A New Paradigm in the Relationship between Melatonin and Breast Cancer: Gut Microbiota Identified as a Potential Regulatory Agent
Abstract
:Simple Summary
Abstract
1. Introduction
2. Search Methods
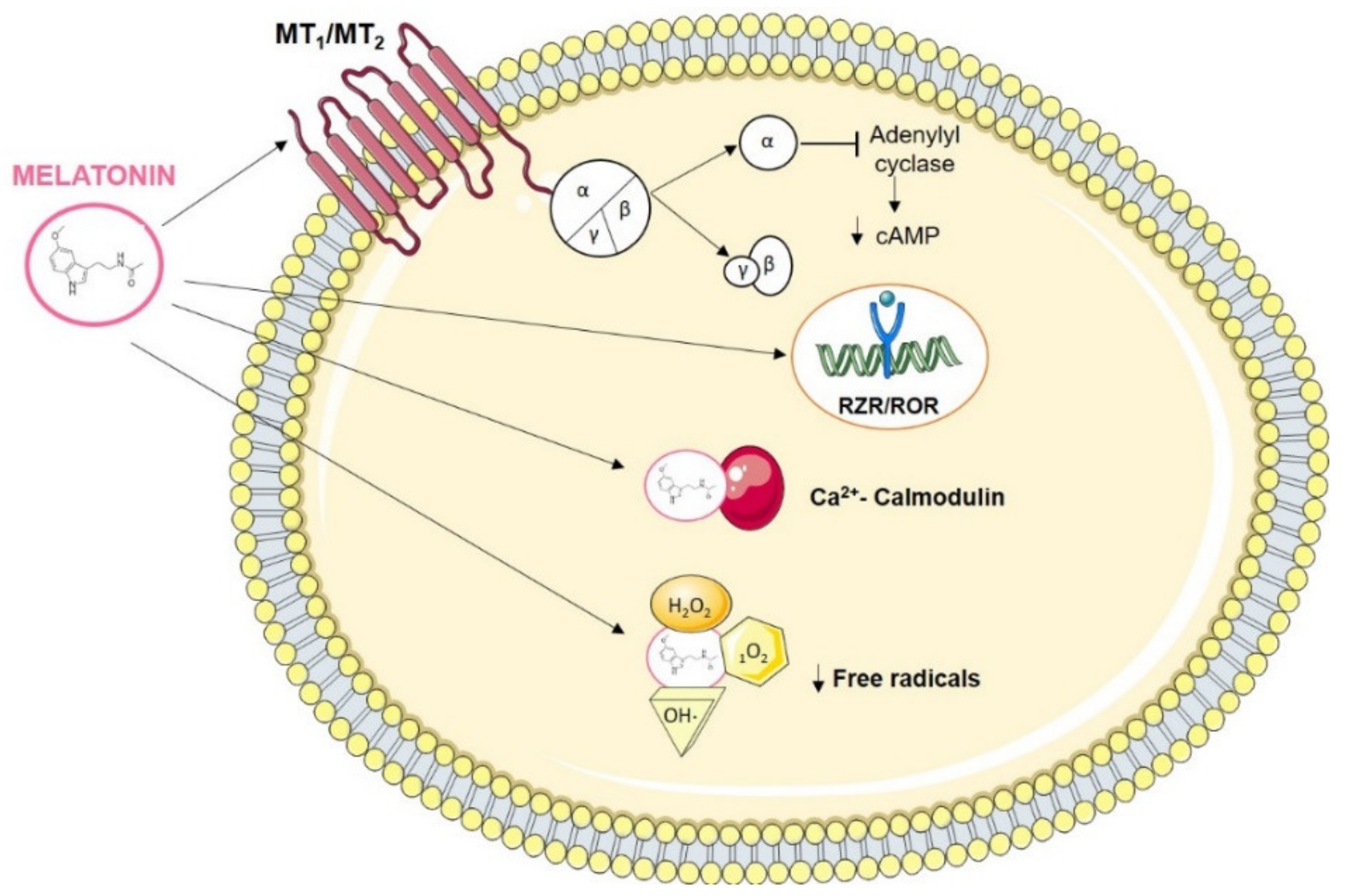
3. Melatonin Interaction Molecules
3.1. Melatonin Actions in Cancer
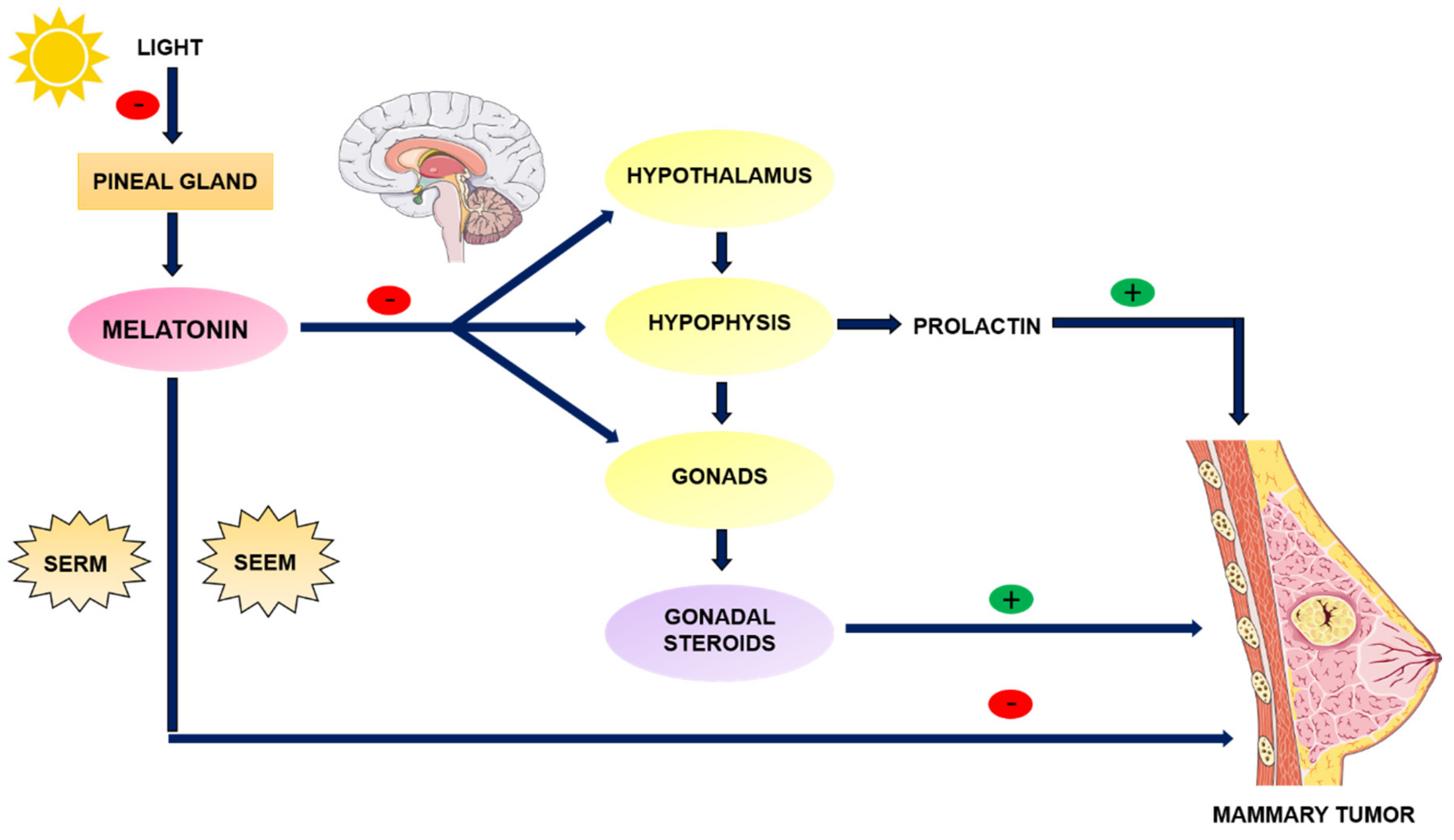
3.2. Melatonin as an Anti-Estrogen: SERM and SEEM Properties
4. Melatonin and Breast Cancer
4.1. Fat Tissue, Estrogens and Breast Cancer
4.2. Melatonin and Desmoplastic Reaction
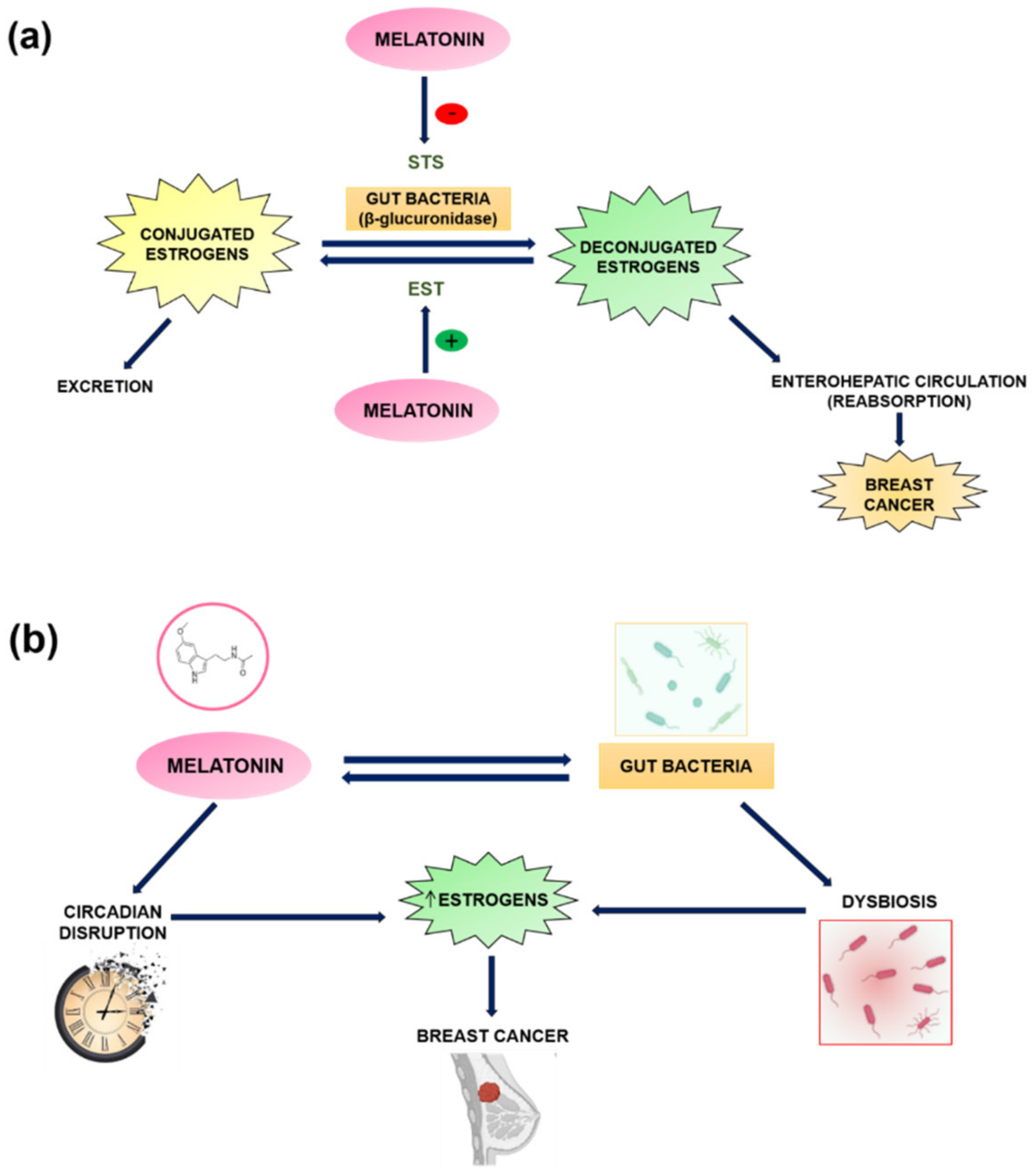
5. Melatonin and the Estrobolome in Breast Cancer
6. Gut Permeability, Intestinal Dysbiosis, and Circadian Disruption
7. Clinical Trials of Melatonin in the Treatment of Breast Cancer
8. Possible Applications of Melatonin in Breast Cancer
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, D.-X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A Hormone, a Tissue Factor, an Autocoid, a Paracoid, and an Antioxidant Vitamin. J. Pineal Res. 2003, 34, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Cos, S.; Sánchez-Barceló, E.J. Melatonin and Mammary Pathological Growth. Front. Neuroendocr. 2000, 21, 133–170. [Google Scholar] [CrossRef]
- Stefulj, J.; Hörtner, M.; Ghosh, M.; Schauenstein, K.; Rinner, I.; Wölfler, A.; Semmler, J.; Liebmann, P.M. Gene Expression of the Key Enzymes of Melatonin Synthesis in Extrapineal Tissues of the Rat. J. Pineal Res. 2001, 30, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, Y.; Fan, F.; Zhang, B. The Role of Microbiome in Insomnia, Circadian Disturbance and Depression. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Autenshlyus, A.I.; Kunts, T.A.; Karpukhina, K.V.; Mikhaylova, E.S.; Varaksin, N.A.; Marinkin, I.O.; Lyakhovich, V.V. Cytokine Pattern of the Breast Tumor Supernatant. Dokl. Biol. Sci. 2016, 470, 247–248. [Google Scholar] [CrossRef]
- Anderson, G. Breast Cancer: Occluded Role of Mitochondria N-Acetylserotonin/Melatonin Ratio in Co-Ordinating Pathophysiology. Biochem. Pharm. 2019, 168, 259–268. [Google Scholar] [CrossRef]
- Aryl Hydrocarbon Receptor/Cytochrome P450 1A1 Pathway Mediates Breast Cancer Stem Cells Expansion through PTEN Inhibition and β-Catenin and Akt Activation|Molecular Cancer|Full Text. Available online: https://molecular-cancer.biomedcentral.com/articles/10.1186/s12943-016-0570-y (accessed on 3 November 2020).
- Asghar, K.; Loya, A.; Rana, I.A.; Tahseen, M.; Ishaq, M.; Farooq, A.; Bakar, M.A.; Masood, I. Indoleamine 2,3-Dioxygenase Expression and Overall Survival in Patients Diagnosed with Breast Cancer in Pakistan. Available online: https://www.dovepress.com/indoleamine-23-dioxygenase-expression-and-overall-survival-in-patients-peer-reviewed-article-CMAR (accessed on 3 November 2020).
- Wei, L.; Zhu, S.; Li, M.; Li, F.; Wei, F.; Liu, J.; Ren, X. High Indoleamine 2,3-Dioxygenase Is Correlated with Microvessel Density and Worse Prognosis in Breast Cancer. Front. Immunol. 2018, 9, 724. [Google Scholar] [CrossRef]
- Kassayová, M.; Bobrov, N.; Strojný, L.; Orendáš, P.; Demečková, V.; Jendželovský, R.; Kubatka, P.; Kisková, T.; Kružliak, P.; Adamkov, M.; et al. Anticancer and Immunomodulatory Effects of Lactobacillus Plantarum LS/07, Inulin and Melatonin in NMU-Induced Rat Model of Breast Cancer. Anticancer Res. 2016, 36, 2719–2728. [Google Scholar]
- Sainz, R.; Mayo, J.; Rodriguez, C.; Tan, D.; López-Burillo, S.; Reiter, R.J. Melatonin and Cell Death: Differential Actions on Apoptosis in Normal and Cancer Cells. Available online: https://pubmed.ncbi.nlm.nih.gov/12943228 (accessed on 20 November 2020).
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.-X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.-F.; Xu, K. Melatonin, a Full Service Anti-Cancer Agent: Inhibition of Initiation, Progression and Metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef]
- Hill, S.M.; Belancio, V.P.; Dauchy, R.T.; Xiang, S.; Brimer, S.; Mao, L.; Hauch, A.; Lundberg, P.W.; Summers, W.; Yuan, L.; et al. Melatonin: An Inhibitor of Breast Cancer. Endocr. Relat. Cancer 2015, 22, R183–R204. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J. Melatonin: The Chemical Expression of Darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158. [Google Scholar] [CrossRef]
- Del Río, B.; García Pedrero, J.M.; Martínez-Campa, C.; Zuazua, P.; Lazo, P.S.; Ramos, S. Melatonin, an Endogenous-Specific Inhibitor of Estrogen Receptor Alpha via Calmodulin. J. Biol. Chem. 2004, 279, 38294–38302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benítez-King, G.; Huerto-Delgadillo, L.; Antón-Tay, F. Binding of 3H-Melatonin to Calmodulin. Life Sci. 1993, 53, 201–207. [Google Scholar] [CrossRef]
- Allegra, M.; Reiter, R.J.; Tan, D.-X.; Gentile, C.; Tesoriere, L.; Livrea, M.A. The Chemistry of Melatonin’s Interaction with Reactive Species. J. Pineal Res. 2003, 34, 1–10. [Google Scholar] [CrossRef]
- Kelleher, F.C.; Rao, A.; Maguire, A. Circadian Molecular Clocks and Cancer. Cancer Lett. 2014, 342, 9–18. [Google Scholar] [CrossRef]
- Cos, S.; Recio, J.; Sánchez-Barceló, E.J. Modulation of the Length of the Cell Cycle Time of MCF-7 Human Breast Cancer Cells by Melatonin. Life Sci. 1996, 58, 811–816. [Google Scholar] [CrossRef]
- Cui, P.; Luo, Z.; Zhang, H.; Su, Y.; Li, A.; Li, H.; Zhang, J.; Yang, Z.; Xiu, R. Effect and Mechanism of Melatonin’s Action on the Proliferation of Human Umbilical Vein Endothelial Cells. J. Pineal Res. 2006, 41, 358–362. [Google Scholar] [CrossRef]
- Martínez-Campa, C.M.; Alonso-González, C.; Mediavilla, M.D.; Cos, S.; González, A.; Sanchez-Barcelo, E.J. Melatonin Down-Regulates HTERT Expression Induced by Either Natural Estrogens (17beta-Estradiol) or Metalloestrogens (Cadmium) in MCF-7 Human Breast Cancer Cells. Cancer Lett. 2008, 268, 272–277. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.-J.; Xu, D.-P.; Li, H.-B. Melatonin for the Prevention and Treatment of Cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef] [Green Version]
- Maroufi, N.F.; Ashouri, N.; Mortezania, Z.; Ashoori, Z.; Vahedian, V.; Amirzadeh-Iranaq, M.T.; Fattahi, A.; Kazemzadeh, H.; Bizzarri, M.; Akbarzadeh, M.; et al. The Potential Therapeutic Effects of Melatonin on Breast Cancer: An Invasion and Metastasis Inhibitor. Pathol. Res. Pract. 2020, 216, 153226. [Google Scholar] [CrossRef]
- Borin, T.F.; Arbab, A.S.; Gelaleti, G.B.; Ferreira, L.C.; Moschetta, M.G.; Jardim-Perassi, B.V.; Iskander, A.S.M.; Varma, N.R.S.; Shankar, A.; Coimbra, V.B.; et al. Melatonin Decreases Breast Cancer Metastasis by Modulating Rho-Associated Kinase Protein-1 Expression. J. Pineal Res. 2016, 60, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, J.; Russo, I.H. The Role of Estrogen in the Initiation of Breast Cancer. J. Steroid Biochem. Mol. Biol. 2006, 102, 89–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cos, S.; González, A.; Martínez-Campa, C.; Mediavilla, M.D.; Alonso-González, C.; Sánchez-Barceló, E.J. Estrogen-Signaling Pathway: A Link between Breast Cancer and Melatonin Oncostatic Actions. Cancer Detect. Prev. 2006, 30, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Cos, S.; González, A.; Martínez-Campa, C.; Mediavilla, M.D.; Alonso-González, C.; Sánchez-Barceló, E.J. Melatonin as a Selective Estrogen Enzyme Modulator. Curr. Cancer Drug Targets 2008, 8, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Campa, C.; González, A.; Mediavilla, M.D.; Alonso-González, C.; Alvarez-García, V.; Sánchez-Barceló, E.J.; Cos, S. Melatonin Inhibits Aromatase Promoter Expression by Regulating Cyclooxygenases Expression and Activity in Breast Cancer Cells. Br. J. Cancer 2009, 101, 1613–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xiao, X.; Zhang, Y.; Shi, D.; Chen, W.; Fu, L.; Liu, L.; Xie, F.; Kang, T.; Huang, W.; et al. Simultaneous Modulation of COX-2, P300, Akt, and Apaf-1 Signaling by Melatonin to Inhibit Proliferation and Induce Apoptosis in Breast Cancer Cells. J. Pineal Res. 2012, 53, 77–90. [Google Scholar] [CrossRef]
- Gonzalez, A.; Cos, S.; Martinez-Campa, C.; Alonso-Gonzalez, C.; Sanchez-Mateos, S.; Mediavilla, M.D.; Sanchez-Barcelo, E.J. Selective Estrogen Enzyme Modulator Actions of Melatonin in Human Breast Cancer Cells. J. Pineal Res. 2008, 45, 86–92. [Google Scholar] [CrossRef]
- Wilson, S.T.; Blask, D.E.; Lemus-Wilson, A.M. Melatonin Augments the Sensitivity of MCF-7 Human Breast Cancer Cells to Tamoxifen In Vitro. J. Clin. Endocrinol. Metab. 1992, 75, 669–670. [Google Scholar] [CrossRef]
- Martínez-Campa, C.; González, A.; Mediavilla, M.D.; Alonso-González, C.; Sánchez-Barceló, E.J.; Cos, S. Melatonin Enhances the Inhibitory Effect of Aminoglutethimide on Aromatase Activity in MCF-7 Human Breast Cancer Cells. Breast Cancer Res. Treat. 2005, 94, 249–254. [Google Scholar] [CrossRef]
- Aydin, M.; Oktar, S.; Ozkan, O.V.; Alçin, E.; Oztürk, O.H.; Nacar, A. Letrozole Induces Hepatotoxicity without Causing Oxidative Stress: The Protective Effect of Melatonin. Gynecol. Endocrinol. 2011, 27, 209–215. [Google Scholar] [CrossRef]
- González-González, A.; Mediavilla, M.D.; Sánchez-Barceló, E.J. Melatonin: A Molecule for Reducing Breast Cancer Risk. Molecules 2018, 23, 336. [Google Scholar] [CrossRef] [Green Version]
- Danforth, D.N.; Tamarkin, L.; Mulvihill, J.J.; Bagley, C.S.; Lippman, M.E. Plasma Melatonin and the Hormone-Dependency of Human Breast Cancer. J. Clin. Oncol. 1985, 3, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Skene, D.J.; Bojkowski, C.J.; Currie, J.E.; Wright, J.; Boulter, P.S.; Arendt, J. 6-Sulphatoxymelatonin Production in Breast Cancer Patients. J. Pineal Res. 1990, 8, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P. Biochemotherapy with Standard Chemotherapies plus the Pineal Hormone Melatonin in the Treatment of Advanced Solid Neoplasms. Pathol. Biol. 2007, 55, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Orendáš, P.; Kubatka, P.; Bojková, B.; Kassayová, M.; Kajo, K.; Výbohová, D.; Kružliak, P.; Péč, M.; Adamkov, M.; Kapinová, A.; et al. Melatonin Potentiates the Anti-Tumour Effect of Pravastatin in Rat Mammary Gland Carcinoma Model. Int. J. Exp. Pathol. 2014, 95, 401–410. [Google Scholar] [CrossRef]
- Cos, S.; Bardasano, J.L.; Mediavilla, M.D.; Sánchez Barceló, E.J. Pineal Gland in Rats with 7,12-Dimethylbenz(a)Anthracene-Induced Mammary Tumors Subjected to Manipulations Known as Enhancers of Pineal Actions. Histol. Histopathol. 1989, 4, 235–239. [Google Scholar]
- Gérard, C.; Brown, K. Obesity and Breast Cancer—Role of Estrogens and the Molecular Underpinnings of Aromatase Regulation in Breast Adipose Tissue. Mol. Cell. Endocrinol. 2018. [Google Scholar] [CrossRef]
- Wang, X.; Simpson, E.R.; Brown, K.A. Aromatase Overexpression in Dysfunctional Adipose Tissue Links Obesity to Postmenopausal Breast Cancer. J. Steroid Biochem. Mol. Biol. 2015, 153, 35–44. [Google Scholar] [CrossRef]
- Szewczyk-Golec, K.; Woźniak, A.; Reiter, R.J. Inter-Relationships of the Chronobiotic, Melatonin, with Leptin and Adiponectin: Implications for Obesity. J. Pineal Res. 2015, 59, 277–291. [Google Scholar] [CrossRef]
- Martínez-Chacón, G.; Brown, K.A.; Docanto, M.M.; Kumar, H.; Salminen, S.; Saarinen, N.; Mäkelä, S. IL-10 Suppresses TNF-α-Induced Expression of Human Aromatase Gene in Mammary Adipose Tissue. FASEB J. 2018, 32, 3361–3370. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.R. Aromatization of Androgens in Women: Current Concepts and Findings. Fertil. Steril. 2002, 77 (Suppl. S4), S6–S10. [Google Scholar] [CrossRef]
- Alvarez-García, V.; González, A.; Martínez-Campa, C.; Alonso-González, C.; Cos, S. Melatonin Modulates Aromatase Activity and Expression in Endothelial Cells. Oncol. Rep. 2013, 29, 2058–2064. [Google Scholar] [CrossRef] [Green Version]
- Cos, S.; González, A.; Alvarez Garcia, V.; Alonso-Gonzalez, C.; Martinez-Campa, C. Melatonin and Breast Cancer: Selective Estrogen Enzyme Modulator. Adv. Cancer Drug Targets 2012, 1, 207–237. [Google Scholar]
- Bulun, S.E.; Lin, Z.; Imir, G.; Amin, S.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; Gurates, B.; et al. Regulation of Aromatase Expression in Estrogen-Responsive Breast and Uterine Disease: From Bench to Treatment. Pharm. Rev. 2005, 57, 359–383. [Google Scholar] [CrossRef]
- Cos, S.; Alvarez-García, V.; González, A.; Alonso-González, C.; Martínez-Campa, C. Melatonin Modulation of Crosstalk among Malignant Epithelial, Endothelial and Adipose Cells in Breast Cancer (Review). Oncol. Lett. 2014, 8, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Rybinska, I.; Agresti, R.; Trapani, A.; Tagliabue, E.; Triulzi, T. Adipocytes in Breast Cancer, the Thick and the Thin. Cells 2020, 9, 560. [Google Scholar] [CrossRef] [Green Version]
- McTernan, P.G.; Anderson, L.A.; Anwar, A.J.; Eggo, M.C.; Crocker, J.; Barnett, A.H.; Stewart, P.M.; Kumar, S. Glucocorticoid Regulation of P450 Aromatase Activity in Human Adipose Tissue: Gender and Site Differences. J. Clin. Endocrinol. Metab. 2002, 87, 1327–1336. [Google Scholar] [CrossRef]
- González, A.; Alvarez-García, V.; Martínez-Campa, C.; Alonso-González, C.; Cos, S. Melatonin Promotes Differentiation of 3T3-L1 Fibroblasts. J. Pineal Res. 2012, 52, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Agarwal, V.R.; Mendelson, C.R.; Simpson, E.R. Estrogen Biosynthesis Proximal to a Breast Tumor Is Stimulated by PGE2 via Cyclic AMP, Leading to Activation of Promoter II of the CYP19 (Aromatase) Gene. Endocrinology 1996, 137, 5739–5742. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Sharma, D. The Microbiome–Estrogen Connection and Breast Cancer Risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut Microbial β-Glucuronidases Reactivate Estrogens as Components of the Estrobolome That Reactivate Estrogens. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Bonmati-Carrion, M.-A.; Tomas-Loba, A. Melatonin and Cancer: A Polyhedral Network Where the Source Matters. Antioxidants 2021, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wang, P.; Yan, J.; Liu, G.; Zeng, B.; Hussain, T.; Peng, C.; Yin, J.; Li, T.; Wei, H.; et al. Melatonin Alleviates Weanling Stress in Mice: Involvement of Intestinal Microbiota. J. Pineal Res. 2018, 64. [Google Scholar] [CrossRef]
- Wu, A.H.; Tseng, C.; Vigen, C.; Yu, Y.; Cozen, W.; Garcia, A.A.; Spicer, D. Gut Microbiome Associations with Breast Cancer Risk Factors and Tumor Characteristics: A Pilot Study. Breast Cancer Res. Treat. 2020, 182, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.F.; Reina-Pérez, I.; Astorga, J.M.; Rodríguez-Carrillo, A.; Plaza-Díaz, J.; Fontana, L. Breast Cancer and Its Relationship with the Microbiota. Int. J. Environ. Res. Public Health 2018, 15, 1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikó, E.; Kovács, T.; Sebő, É.; Tóth, J.; Csonka, T.; Ujlaki, G.; Sipos, A.; Szabó, J.; Méhes, G.; Bai, P. Microbiome—Microbial Metabolome—Cancer Cell Interactions in Breast Cancer—Familiar, But Unexplored. Cells 2019, 8, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapira, I.; Sultan, K.; Lee, A.; Taioli, E. Evolving Concepts: How Diet and the Intestinal Microbiome Act as Modulators of Breast Malignancy. Available online: https://www.hindawi.com/journals/isrn/2013/693920 (accessed on 17 December 2020).
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.-W.; Zhao, B.-C.; Yang, X.; Lei, S.-H.; Jiang, Y.-M.; Liu, K.-X. Relationships of Sleep Disturbance, Intestinal Microbiota, and Postoperative Pain in Breast Cancer Patients: A Prospective Observational Study. Sleep Breath 2020. [Google Scholar] [CrossRef]
- Li, G.; Yao, W.; Jiang, H. Short-Chain Fatty Acids Enhance Adipocyte Differentiation in the Stromal Vascular Fraction of Porcine Adipose Tissue. J. Nutr. 2014, 144, 1887–1895. [Google Scholar] [CrossRef] [Green Version]
- Yoo, E.J.; Chung, J.-J.; Choe, S.S.; Kim, K.H.; Kim, J.B. Down-Regulation of Histone Deacetylases Stimulates Adipocyte Differentiation. J. Biol. Chem. 2006, 281, 6608–6615. [Google Scholar] [CrossRef] [Green Version]
- Xiang, S.; Dauchy, R.T.; Hoffman, A.E.; Pointer, D.; Frasch, T.; Blask, D.E.; Hill, S.M. Epigenetic Inhibition of the Tumor Suppressor ARHI by Light at Night-Induced Circadian Melatonin Disruption Mediates STAT3-Driven Paclitaxel Resistance in Breast Cancer. J. Pineal Res. 2019, 67, e12586. [Google Scholar] [CrossRef]
- Subbaramaiah, K.; Howe, L.R.; Bhardwaj, P.; Du, B.; Gravaghi, C.; Yantiss, R.K.; Zhou, X.K.; Blaho, V.A.; Hla, T.; Yang, P.; et al. Obesity Is Associated with Inflammation and Elevated Aromatase Expression in the Mouse Mammary Gland. Cancer Prev. Res. 2011, 4, 329–346. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Álvarez, F.; Marzo-Sola, M.E. Role of Intestinal Microbiota in the Development of Multiple Sclerosis. Neurologia 2017, 32, 175–184. [Google Scholar] [CrossRef]
- Suraya, R.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Microbiome as a Target for Cancer Therapy. Integr. Cancer Ther. 2020, 19, 1534735420920721. [Google Scholar] [CrossRef]
- De Moreno de Leblanc, A.; Perdigón, G. The Application of Probiotic Fermented Milks in Cancer and Intestinal Inflammation. Proc. Nutr. Soc. 2010, 69, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G. Gut Dysbiosis Dysregulates Central and Systemic Homeostasis via Decreased Me Latonin and Suboptimal Mitochondria Functioning: Pathoetiological and Pathophysiological Implications. Melatonin Res. 2019, 2, 70–85. [Google Scholar] [CrossRef]
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Mutlu, E.; Engen, P.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. Circadian Disorganization Alters Intestinal Microbiota. PLoS ONE 2014, 9, e97500. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Reiter, R.J. COVID-19 Pathophysiology: Interactions of Gut Microbiome, Melatonin, Vitamin D, Stress, Kynurenine and the Alpha 7 Nicotinic Receptor: Treatment Implications. Melatonin Res. 2020, 3, 322–345. [Google Scholar] [CrossRef]
- Jing, Y.; Yang, D.; Bai, F.; Zhang, C.; Qin, C.; Li, D.; Wang, L.; Yang, M.; Chen, Z.; Li, J. Melatonin Treatment Alleviates Spinal Cord Injury-Induced Gut Dysbiosis in Mice. J. Neurotrauma 2019, 36, 2646–2664. [Google Scholar] [CrossRef] [PubMed]
- Ghareghani, M.; Reiter, R.J.; Zibara, K.; Farhadi, N. Latitude, Vitamin D, Melatonin, and Gut Microbiota Act in Concert to Initiate Multiple Sclerosis: A New Mechanistic Pathway. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M. The Gut–Brain Axis: The Role of Melatonin in Linking Psychiatric, Inflammatory and Neurodegenerative Conditions. Adv. Integr. Med. 2015, 2, 31–37. [Google Scholar] [CrossRef]
- Receptores de Reconocimiento de La Microbiota Intestinal En Situaciones Fisiopatológicas Del Epitelio Intestinal. Alteraciones Del Sistema Serotoninérgico—Repositorio Institucional de Documentos. Available online: https://zaguan.unizar.es/record/30690?ln=es# (accessed on 14 October 2020).
- Mannino, G.; Caradonna, F.; Cruciata, I.; Lauria, A.; Perrone, A.; Gentile, C. Melatonin Reduces Inflammatory Response in Human Intestinal Epithelial Cells Stimulated by Interleukin-1β. J. Pineal Res. 2019, 67, e12598. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Endometriosis Pathoetiology and Pathophysiology: Roles of Vitamin A, Estrogen, Immunity, Adipocytes, Gut Microbiome and Melatonergic Pathway on Mitochondria Regulation. Biomol. Concepts 2019, 10, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.R.; Siskin, J.; Gorenz, A.; Shaikh, M.; Raeisi, S.; Fogg, L.; Forsyth, C.; Keshavarzian, A. Disrupted Diurnal Oscillation of Gut-Derived Short Chain Fatty Acids in Shift Workers Drinking Alcohol: Possible Mechanism for Loss of Resiliency of Intestinal Barrier in Disrupted Circadian Host. Transl. Res. 2020, 221, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Wootla, B.; Anderson, G. Multiple Sclerosis, Gut Microbiota and Permeability: Role of Tryptophan Catabolites, Depression and the Driving Down of Local Melatonin. Curr. Pharm. Des. 2016, 22, 6134–6141. [Google Scholar] [CrossRef]
- Mei, Q.; Diao, L.; Xu, J.; Liu, X.; Jin, J. A Protective Effect of Melatonin on Intestinal Permeability Is Induced by Diclofenac via Regulation of Mitochondrial Function in Mice. Acta Pharm. Sin. 2011, 32, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Schernhammer, E.S.; Giobbie-Hurder, A.; Gantman, K.; Savoie, J.; Scheib, R.; Parker, L.M.; Chen, W.Y. A Randomized Controlled Trial of Oral Melatonin Supplementation and Breast Cancer Biomarkers. Cancer Causes Control 2012, 23, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Lobo, R.A. Hormone-Replacement Therapy: Current Thinking. Nat. Rev. Endocrinol. 2017, 13, 220–231. [Google Scholar] [CrossRef]
- Witt-Enderby, P.A.; Davis, V.L. Combination Hormone Replacement Therapy (HRT) and Melatonin to Prevent and Treat Mammary Cancer. U.S. Patent 8,618,083, 31 December 2013. [Google Scholar]
- Nduhirabandi, F.; Du Toit, E.F.; Blackhurst, D.; Marais, D.; Lochner, A. Chronic Melatonin Consumption Prevents Obesity-Related Metabolic Abnormalities and Protects the Heart against Myocardial Ischemia and Reperfusion Injury in a Prediabetic Model of Diet-Induced Obesity. J. Pineal Res. 2011, 50, 171–182. [Google Scholar] [CrossRef]
- Cos, S.; Martínez-Campa, C.; Mediavilla, M.D.; Sánchez-Barceló, E.J. Melatonin Modulates Aromatase Activity in MCF-7 Human Breast Cancer Cells. J. Pineal Res. 2005, 38, 136–142. [Google Scholar] [CrossRef]
- Alonso-Gonzalez, C.; Mediavilla, D.; Martinez-Campa, C.; Gonzalez, A.; Cos, S.; Sanchez-Barcelo, E.J. Melatonin Modulates the Cadmium-Induced Expression of MT-2 and MT-1 Metallothioneins in Three Lines of Human Tumor Cells (MCF-7, MDA-MB-231 and HeLa). Toxicol. Lett. 2008, 181, 190–195. [Google Scholar] [CrossRef]
- Alonso-González, C.; González, A.; Mazarrasa, O.; Güezmes, A.; Sánchez-Mateos, S.; Martínez-Campa, C.; Cos, S.; Sánchez-Barceló, E.J.; Mediavilla, M.D. Melatonin Prevents the Estrogenic Effects of Sub-Chronic Administration of Cadmium on Mice Mammary Glands and Uterus. J. Pineal Res. 2007, 42, 403–410. [Google Scholar] [CrossRef]
- Hansen, J. Night Shift Work and Risk of Breast Cancer. Curr. Environ. Health Rep. 2017, 4, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dauchy, R.T.; Tirrell, P.C.; Wu, S.S.; Lynch, D.T.; Jitawatanarat, P.; Burrington, C.M.; Dauchy, E.M.; Blask, D.E.; Greene, M.W. Light at Night Activates IGF-1R/PDK1 Signaling and Accelerates Tumor Growth in Human Breast Cancer Xenografts. Cancer Res. 2011, 71, 2622–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt-Enderby, P.A.; Davis, V.L.; Lapinsky, D. Anti-Cancer Tamoxifen-Melatonin Hybrid Ligand. U.S. Patent 8,785,501, 22 July 2014. [Google Scholar]
- Hasan, M.; Leak, R.K.; Stratford, R.E.; Zlotos, D.P.; Witt-Enderby, P.A. Drug Conjugates-an Emerging Approach to Treat Breast Cancer. Pharm. Res. Perspect. 2018, 6, e00417. [Google Scholar] [CrossRef] [PubMed]
- Innominato, P.F.; Lim, A.S.; Palesh, O.; Clemons, M.; Trudeau, M.; Eisen, A.; Wang, C.; Kiss, A.; Pritchard, K.I.; Bjarnason, G.A. The Effect of Melatonin on Sleep and Quality of Life in Patients with Advanced Breast Cancer. Support. Care Cancer 2016, 24, 1097–1105. [Google Scholar] [CrossRef]
- Hansen, M.V.; Andersen, L.T.; Madsen, M.T.; Hageman, I.; Rasmussen, L.S.; Bokmand, S.; Rosenberg, J.; Gögenur, I. Effect of Melatonin on Depressive Symptoms and Anxiety in Patients Undergoing Breast Cancer Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. Breast Cancer Res. Treat. 2014, 145, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Giobbie-Hurder, A.; Gantman, K.; Savoie, J.; Scheib, R.; Parker, L.M.; Schernhammer, E.S. A Randomized, Placebo-Controlled Trial of Melatonin on Breast Cancer Survivors: Impact on Sleep, Mood, and Hot Flashes. Breast Cancer Res. Treat. 2014, 145, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Chen, X.; Yan, J.; Li, M.; Liu, T.; Zhu, C.; Pan, G.; Guo, Q.; Yang, H.; Pei, M.; et al. Melatonin at Pharmacological Concentrations Suppresses Osteoclastogenesis via the Attenuation of Intracellular ROS. Osteoporos. Int. 2017, 28, 3325–3337. [Google Scholar] [CrossRef]
- Maria, S.; Witt-Enderby, P.A. Melatonin Effects on Bone: Potential Use for the Prevention and Treatment for Osteopenia, Osteoporosis, and Periodontal Disease and for Use in Bone-Grafting Procedures. J. Pineal Res. 2014, 56, 115–125. [Google Scholar] [CrossRef]
- Ben-David, M.A.; Elkayam, R.; Gelernter, I.; Pfeffer, R.M. Melatonin for Prevention of Breast Radiation Dermatitis: A Phase II, Prospective, Double-Blind Randomized Trial. Isr. Med. Assoc. J. 2016, 18, 188–192. [Google Scholar]
- Lissoni, P.; Tancini, G.; Paolorossi, F.; Mandalà, M.; Ardizzoia, A.; Malugani, F.; Giani, L.; Barni, S. Chemoneuroendocrine Therapy of Metastatic Breast Cancer with Persistent Thrombocytopenia with Weekly Low-Dose Epirubicin plus Melatonin: A Phase II Study. J. Pineal Res. 1999, 26, 169–173. [Google Scholar] [CrossRef]
- Liu, S.; Madu, C.O.; Lu, Y. The Role of Melatonin in Cancer Development. Oncomedicine 2018, 3, 37–47. [Google Scholar] [CrossRef]
- Malhotra, S.; Sawhney, G.; Pandhi, P. The Therapeutic Potential of Melatonin: A Review of the Science. Medscape Gen. Med. 2004, 6, 42. [Google Scholar]
- Madsen, M.T.; Hansen, M.V.; Andersen, L.T.; Hageman, I.; Rasmussen, L.S.; Bokmand, S.; Rosenberg, J.; Gögenur, I. Effect of Melatonin on Sleep in the Perioperative Period after Breast Cancer Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Sleep Med. 2016, 12, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Eslami-S, Z.; Majidzadeh-A, K.; Halvaei, S.; Babapirali, F.; Esmaeili, R. Microbiome and Breast Cancer: New Role for an Ancient Population. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakritz, J.R.; Poutahidis, T.; Levkovich, T.; Varian, B.J.; Ibrahim, Y.M.; Chatzigiagkos, A.; Mirabal, S.; Alm, E.J.; Erdman, S.E. Beneficial Bacteria Stimulate Host Immune Cells to Counteract Dietary and Genetic Predisposition to Mammary Cancer in Mice. Int. J. Cancer 2014, 135, 529–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdi, M.H.; Dallal, M.M.S.; Hassan, Z.M.; Holakuyee, M.; Amiri, S.A.; Abolhassani, M.; Mahdavi, M. Oral Administration of Lactobacillus Acidophilus Induces IL-12 Production in Spleen Cell Culture of BALB/c Mice Bearing Transplanted Breast Tumour. Br. J. Nutr. 2010, 104, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Masakazu, T.; Saya, H.; Ai, T.; Nobuaki, S.; Yasuo, H.; Keisei, A.; Takeshi, N.; Yutaka, T.; Norikazu, M.; Shozo, O.; et al. Probiotic Beverage with Soy Isoflavone Consumption for Breast Cancer Prevention: A Case-Control Study. Curr. Nutr. Food Sci. 2013, 9, 194–200. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laborda-Illanes, A.; Sánchez-Alcoholado, L.; Boutriq, S.; Plaza-Andrades, I.; Peralta-Linero, J.; Alba, E.; González-González, A.; Queipo-Ortuño, M.I. A New Paradigm in the Relationship between Melatonin and Breast Cancer: Gut Microbiota Identified as a Potential Regulatory Agent. Cancers 2021, 13, 3141. https://doi.org/10.3390/cancers13133141
Laborda-Illanes A, Sánchez-Alcoholado L, Boutriq S, Plaza-Andrades I, Peralta-Linero J, Alba E, González-González A, Queipo-Ortuño MI. A New Paradigm in the Relationship between Melatonin and Breast Cancer: Gut Microbiota Identified as a Potential Regulatory Agent. Cancers. 2021; 13(13):3141. https://doi.org/10.3390/cancers13133141
Chicago/Turabian StyleLaborda-Illanes, Aurora, Lidia Sánchez-Alcoholado, Soukaina Boutriq, Isaac Plaza-Andrades, Jesús Peralta-Linero, Emilio Alba, Alicia González-González, and María Isabel Queipo-Ortuño. 2021. "A New Paradigm in the Relationship between Melatonin and Breast Cancer: Gut Microbiota Identified as a Potential Regulatory Agent" Cancers 13, no. 13: 3141. https://doi.org/10.3390/cancers13133141
APA StyleLaborda-Illanes, A., Sánchez-Alcoholado, L., Boutriq, S., Plaza-Andrades, I., Peralta-Linero, J., Alba, E., González-González, A., & Queipo-Ortuño, M. I. (2021). A New Paradigm in the Relationship between Melatonin and Breast Cancer: Gut Microbiota Identified as a Potential Regulatory Agent. Cancers, 13(13), 3141. https://doi.org/10.3390/cancers13133141





