Margin Accentuation Irreversible Electroporation in Stage III Pancreatic Cancer: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
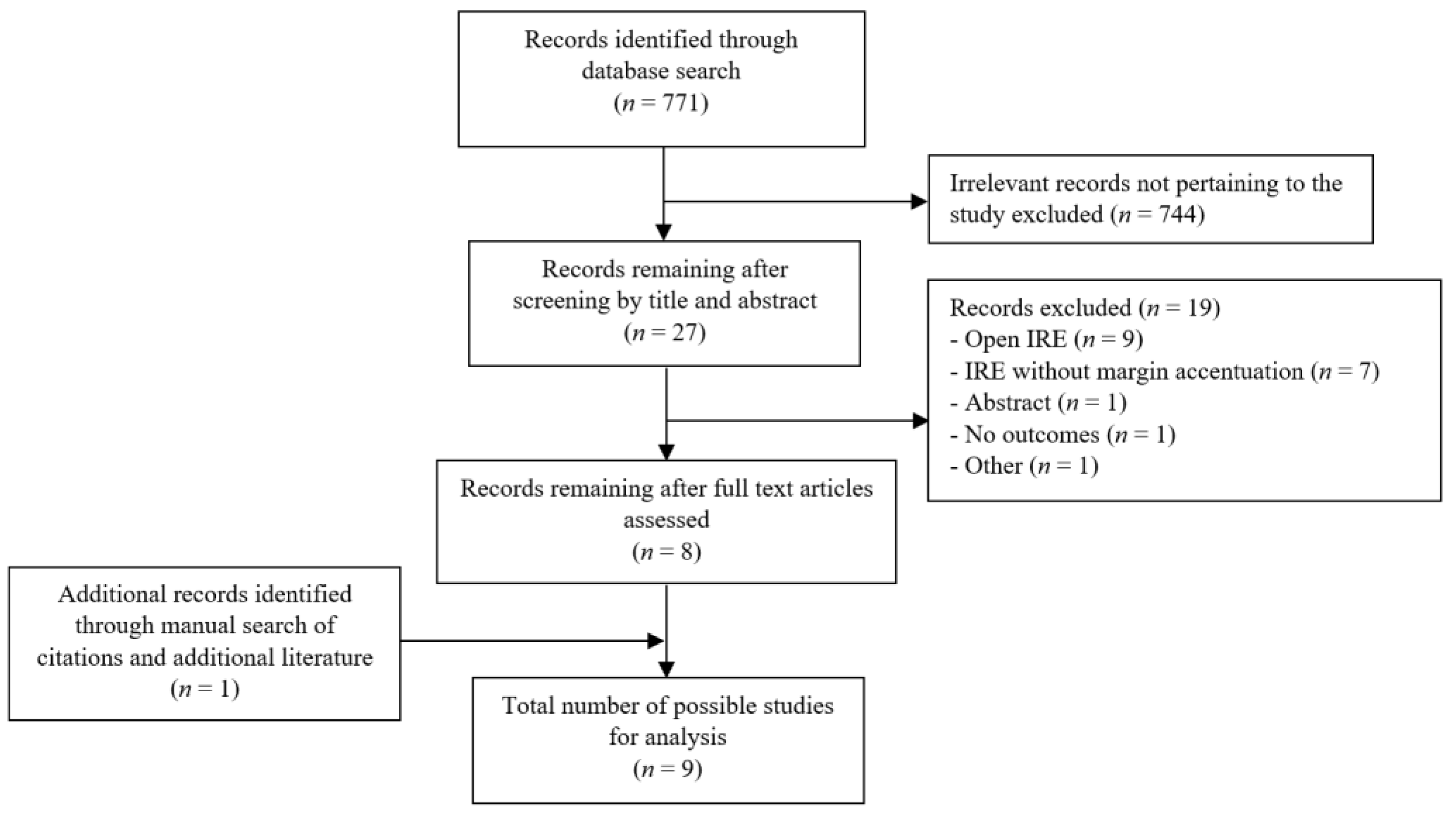
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Selection and Extraction
2.4. Definitions
2.5. Statistical Methodology and Risk of Bias Assessment
3. Results
3.1. Tumour Characteristics and Pancreatic Resections
3.2. Survival and Recurrence
3.3. Pathological Outcomes, Complications and Length of Stay
3.4. Heterogeneity and Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Bilimora, K.Y.; Bentrem, D.J.; Ko, C.Y.; Steewart, A.K.; Winchester, D.P.; Talamonti, M.S. National Failure to Operate on Early Stage Pancreatic Cancer. Ann. Surg. 2007, 246, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Perysinakis, I.; Avlonitis, S.; Georgiadou, D.; Tsipras, H.; Margaris, I. Five-year actual survival after pancreatoduodenectomy for pancreatic head cancer. ANZ J. Surg. 2015, 85, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Behrman, S.W.; Benson, A.B.; Casper, E.S.; Chiorean, E.G.; Chung, V.; Cohen, S.J.; Czito, B.; Engebretson, A.; et al. Pancreatic adenocarcinoma, version 2.2014: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2014, 12, 1083–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombouts, S.J.; Mungroop, T.H.; Heilmann, M.N.; van Laarhoven, H.W.; Busch, O.R.; Molenaar, I.Q.; Besselink, M.G.; Wilmink, J.W. FOLFIRINOX in Locally Advanced and Metastatic Pancreatic Cancer: A Single Centre Cohort Study. J. Cancer 2016, 7, 1861–1866. [Google Scholar] [CrossRef] [Green Version]
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016, 17, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Barreto, S.G.; Loveday, B.; Windsor, J.A.; Pandanaboyana, S. Detecting tumour response and predicting resectability after neoadjuvant therapy for borderline resectable and locally advanced pancreatic cancer. ANZ J. Surg. 2019, 89, 481–487. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2008, 45, 228–247. [Google Scholar] [CrossRef]
- Hank, T.; Strobel, O. Conversion Surgery for Advanced Pancreatic Cancer. J. Clin. Med. 2019, 8, 1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbeke, C.; Löhr, M.; Severin Karlsson, J.; Del Chiaro, M. Pathology reporting of pancreatic cancer following neoadjuvant therapy: Challenges and uncertainties. Cancer Treat. Rev. 2014, 41, 17–26. [Google Scholar]
- Lee, E.W.; Thai, S.; Kee, S.T. Irreversible Electroporation: A Novel Image-Guided Cancer Therapy. Gut Liver 2010, 4 (Suppl. 1), 99–104. [Google Scholar] [CrossRef]
- Cannon, R.; Ellis, S.; Hayes, D.; Narayanan, G.; Martin, R.C.G. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J. Surg. Oncol. 2013, 107, 544–549. [Google Scholar] [CrossRef]
- Martin, R.C.G.; McFarland, K.; Ellis, S.; Velanovich, V. Irreversible Electroporation Therapy in the Management of Locally Advanced Pancreatic Adenocarcinoma. J. Am. Coll. Surg. 2012, 215, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Kristoffersson, S.; Andersson, R.; Bergenfeldt, M. The role of irreversible electroporation (IRE) for locally advanced pancreatic cancer: A systematic review of safety and efficacy. Scand. J. Gastroenterol. 2017, 52, 1165–1171. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callery, M.P.; Chang, K.J.; Fishman, E.K.; Talamonti, M.S.; William Traverso, L.; Linehan, D.C. Pretreatment Assessment of Resectable and Borderline Resectable Pancreatic Cancer: Expert Consensus Statement. Ann. Surg. Oncol. 2009, 16, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; McFarland, K.; Velanovich, V.; Martin, R. Borderline and locally advanced pancreatic adenocarcinoma margin accentuation with intraoperative irreversible electroporation. Surgery 2014, 156, 910–922. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; Graf, R.; Vonlanthen, R.; Padbury, R.; Cameron, K.L.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.C.; Kwon, D.; Chalikonda, S.; Sellers, M.; Kotz, E.; Scoggins, C.; McMasters, K.M.; Watkins, K. Treatment of 200 Locally Advanced (Stage III) Pancreatic Adenocarcinoma Patients with Irreversible Electroporation: Safety and Efficacy. Ann. Surg. 2015, 262, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Hermanek, P.; Wittekind, C. Residual tumor (R) classification and prognosis. Semin. Surg. Oncol. 1994, 10, 12–20. [Google Scholar] [CrossRef]
- Bowden, J.; Jackson, C. MetaAnalyser: An Interactive Visualisation of Meta-Analysis as a Physical Weighing Machine. 2016. Available online: https://cran.r-project.org/web/packages/MetaAnalyser/index.html (accessed on 5 February 2021).
- Ankit, R. WebPlotDigitizer 4.0. 2017. Available online: https://automeris.io/WebPlotDigitizer (accessed on 5 February 2021).
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Kluger, M.D.; Rashid, M.F.; Rosario, V.L.; Schrope, B.A.; Steinman, J.A.; Hecht, E.M.; Chabot, J.A. Resection of Locally Advanced Pancreatic Cancer without Regression of Arterial Encasement After Modern-Era Neoadjuvant. Ther. J. Gastrointest. Surg. 2018, 22, 235–241. [Google Scholar] [CrossRef]
- Martin, R.C.G., II; McFarland, K.; Ellis, S.; Velanovich, V. Irreversible Electroporation in Locally Advanced Pancreatic Cancer: Potential Improved Overall Survival. Ann. Surg. Oncol. 2013, 20, 443–449. [Google Scholar] [CrossRef]
- Papoulas, M.; Abdul-Hamid, S.; Peddu, P.; Cotoi, C.; Heaton, N.; Menon, K. Irreversible electroporation in borderline resectable pancreatic adenocarcinoma for margin accentuation. J. Surg. Case Rep. 2018, 2018, rjy127. [Google Scholar] [CrossRef] [PubMed]
- Kluger, M.D.; Epelboym, I.; Schrope, B.A.; Mahendraraj, K.; Hecht, E.M.; Susman, J.; Weintraub, J.L.; Chabot, J.A. Single-Institution Experience with Irreversible Electroporation for T4 Pancreatic Cancer: First 50 Patients. Ann. Surg. Oncol. 2016, 23, 1736–1743. [Google Scholar] [CrossRef]
- Marsanic, P.; Mellano, A.; Sottile, A.; De Simone, M. Irreversible electroporation as treatment of locally advanced and as margin accentuation in borderline resectable pancreatic adenocarcinoma. Med. Biol. Eng. Comput. 2017, 55, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Dunki-Jacobs, E.M.; Philips, P.; Martin, R.C. Evaluation of Resistance as a Measure of Successful Tumor Ablation During Irreversible Electroporation of the Pancreas. J. Am. Coll. Surg. 2014, 218, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Varadhachary, G.; Tamm, E.; Abbruzzese, J.; Xiong, H.; Crane, C.; Wang, H.; Lee, J.E.; Pisters, P.W.; Evans, D.B.; Wolff, R.A.; et al. Borderline Resectable Pancreatic Cancer: Definitions, Management, and Role of Preoperative Therapy. Ann. Surg. Oncol. 2006, 13, 1035–1046. [Google Scholar] [CrossRef]
- Paiella, S.; De Pastena, M.; D’Onofrio, M.; Crinò, S.F.; Pan, T.L.; De Robertis, R.; Elio, G.; Martone, E.; Bassi, C.; Salvia, R.; et al. Palliative therapy in pancreatic cancer-interventional treatment with radiofrequency ablation/irreversible electroporation. Transl. Gastroenterol. Hepatol. 2018, 3, 80. [Google Scholar] [CrossRef]
- Bagla, S.; Papadouris, D. Percutaneous Irreversible Electroporation of Surgically Unresectable Pancreatic Cancer: A Case Report. J. Vasc. Interv. Radiol. 2012, 23, 142–145. [Google Scholar] [CrossRef]
- Maor, E.; Ivorra, A.; Leor, J.; Rubinsky, B. The Effect of Irreversible Electroporation on Blood Vessels. Technol. Cancer Res. Treat. 2007, 6, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Lafranceschina, S.; Brunetti, O.; Delvecchio, A.; Conticchio, M.; Ammendola, M.; Currò, G.; Piardi, T.; de’Angelis, N.; Silvestris, N.; Memeo, R.; et al. Systematic Review of Irreversible Electroporation Role in Management of Locally Advanced Pancreatic Cancer. Cancers 2019, 11, 1718. [Google Scholar] [CrossRef] [Green Version]
- Klaiber, U.; Schnaidt, E.; Hinz, U.; Gaida, M.; Heger, U.; Hank, T.; Strobel, O.; Neoptolemos, J.P.; Mihaljevic, A.L.; Büchler, M.W.; et al. Prognostic Factors of Survival After Neoadjuvant Treatment and Resection for Initially Unresectable Pancreatic Cancer. Ann. Surg. 2019, 273, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.A.; Rombouts, S.J.; de Rooij, T.; van Delden, O.M.; Dijkgraaf, M.G.; van Gulik, T.M.; van Hooft, J.E.; van Laarhoven, H.W.; Martin, R.C.; Schoorlemmer, A.; et al. Induction Chemotherapy Followed by Resection or Irreversible Electroporation in Locally Advanced Pancreatic Cancer (IMPALA): A Prospective Cohort Study. Ann. Surg. Oncol. 2017, 24, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, B.; Savastyuk, A.Y.; Nayar, M.; Wilson, C.H.; Windsor, J.A.; Roberts, K.; French, J.J.; Pandanaboyana, S. Recurrence Patterns for Pancreatic Ductal Adenocarcinoma after Upfront Resection Versus Resection Following Neoadjuvant Therapy: A Comprehensive Meta-Analysis. J. Clin. Med. 2020, 9, 2132. [Google Scholar] [CrossRef]
- Scheffer, H.J.; Stam, A.G.M.; Geboers, B.; Vroomen, L.G.P.H.; Ruarus, A.; de Bruijn, B.; van den Tol, M.P.; Kazemier, G.; Meijerink, M.R.; de Gruijl, T.D.; et al. Irreversible electroporation of locally advanced pancreatic cancer transiently alleviates immune suppression and creates a window for antitumor T cell activation. Oncoimmunology 2019, 8, 1652532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, J.S.S.; Ray, P.; Hayashi, T.; Whisenant, T.C.; Vicente, D.; Carson, D.A.; Miller, A.M.; Schoenberger, S.P.; White, R.R. Irreversible Electroporation Combined with Checkpoint Blockade and TLR7 Stimulation Induces Antitumor Immunity in a Murine Pancreatic Cancer Model. Cancer Immunol. Res. 2019, 7, 1714–1726. [Google Scholar] [CrossRef]
- Izzo, F.; Granata, V.; Fusco, R.; D’Alessio, V.; Petrillo, A.; Lastoria, S.; Piccirillo, M.; Albino, V.; Belli, A.; Tafuto, S.; et al. Clinical Phase I/II Study: Local Disease Control and Survival in Locally Advanced Pancreatic Cancer Treated with Electrochemotherapy. J. Clin. Med. 2021, 10, 1305. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Piccirillo, M.; Leongito, M.; Palaia, R.; Granata, F.; Lastoria, S.; Izzo, F.; Petrillo, A.; et al. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J. Gastroenterol. WJG 2017, 23, 4767–4778. [Google Scholar] [CrossRef]
- Casadei, R.; Ricci, C.; Ingaldi, C.; Alberici, L.; Di Marco, M.; Guido, A.; Minni, F.; Serra, C. Intraoperative electrochemotherapy in locally advanced pancreatic cancer: Indications, techniques and results-a single-center experience. Updates Surg. 2020, 72, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
| Author | Publication Year | Type of Study | Location of Publication | Total Cohort | MA IRE Cohort |
|---|---|---|---|---|---|
| Kluger et al. [25] | 2018 | Observational Cohort | USA | 56 | 56 |
| Papoulas et al. [27] | 2018 | Case Report | United Kingdom | 1 | 1 |
| Marsanic et al. [29] | 2017 | Observational Cohort | Italy | 7 | 5 |
| Kluger et al. [28] | 2016 | Comparative Cohort | USA | 50 | 24 |
| Martin et al. [19] | 2015 | Comparative Cohort | USA | 200 | 50 |
| Dunki-Jacobs et al. [30] | 2014 | Observational Cohort | USA | 65 | 24 |
| Kwon et al. [17] | 2014 | Observational Cohort | USA | 48 | 48 |
| Martin et al. [26] | 2013 | Comparative Cohort | USA | 139 | 19 |
| Martin et al. [13] | 2012 | Observational Cohort | USA | 27 | 8 |
| Author | Publication Year | LAPC/BRPC | Neoadjuvant | Vascular Involvement | PD/DP | Arterial/Venous Resection |
|---|---|---|---|---|---|---|
| Kluger et al. [25] | 2018 | 56/0 | 56 | 49 | 34/22 | - |
| Papoulas et al. [27] | 2018 | 0/1 | 1 | 1 | 1/0 | 0/1 |
| Marsanic et al. [29] | 2017 | 0/5 | 5 | 5 | 5/0 | - |
| Kluger et al. [28] | 2016 | 24/0 | 22 | 24 | 15/9 | 0/12 |
| Martin et al. [19] | 2015 | 50/0 | 8 | 50 | 13/37 | 37/25 |
| Dunki-Jacobs et al. [30] | 2014 | 24/0 | 24 | 24 | 8/16 | - |
| Kwon et al. [17] | 2014 | 11/37 | 18 | 48 | 31/17 | 10/25 |
| Martin et al. [26] | 2013 | 19/0 | 19 | 19 | 9/10 | 19/0 |
| Martin et al. [13] | 2012 | 8/0 | 8 | 8 | 4/4 | - |
| Author | Publication Year | MA IRE Cohort | R0 Resection | LOS (Days) * | PFS (Months) ¥ | Overall Recurrence | Overall Survival MA IRE (Months) ¥ | Overall Survival No MA IRE Cohort (Months) ¥ |
|---|---|---|---|---|---|---|---|---|
| Kluger et al. [25] | 2018 | 56 | 45 | 7 (5–11) | 8.5 (6–15) | 26 | 18.5 (12–32) | - |
| Papoulas et al. [27] | 2018 | 1 | 1 | - | - | 0 | - | - |
| Marsanic et al. [29] | 2017 | 5 | - | - | - | 0 | - | - |
| Kluger et al. [28] | 2016 | 24 | - | 8 (3–40) | - | - | - | 7.7 (6–12) |
| Martin et al. [19] | 2015 | 50 | - | 7 (4–26) | - | 6 | 28.3 (9–85) * | 23.2 (5–76) * |
| Dunki-Jacobs et al. [30] | 2014 | 24 | - | 6 (5–58) | - | 3 | - | - |
| Kwon et al. [17] | 2014 | 48 | 31 | 9 (4–58) | 10.7 (3–30) | 28 | 22.4 (18–25) | - |
| Martin et al. [26] | 2013 | 19 | - | - | - | - | - | - |
| Martin et al. [13] | 2012 | 8 | - | - | - | 0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratnayake, B.; Al-Leswas, D.; Mohammadi-Zaniani, G.; Littler, P.; Sen, G.; Manas, D.; Pandanaboyana, S. Margin Accentuation Irreversible Electroporation in Stage III Pancreatic Cancer: A Systematic Review. Cancers 2021, 13, 3212. https://doi.org/10.3390/cancers13133212
Ratnayake B, Al-Leswas D, Mohammadi-Zaniani G, Littler P, Sen G, Manas D, Pandanaboyana S. Margin Accentuation Irreversible Electroporation in Stage III Pancreatic Cancer: A Systematic Review. Cancers. 2021; 13(13):3212. https://doi.org/10.3390/cancers13133212
Chicago/Turabian StyleRatnayake, Bathiya, Dhya Al-Leswas, Ghazaleh Mohammadi-Zaniani, Peter Littler, Gourab Sen, Derek Manas, and Sanjay Pandanaboyana. 2021. "Margin Accentuation Irreversible Electroporation in Stage III Pancreatic Cancer: A Systematic Review" Cancers 13, no. 13: 3212. https://doi.org/10.3390/cancers13133212
APA StyleRatnayake, B., Al-Leswas, D., Mohammadi-Zaniani, G., Littler, P., Sen, G., Manas, D., & Pandanaboyana, S. (2021). Margin Accentuation Irreversible Electroporation in Stage III Pancreatic Cancer: A Systematic Review. Cancers, 13(13), 3212. https://doi.org/10.3390/cancers13133212







