 3271. https://doi.org/10.3390/cancers13133271









