Impact of Race and Socioeconomics Disparities on Survival in Young-Onset Colorectal Adenocarcinoma—A SEER Registry Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Source
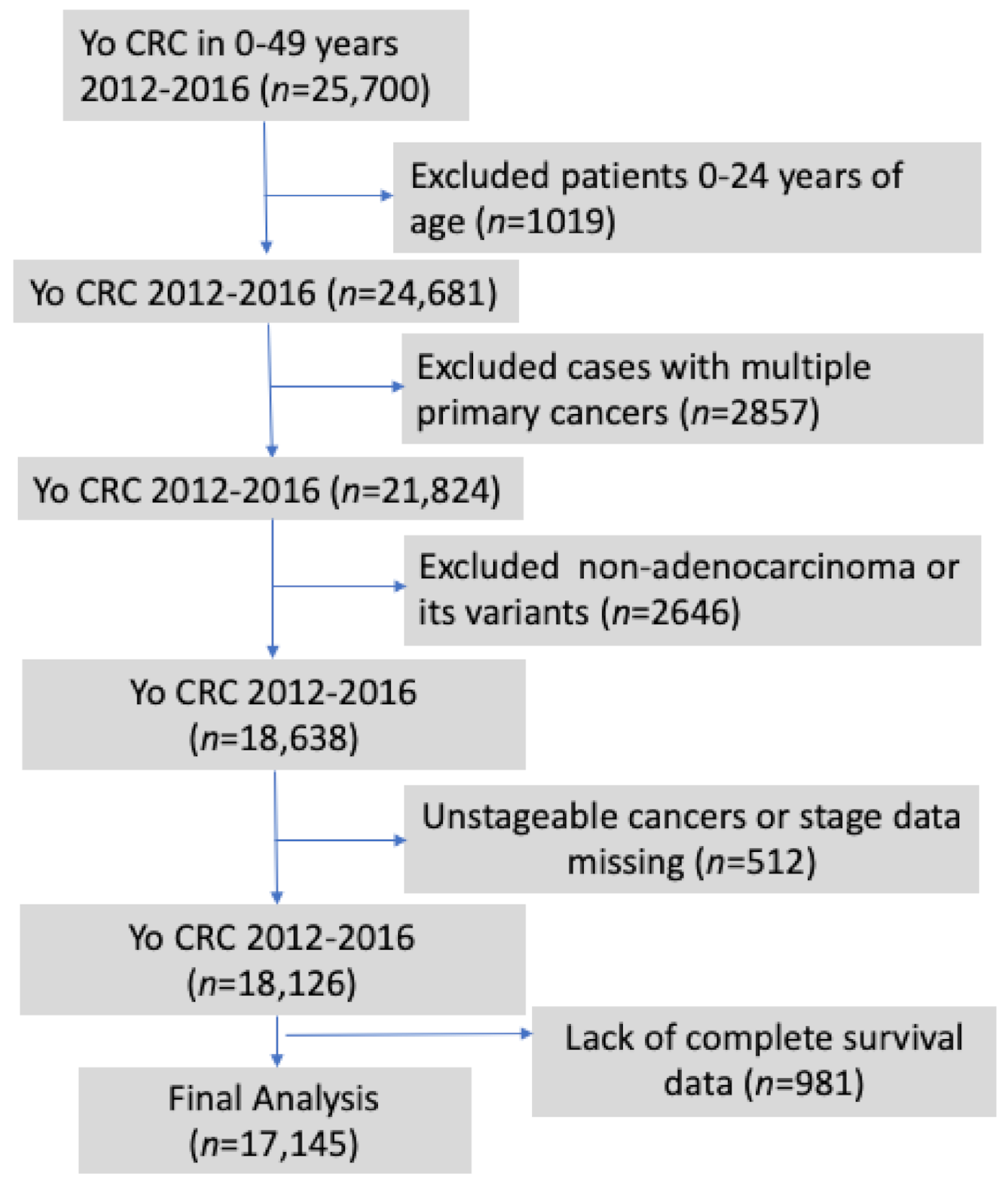
2.3. Patient Selection
2.4. Socioeconomic Determinants of Health (SEDH)
2.5. Statistical Analysis
3. Results
3.1. Completion of High School Education (above vs. below 50th Centile)
3.2. Poverty (above vs. below 50th Centile)
3.3. Employment Status (above vs. below 50th Centile)
3.4. Household Income with Reference to the Median (above vs. below 50th Centile
3.5. Marital (Married) Status (above vs. below 50th Centile)
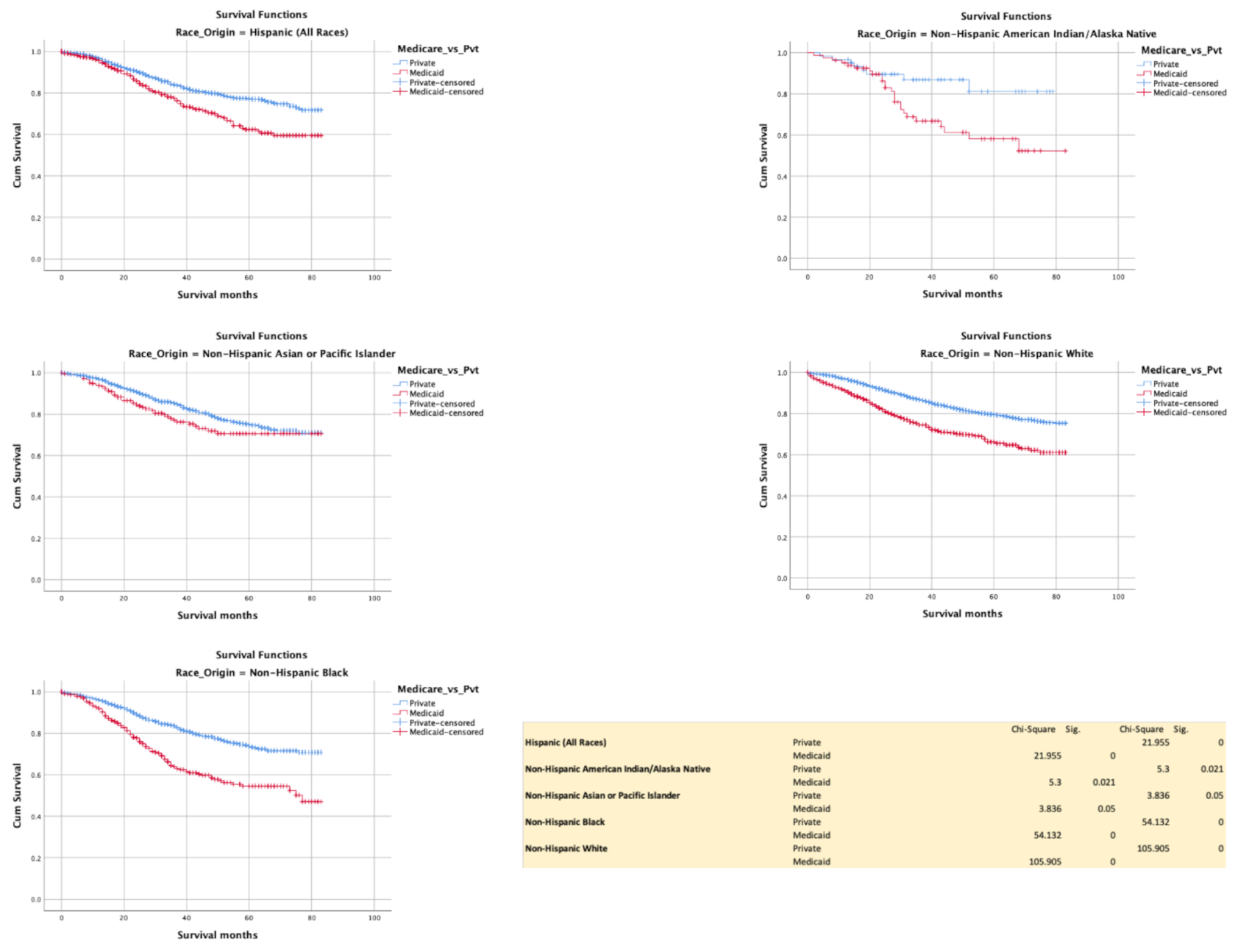
3.6. Insurance (Commercial vs. Medicaid)
3.7. Recent Immigration to the U.S. (above vs. below 50th Centile)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Colorectal Cancer Facts & Figures 2020–2022. Atlanta; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- Surveillance, Epidemiology, and End Results (SEER) Program Cancer Statistics Review 1975–2017. Available online: www.seer.cancer.gov (accessed on 3 March 2021).
- Murphy, C.C.; Sandler, R.S.; Sanoff, H.K.; Yang, Y.C.; Lund, J.L.; Baron, J.A. Decrease in incidence of colorectal cancer among individuals 50 years or older after recommendations for population-based screening. Clin. Gastroenterol. Hepatol. 2017, 15, 903–909.e906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, H.; Desai, R.; Aloysius, M.M.; Jecmenica, M.; Enders, G.H.; Bansal, P. Young-onset colorectal cancer: Thospitalization trends and gender disparities in the United States 2010–2014. Int. J. Colorectal. Dis. 2019, 34, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Jemal, A.; Ward, E.M. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol. Prev. Biomark. 2009, 18, 1695–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, C.E.; Hu, C.Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [Green Version]
- Ellis, L.; Canchola, A.J.; Spiegel, D.; Ladabaum, U.; Haile, R.; Gomez, S.L. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J. Clin. Oncol. 2018, 36, 25–33. [Google Scholar] [CrossRef]
- Holowatyj, A.N.; Ruterbusch, J.J.; Rozek, L.S.; Cote, M.L.; Stoffel, E.M. Racial/Ethnic Disparities in Survival Among Patients With Young-Onset Colorectal Cancer. J. Clin. Oncol. 2016, 34, 2148–2156. [Google Scholar] [CrossRef]
- Warren Andersen, S.; Blot, W.J.; Lipworth, L.; Steinwandel, M.; Murff, H.J.; Zheng, W. Association of Race and Socioeconomic Status With Colorectal Cancer Screening, Colorectal Cancer Risk, and Mortality in Southern US Adults. JAMA Netw. Open. 2019, 2, e1917995. [Google Scholar] [CrossRef]
- Saldana-Ruiz, N.; Clouston, S.A.; Rubin, M.S.; Colen, C.G.; Link, B.G. Fundamental causes of colorectal cancer mortality in the United States: Understanding the importance of socioeconomic status in creating inequality in mortality. Am. J. Public Health 2013, 103, 99–104. [Google Scholar] [CrossRef]
- Provenzale, D.; Ness, R.M.; Llor, X.; Weiss, J.M.; Abbadessa, B.; Cooper, G.; Early, D.S.; Friedman, M.; Giardiello, F.M.; Glaser, K.; et al. NCCN Guidelines Insights: Colorectal Cancer Screening, Version 2.2020. J. Natl. Compr. Canc. Netw. 2020, 18, 1312–1320. [Google Scholar] [CrossRef]
- Mannucci, A.; Zuppardo, R.A.; Rosati, R.; Leo, M.D.; Perea, J.; Cavestro, G.M. Colorectal cancer screening from 45 years of age: Thesis, antithesis and synthesis. World J. Gastroenterol. 2019, 25, 2565–2580. [Google Scholar] [CrossRef]
- Marcella, S.; Miller, J.E. Racial differences in colorectal cancer mortality. The importance of stage and socioeconomic status. J. Clin. Epidemiol. 2001, 54, 359–366. [Google Scholar] [CrossRef]
- Le, H.; Ziogas, A.; Lipkin, S.M.; Zell, J.A. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1950–1962. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Hargreaves, M.; Liu, J.; Kenerson, D.; Neal, R.; Takizala, Z.; Beard, K.; Pinkerton, H.; Burress, M.; Blot, B. Factors influencing colorectal cancer screening in low-income African Americans in Tennessee. J. Community Health 2012, 37, 673–679. [Google Scholar] [CrossRef]
- Patel, A.; Gantz, O.; Zagadailov, P.; Merchant, A.M. The role of socioeconomic disparity in colorectal cancer stage at presentation. Updates Surg. 2019, 71, 523–531. [Google Scholar] [CrossRef]
- Hayat, M.J.; Howlader, N.; Reichman, M.E.; Edwards, B.K. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 2007, 12, 20–37. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Available online: http://www.seer.cancer.gov (accessed on 3 March 2021).
- American Community Survey (ACS). Available online: https://www.census.gov/programs-surveys/acs. (accessed on 13 February 2021).
- Soneji, S.; Iyer, S.S.; Armstrong, K.; Asch, D.A. Racial disparities in stage-specific colorectal cancer mortality: 1960–2005. Am. J. Public Health 2010, 100, 1912–1916. [Google Scholar] [CrossRef]
- Wu, J.; Ye, J.; Wu, W.; Fang, D.; Wang, K.; Yang, L.; Jiang, X.; Wang, Q.; Li, L. Racial disparities in young-onset patients with colorectal, breast and testicular cancer. J. Cancer 2019, 10, 5388–5396. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Sakhuja, S.; Waterbor, J.; Pisu, M.; Altekruse, S.F. Racial/ethnic disparities in de novo metastases sites and survival outcomes for patients with primary breast, colorectal, and prostate cancer. Cancer Med. 2018, 7, 1183–1193. [Google Scholar] [CrossRef]
- Murphy, C.C.; Lund, J.L.; Sandler, R.S. Young-Onset Colorectal Cancer: Earlier Diagnoses or Increasing Disease Burden? Gastroenterology 2017, 152, 1809–1812.e1803. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [Green Version]
- Fedewa, S.A.; Flanders, W.D.; Ward, K.C.; Lin, C.C.; Jemal, A.; Goding Sauer, A.; Doubeni, C.A.; Goodman, M. Racial and Ethnic Disparities in Interval Colorectal Cancer Incidence: A Population-Based Cohort Study. Ann. Intern. Med. 2017, 166, 857–866. [Google Scholar] [CrossRef]
- Laiyemo, A.O.; Doubeni, C.; Pinsky, P.F.; Doria-Rose, V.P.; Bresalier, R.; Lamerato, L.E.; Crawford, E.D.; Kvale, P.; Fouad, M.; Hickey, T.; et al. Race and colorectal cancer disparities: Health-care utilization vs. different cancer susceptibilities. J. Natl. Cancer Inst. 2010, 102, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Semega, J.; Kollar, M.; Shrider, E.; Creamer, J. Income and Poverty in the United States. U.S. Census Bur. Current Popul. Rep. 2020, 60–270. [Google Scholar]
- Doubeni, C.A.; Major, J.M.; Laiyemo, A.O.; Schootman, M.; Zauber, A.G.; Hollenbeck, A.R.; Sinha, R.; Allison, J. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J. Natl. Cancer Inst. 2012, 104, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Perisetti, A.; Khan, H.; George, N.E.; Yendala, R.; Rafiq, A.; Blakely, S.; Rasmussen, D.; Villalpando, N.; Goyal, H. Colorectal cancer screening use among insured adults: Is out-of-pocket cost a barrier to routine screening? World J. Gastrointest Pharmacol. Ther. 2018, 9, 31–38. [Google Scholar] [CrossRef]
- Scally, B.J.; Krieger, N.; Chen, J.T. Racialized economic segregation and stage at diagnosis of colorectal cancer in the United States. Cancer Causes Control 2018, 29, 527–537. [Google Scholar] [CrossRef]
- Wang, L.; Wilson, S.E.; Stewart, D.B.; Hollenbeak, C.S. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: Does marriage affect cancer survival by gender and stage? Cancer Epidemiol. 2011, 35, 417–422. [Google Scholar] [CrossRef] [PubMed]
- El-Shami, K.; Oeffinger, K.C.; Erb, N.L.; Willis, A.; Bretsch, J.K.; Pratt-Chapman, M.L.; Cannady, R.S.; Wong, S.L.; Rose, J.; Barbour, A.L.; et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J. Clin. 2015, 65, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Cancer Facts & Figures 2020. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html (accessed on 14 February 2021).
- Myerson, R.M.; Tucker-Seeley, R.D.; Goldman, D.P.; Lakdawalla, D.N. Does Medicare Coverage Improve Cancer Detection and Mortality Outcomes? J. Policy Anal. Manage. 2020, 39, 577–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sineshaw, H.M.; Ng, K.; Flanders, W.D.; Brawley, O.W.; Jemal, A. Factors That Contribute to Differences in Survival of Black vs. White Patients With Colorectal Cancer. Gastroenterology 2018, 154, 906–915.e907. [Google Scholar] [CrossRef] [Green Version]
- Phimha, S.; Promthet, S.; Suwanrungruang, K.; Chindaprasirt, J.; Bouphan, P.; Santong, C.; Vatanasapt, P. Health Insurance and Colorectal Cancer Survival in Khon Kaen, Thailand. Asian Pac. J. Cancer Prev. 2019, 20, 1797–1802. [Google Scholar] [CrossRef] [Green Version]
- Wallace, K.; Sterba, K.R.; Gore, E.; Lewin, D.N.; Ford, M.E.; Thomas, M.B.; Alberg, A.J. Prognostic factors in relation to racial disparity in advanced colorectal cancer survival. Clin. Colorectal. Cancer 2013, 12, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Kupfer, S.S.; Carr, R.M.; Carethers, J.M. Reducing colorectal cancer risk among African Americans. Gastroenterology 2015, 149, 1302–1304. [Google Scholar] [CrossRef] [Green Version]
- Rogers, C.R.; Robinson, C.D.; Arroyo, C.; Obidike, O.J.; Sewali, B.; Okuyemi, K.S. Colorectal Cancer Screening Uptake’s Association With Psychosocial and Sociodemographic Factors Among Homeless Blacks and Whites. Health Educ. Behav. 2017, 44, 928–936. [Google Scholar] [CrossRef] [Green Version]
- Jean-Pierre, P.; Cheng, Y.; Wells, K.J.; Freund, K.M.; Snyder, F.R.; Fiscella, K.; Holden, A.E.; Paskett, E.D.; Dudley, D.J.; Simon, M.A.; et al. Satisfaction with cancer care among underserved racial-ethnic minorities and lower-income patients receiving patient navigation. Cancer 2016, 122, 1060–1067. [Google Scholar] [CrossRef] [Green Version]
- Jean-Pierre, P.; Hendren, S.; Fiscella, K.; Loader, S.; Rousseau, S.; Schwartzbauer, B.; Sanders, M.; Carroll, J.; Epstein, R. Understanding the processes of patient navigation to reduce disparities in cancer care: Perspectives of trained navigators from the field. J. Cancer Educ. 2011, 26, 111–120. [Google Scholar] [CrossRef] [Green Version]







| Hispanic | Non-Hispanic American Indian | Non-Hispanic Asian Pacific Islander | Non-Hispanic Black | Non-Hispanic White | Total | ||
|---|---|---|---|---|---|---|---|
| n = 2874 | n = 164 | n = 1676 | n = 2305 | n =10,126 | n = 17,145 | ||
| Age Range | 25–29 years | 127 | 7 | 52 | 61 | 292 | 539 |
| 30–34 years | 288 | 1 4 | 113 | 141 | 717 | 1273 | |
| 35–39 years | 487 | 28 | 217 | 294 | 1287 | 2313 | |
| 40–44 years | 755 | 40 | 464 | 595 | 2702 | 4556 | |
| 45–49 years | 1217 | 75 | 830 | 1214 | 5128 | 8464 | |
| Sex | Female | 1371 | 77 | 846 | 1201 | 4754 | 8249 |
| Male | 1503 | 87 | 830 | 1104 | 5372 | 8896 | |
| Primary Site | Appendix | 95 | 8 | 37 | 57 | 304 | 501 |
| Ascending Colon | 263 | 15 | 121 | 345 | 803 | 1547 | |
| Cecum | 252 | 9 | 115 | 348 | 922 | 1646 | |
| Colon NOS | 23 | 1 | 7 | 9 | 55 | 95 | |
| Descending | 182 | 8 | 126 | 179 | 526 | 1021 | |
| Hepatic Flexure | 73 | 5 | 52 | 64 | 209 | 403 | |
| Overlapping | 30 | 1 | 11 | 24 | 67 | 133 | |
| Rectosigmoid | 274 | 19 | 190 | 159 | 1118 | 1760 | |
| Rectum | 668 | 42 | 457 | 353 | 2807 | 4327 | |
| Sigmoid | 751 | 45 | 437 | 534 | 2569 | 4336 | |
| Splenic Flexure | 69 | 2 | 42 | 65 | 226 | 404 | |
| Transverse Colon | 194 | 9 | 81 | 168 | 520 | 972 | |
| Histology | Adenocarcinoma, NOS | 1923 | 119 | 1224 | 1554 | 6839 | 11,659 |
| Adenocarcinoma In adenomatous polyp | 198 | 11 | 105 | 186 | 994 | 1494 | |
| Tubular Adenocarcinoma | 1 | 0 | 0 | 1 | 4 | 6 | |
| Serrated Adenocarcinoma | 0 | 0 | 0 | 0 | 1 | 1 | |
| Adenocarcinoma in adenomatous polyposis coli | 11 | 0 | 3 | 4 | 17 | 35 | |
| Adenocarcinoma in multiple Adenomatous polyps | 3 | 0 | 2 | 2 | 2 | 9 | |
| Adenocarcinoma in villous adenoma | 79 | 1 | 25 | 54 | 213 | 372 | |
| Villous Adenocarcinoma | 1 | 0 | 1 | 2 | 2 | 6 | |
| Adenocarcinoma in tubulovillous adenoma | 267 | 17 | 154 | 248 | 1039 | 1725 | |
| Clear cell Adenocarcinoma, NOS | 1 | 0 | 0 | 0 | 2 | 3 | |
| Cystadenocarcinoma, NOS | 1 | 0 | 0 | 0 | 1 | 2 | |
| Mucinous cystadenocarcinoma, NOS | 2 | 0 | 1 | 2 | 8 | 13 | |
| Mucinous adenocarcinoma | 308 | 11 | 117 | 190 | 788 | 1414 | |
| Mucin- producing adenocarcinoma | 27 | 1 | 6 | 22 | 52 | 108 | |
| Signet ring cell carcinoma | 51 | 4 | 37 | 40 | 161 | 293 | |
| Grade | Well differentiated; Grade I | 279 | 14 | 111 | 157 | 698 | 1259 |
| Moderately differentiated; Grade II | 1901 | 110 | 1126 | 1592 | 6884 | 11,613 | |
| Poorly differentiated; Grade Ill | 420 | 23 | 287 | 316 | 1452 | 2498 | |
| Undifferentiated; anaplastic; Grade IV | 79 | 4 | 38 | 73 | 338 | 532 | |
| Mucinous | Non-Mucinous | 2566 | 153 | 1559 | 2115 | 9338 | 15,731 |
| Mucinous | 308 | 11 | 117 | 190 | 788 | 1414 | |
| AJCC-7 TNM | 0 | 96 | 2 | 48 | 77 | 293 | 516 |
| I | 469 | 30 | 276 | 385 | 1917 | 3077 | |
| IIA | 589 | 42 | 341 | 429 | 1922 | 3323 | |
| IIB | 70 | 1 | 28 | 49 | 212 | 360 | |
| IIC | 84 | 4 | 27 | 50 | 192 | 357 | |
| IIIA | 109 | 7 | 56 | 112 | 452 | 736 | |
| IIIB | 684 | 27 | 426 | 524 | 2409 | 4070 | |
| IIIC | 281 | 15 | 161 | 202 | 890 | 1549 | |
| IVA | 284 | 20 | 161 | 259 | 1058 | 1782 | |
| IVB | 184 | 14 | 1 43 | 204 | 702 | 1247 | |
| IVC | 24 | 2 | 9 | 14 | 79 | 128 | |
| Procedure | Colectomy or proctocolectomy resection in continuity | 61 | 0 | 27 | 27 | 150 | 265 |
| Colectomy, NOS; Proctectomy, NOS | 11 | 1 | 7 | 11 | 22 | 52 | |
| Cryosurgery | 0 | 0 | 0 | 0 | 1 | 1 | |
| Electrocautery fulguration (hot forceps) | 2 | 0 | 0 | 0 | 2 | 4 | |
| Endoscopic polypectomy | 42 | 1 | 32 | 36 | 144 | 255 | |
| Excisional biopsy | 16 | 2 | 9 | 14 | 104 | 145 | |
| Hemicolectomy or subtotal colectomy | 844 | 47 | 429 | 917 | 2769 | 5006 | |
| Hemicolectomy or subtotal colectomy (and resection) | 81 | 8 | 40 | 100 | 233 | 462 | |
| Local tumor destruction, NOS | 1 | 0 | 1 | 1 | 0 | 3 | |
| Local tumor excision, electrocautery | 11 | 2 | 4 | 5 | 35 | 57 | |
| Local tumor excision, laser ablation | 1 | 0 | 0 | 0 | 1 | 2 | |
| Local tumor excision, laser excision | 1 | 0 | 0 | 0 | 0 | 1 | |
| Local tumor excision, NOS | 13 | 1 | 4 | 8 | 32 | 58 | |
| Partial colectomy (segmental resection) | 1369 | 78 | 890 | 869 | 5145 | 8351 | |
| Polypectomy, NOS | 56 | 2 | 48 | 63 | 246 | 415 | |
| Surgical polypectomy | 6 | 1 | 1 | 7 | 16 | 31 | |
| Total colectomy | 209 | 12 | 107 | 122 | 679 | 1129 | |
| Total colectomy (& resection of a contiguous organ) | 11 | 1 | 6 | 8 | 52 | 78 | |
| Total colectomy with ileorectal reconstruction | 0 | 0 | 0 | 1 | 2 | 3 | |
| Total colectomy with ileostomy, NOS | 1 | 0 | 1 | 0 | 4 | 6 | |
| Total colectomy with Pouch | 1 | 0 | 0 | 0 | 1 | 2 | |
| Total proctocolectomy (& resection of a contiguous organ) | 3 | 0 | 1 | 5 | 10 | 19 | |
| Total proctocolectomy with ileostomy (NOS) | 1 | 1 | 0 | 2 | 6 | 10 | |
| Total proctocolectomy with ileostomy & Pouch | 3 | 0 | 0 | 0 | 7 | 10 | |
| Total proctocolectomy, NOS | 36 | 0 | 28 | 29 | 184 | 277 | |
| Unknown if surgery performed; death certificate ON | 26 | 3 | 8 | 17 | 56 | 110 | |
| Wedge resection (segmental resection) & resection | 68 | 4 | 33 | 63 | 225 | 393 |
| Hispanic | Non-Hispanic American | Non-Hispanic Asian or Pacific Islander | Non-Hispanic Black | Non-Hispanic White | |
|---|---|---|---|---|---|
| Indian/Alaskan | |||||
| Native | |||||
| Marital Status (Unmarried vs. Married) |  |  |  |  |  |
| Immigration (Above vs. Below 50th centile) |  |  |  |  |  |
| Employment Status (Above vs. Below 50th centile) |  |  |  |  |  |
| Poverty (Above vs. Below 50th centile) |  |  |  |  |  |
| High School Education (Above vs. Below 50th centile) |  |  |  |  |  |
| Insurance (Medicaid vs. Commercial) |  |  |  |  |  |
| Household Income (Above vs. Below 50th centile) |  |  |  |  |  |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Socioeconomic Determinant | HR | CI | p | Socioeconomic Determinant | HR | CI | p |
| Immigration | 1.081 | 1.002–1.165 | 0.043 | Immigration | 1.011 | 0.927–1.102 | 0.808 |
| Employment Status | 0.89 | 0.826–0.96 | 0.002 | Unemployment | 1.006 | 0.91–1.112 | 0.809 |
| Poverty | 0.811 | 0.753–0.875 | 0.001 | Poverty | 0.887 | 0.787–0.999 | 0.049 |
| High School Education | 0.795 | 0.738–0.857 | <0.0001 | High School Education | 0.875 | 0.791–0.967 | 0.009 |
| Insurance (Commercial vs. Medicaid) | 0.54 | 0.494–0.592 | <0.0001 | Insurance (Commercial vs. Medicaid) | 0.597 | 0.542–0.659 | <0.0001 |
| Unmarried vs. Married | 1.436 | 1.331–1.55 | <0.0001 | Unmarried vs. Married | 1.305 | 1.201–1.419 | <0.0001 |
| Household Income | 1.26 | 1.169–1.357 | 0.001 | Household Income | 1.136 | 0.994–1.1298 | 0.062 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloysius, M.M.; Goyal, H.; Shah, N.J.; Pallav, K.; John, N.; Gajendran, M.; Perisetti, A.; Tharian, B. Impact of Race and Socioeconomics Disparities on Survival in Young-Onset Colorectal Adenocarcinoma—A SEER Registry Analysis. Cancers 2021, 13, 3262. https://doi.org/10.3390/cancers13133262
Aloysius MM, Goyal H, Shah NJ, Pallav K, John N, Gajendran M, Perisetti A, Tharian B. Impact of Race and Socioeconomics Disparities on Survival in Young-Onset Colorectal Adenocarcinoma—A SEER Registry Analysis. Cancers. 2021; 13(13):3262. https://doi.org/10.3390/cancers13133262
Chicago/Turabian StyleAloysius, Mark M., Hemant Goyal, Niraj J. Shah, Kumar Pallav, Nimy John, Mahesh Gajendran, Abhilash Perisetti, and Benjamin Tharian. 2021. "Impact of Race and Socioeconomics Disparities on Survival in Young-Onset Colorectal Adenocarcinoma—A SEER Registry Analysis" Cancers 13, no. 13: 3262. https://doi.org/10.3390/cancers13133262
APA StyleAloysius, M. M., Goyal, H., Shah, N. J., Pallav, K., John, N., Gajendran, M., Perisetti, A., & Tharian, B. (2021). Impact of Race and Socioeconomics Disparities on Survival in Young-Onset Colorectal Adenocarcinoma—A SEER Registry Analysis. Cancers, 13(13), 3262. https://doi.org/10.3390/cancers13133262







