Analyzing the Opportunities to Target DNA Double-Strand Breaks Repair and Replicative Stress Responses to Improve Therapeutic Index of Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Standard of Care of Colorectal Cancer
1.2. Molecular Classification of CRC Tumors
2. Analyzing the Opportunities to Increase Therapeutic Index for CRC by Using DSB Repair Inhibitors
2.1. HR-Deficient Phenotypes in Colorectal Cancers
2.2. MMR-Deficient Phenotypes in Colorectal Cancers
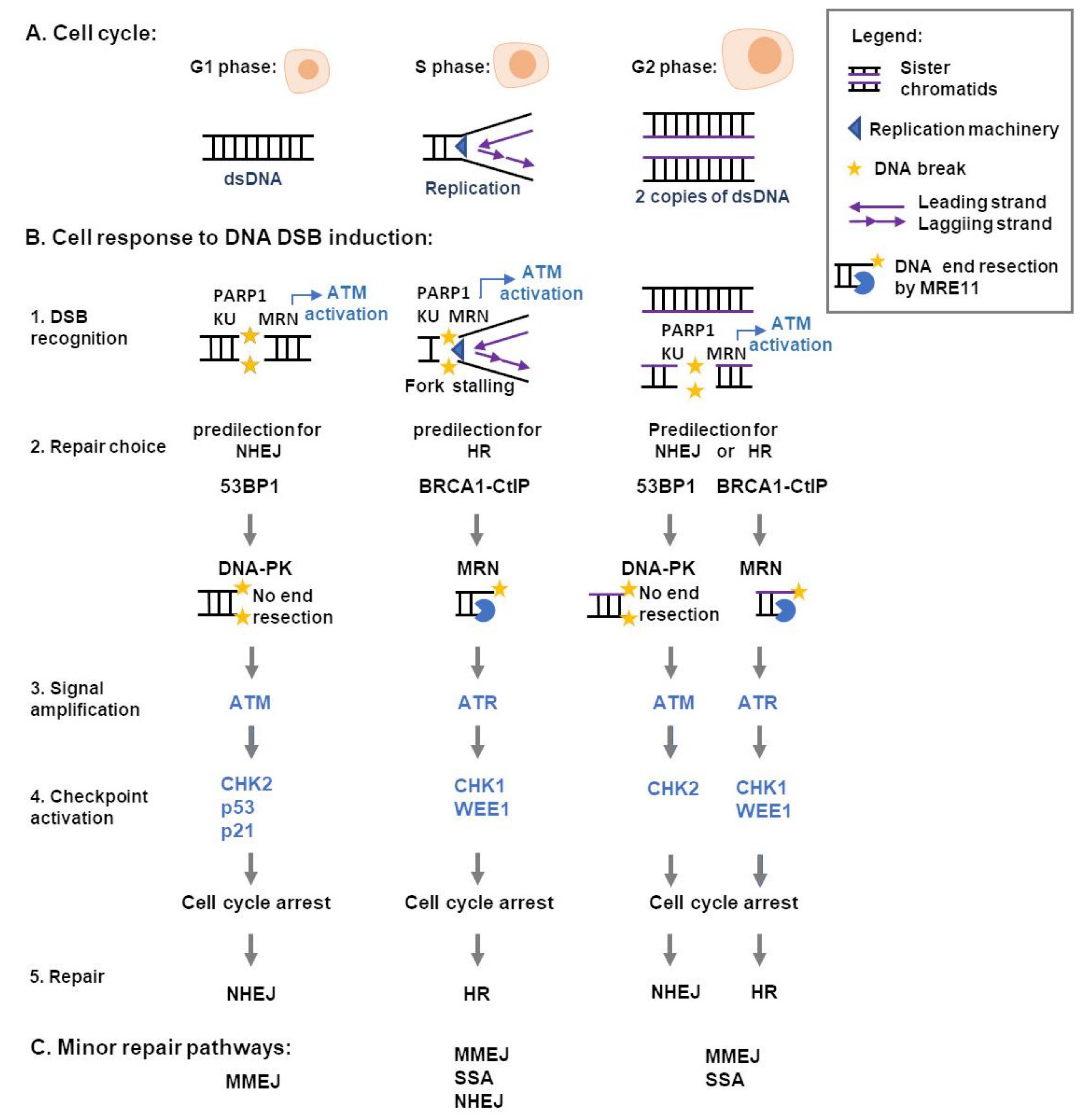
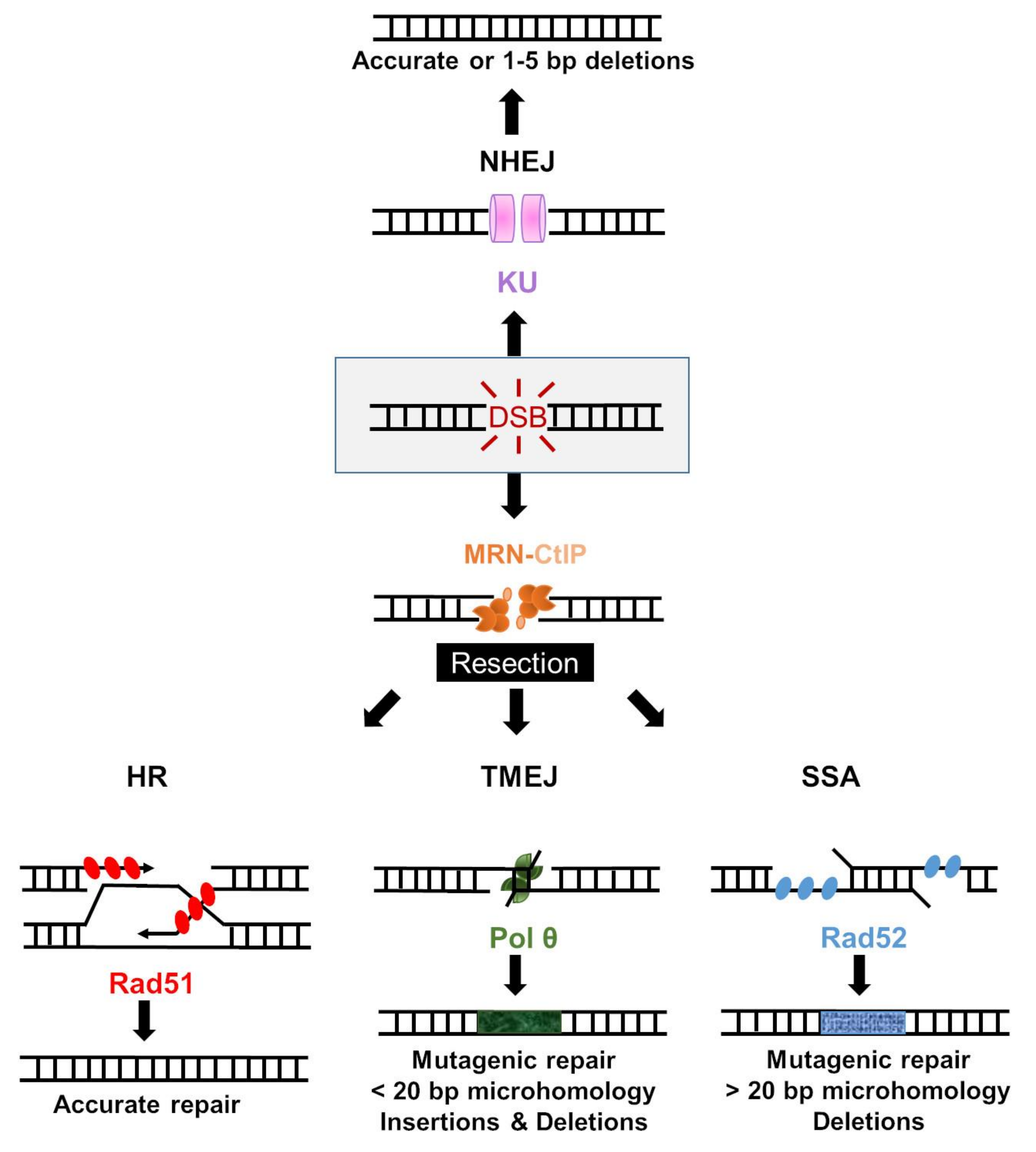
3. Cellular Responses to DNA DSB
4. DNA Replication Fork Arrest, Replication Stress, Checkpoint Activation, and Genome Instability in Cancer
5. Emerging DSB Repair-Targeting Therapies for Colorectal Cancer
5.1. Homologous Recombination Repair
5.1.1. Targeting MRE11 in CRC
5.1.2. RAD51 Inhibition
5.2. Alternative End-Joining
5.2.1. Polymerase Theta-Mediated End Joining
5.2.2. Polymerase Theta (Polθ) Inhibition
5.3. Single-Strand Annealing
RAD52 Inhibition
5.4. Cell Cycle Checkpoint Inhibition
5.4.1. ATR Inhibition
5.4.2. CHK1 Inhibition
5.4.3. ATM Inhibition
5.4.4. WEE1 Inhibition
6. Clinical Trials of DDR Inhibitors in CRC Patients
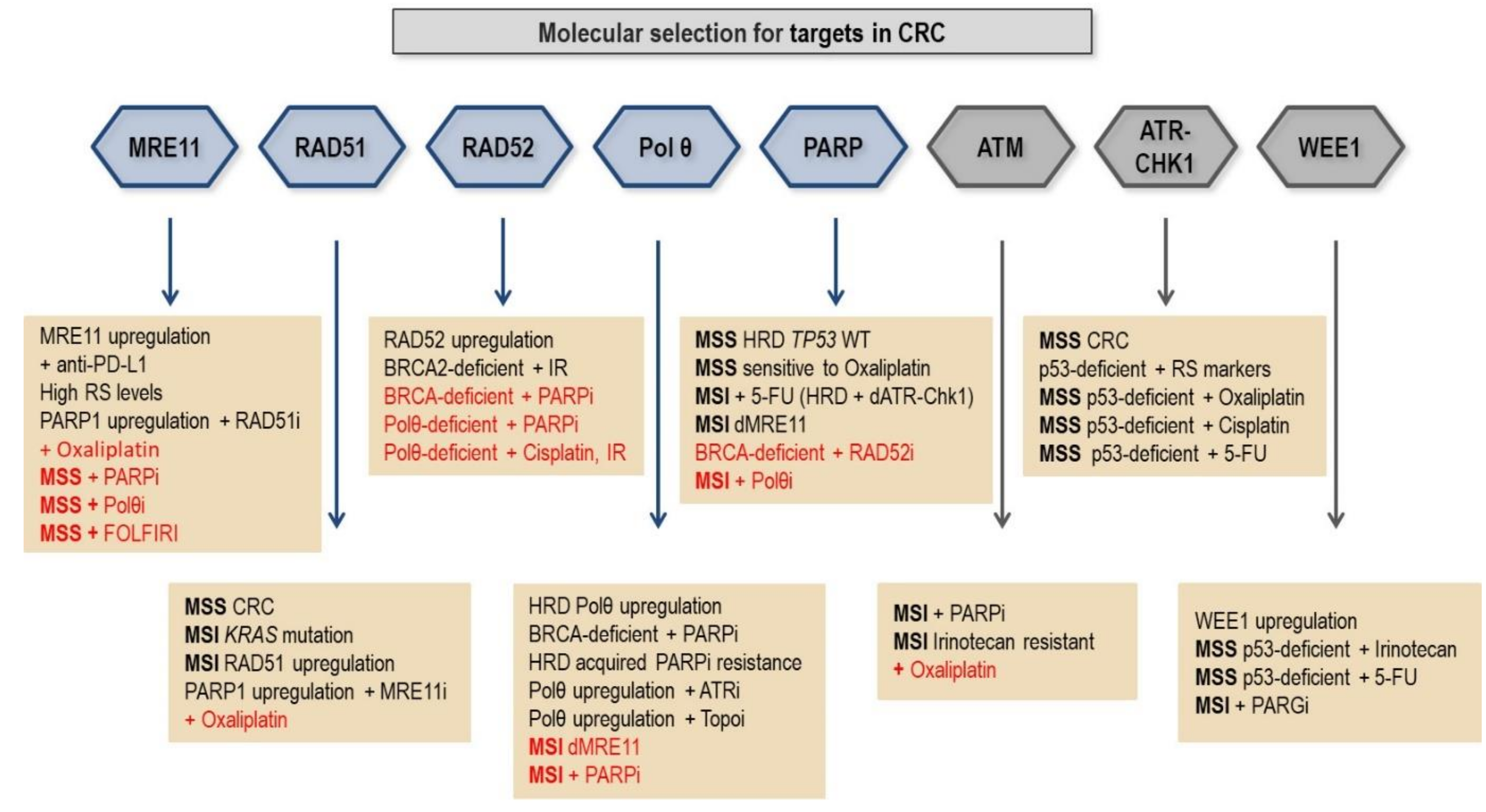
7. Molecular Selection of CRC Patients for Clinical Trials with DDR Inhibitors
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, C.E.; Hu, C.Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Arvelo, F.; Sojo, F.; Cotte, C. Biology of colorectal cancer. Ecancermedicalscience 2015, 9, 520. [Google Scholar] [CrossRef] [Green Version]
- Shaukat, A.; Kahi, C.J.; Burke, C.A.; Rabeneck, L.; Sauer, B.G.; Rex, D.K. ACG clinical guidelines: Colorectal cancer screening 2021. Am. J. Gastroenterol. 2021, 116, 458–479. [Google Scholar] [CrossRef]
- Feo, L.; Polcino, M.; Nash, G.M. Resection of the primary tumor in stage iv colorectal cancer: When is it necessary? Surg. Clin. N. Am. 2017, 97, 657–669. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, Y.L.; Chuang, J.P.; Lee, J.C. Differences in survival between colon and rectal cancer from SEER data. PLoS ONE 2013, 8, e78709. [Google Scholar] [CrossRef] [Green Version]
- West, N.P.; Hohenberger, W.; Weber, K.; Perrakis, A.; Finan, P.J.; Quirke, P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J. Clin. Oncol. 2010, 28, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.; Loprinzi, C.L.; Sargent, D.J.; Thomé, S.D.; Alberts, S.R.; Haller, D.G.; Benedetti, J.; Francini, G.; Shepherd, L.E.; Seitz, J.F.; et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J. Clin. Oncol. 2004, 22, 1797–1806. [Google Scholar] [CrossRef]
- Li, H.; Fu, G.; Wei, W.; Huang, Y.; Wang, Z.; Liang, T.; Tian, S.; Chen, H.; Zhang, W. Re-evaluation of the survival paradox between stage IIB/IIC and stage IIIA colon cancer. Front. Oncol. 2020, 10, 2468. [Google Scholar] [CrossRef]
- Benson, A.B.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Deming, D.; Farkas, L.; Garrido-Laguna, I.; Grem, J.L.; et al. NCCN guidelines version 2.2021 colon cancer nccn evidence blocks tm continue nccn guidelines panel disclosures. Cancers 2021, 13, 2638. [Google Scholar]
- André, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef] [Green Version]
- André, T.; De Gramont, A.; Vernerey, D.; Chibaudel, B.; Bonnetain, F.; Tijeras-Raballand, A.; Scriva, A.; Hickish, T.; Tabernero, J.; Van Laethem, J.L.; et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: Updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J. Clin. Oncol. 2015, 33, 4176–4187. [Google Scholar] [CrossRef]
- Grothey, A.; Sobrero, A.F.; Shields, A.F.; Yoshino, T.; Paul, J.; Taieb, J.; Souglakos, J.; Shi, Q.; Kerr, R.; Labianca, R.; et al. Duration of adjuvant chemotherapy for stage III colon cancer. N. Engl. J. Med. 2018, 378, 1177–1188. [Google Scholar] [CrossRef]
- Böckelman, C.; Engelmann, B.E.; Kaprio, T.; Hansen, T.F.; Glimelius, B. Risk of recurrence in patients with colon cancer stage II and III: A systematic review and meta-analysis of recent literature. Acta Oncol. 2015, 54, 5–16. [Google Scholar] [CrossRef]
- Dienstmann, R.; Mason, M.J.; Sinicrope, F.A.; Phipps, A.I.; Tejpar, S.; Nesbakken, A.; Danielsen, S.A.; Sveen, A.; Buchanan, D.D.; Clendenning, M.; et al. Prediction of overall survival in stage II and III colon cancer beyond TNM system: A retrospective, pooled biomarker study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1023–1031. [Google Scholar] [CrossRef]
- Chu, Q.D.; Zhou, M.; Medeiros, K.L.; Peddi, P.; Kavanaugh, M.; Wu, X.C. Poor survival in stage IIB/C (T4N0) compared to stage IIIA (T1-2 N1, T1N2a) colon cancer persists even after adjusting for adequate lymph nodes retrieved and receipt of adjuvant chemotherapy. BMC Cancer 2016, 16, 460. [Google Scholar] [CrossRef] [Green Version]
- Figueredo, A.; Charette, M.L.; Maroun, J.; Brouwers, M.C.; Zuraw, L. Adjuvant therapy for stage II colon cancer: A systematic review from the cancer care Ontario program in evidence-based care’s gastrointestinal cancer disease site group. J. Clin. Oncol. 2004, 22, 3395–3407. [Google Scholar] [CrossRef]
- Sepulveda, A.R.; Hamilton, S.R.; Allegra, C.J.; Grody, W.; Cushman-Vokoun, A.M.; Funkhouser, W.K.; Kopetz, S.E.; Lieu, C.; Lindor, N.M.; Minsky, B.D.; et al. Molecular biomarkers for the evaluation of colorectal cancer: Guideline from the american society for clinical pathology, college of american pathologists, association for molecular pathology, and american society of clinical oncology. J. Mol. Diagn. 2017, 19, 187–225. [Google Scholar] [CrossRef] [Green Version]
- Sorich, M.J.; Wiese, M.D.; Rowland, A.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: A meta-analysis of randomized, controlled trials. Ann. Oncol. 2015, 26, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Petrelli, F.; Coinu, A.; Di Bartolomeo, M.; Borgonovo, K.; Maggi, C.; Cabiddu, M.; Iacovelli, R.; Bossi, I.; Lonati, V.; et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur. J. Cancer 2015, 51, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–Mutated colorectal cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Raghav, K.; Loree, J.M.; Morris, J.S.; Overman, M.J.; Yu, R.; Meric-Bernstam, F.; Menter, D.; Korphaisarn, K.; Kee, B.; Muranyi, A.; et al. Validation of HER2 amplification as a predictive biomarker for anti–epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. JCO Precis. Oncol. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular basis of colorectal cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef] [Green Version]
- Mur, P.; García-Mulero, S.; Del Valle, J.; Magraner-Pardo, L.; Vidal, A.; Pineda, M.; Cinnirella, G.; Martín-Ramos, E.; Pons, T.; López-Doriga, A.; et al. Role of POLE and POLD1 in familial cancer. Genet. Med. 2020, 22, 2089–2100. [Google Scholar] [CrossRef]
- Weren, R.D.A.; Ligtenberg, M.J.L.; Kets, C.M.; De Voer, R.M.; Verwiel, E.T.P.; Spruijt, L.; Van Zelst-Stams, W.A.G.; Jongmans, M.C.; Gilissen, C.; Hehir-Kwa, J.Y.; et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat. Genet. 2015, 47, 668–671. [Google Scholar] [CrossRef]
- Pilati, C.; Shinde, J.; Alexandrov, L.B.; Assié, G.; André, T.; Hélias-Rodzewicz, Z.; Ducoudray, R.; Le Corre, D.; Zucman-Rossi, J.; Emile, J.F.; et al. Mutational signature analysis identifies MUTYH deficiency in colorectal cancers and adrenocortical carcinomas. J. Pathol. 2017, 242, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Becht, E.; De Reyniès, A.; Giraldo, N.A.; Pilati, C.; Buttard, B.; Lacroix, L.; Selves, J.; Sautès-Fridman, C.; Laurent-Puig, P.; Fridman, W.H. Immune and stromal classification of Colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin. Cancer Res. 2016, 22, 4057–4066. [Google Scholar] [CrossRef] [Green Version]
- Vodicka, P.; Vodenkova, S.; Buchler, T.; Vodickova, L. DNA repair capacity and response to treatment of colon cancer. Pharmacogenomics 2019, 20, 1225–1233. [Google Scholar] [CrossRef]
- Punt, C.J.A.; Koopman, M.; Vermeulen, L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2017, 14, 235–246. [Google Scholar] [CrossRef]
- Mouillet-Richard, S.; Laurent-Puig, P. YAP/TAZ signalling in colorectal cancer: Lessons from consensus molecular subtypes. Cancers 2020, 12, 3160. [Google Scholar] [CrossRef]
- Jongen, J.M.J.; Van der Waals, L.M.; Trumpi, K.; Laoukili, J.; Peters, N.A.; van Schelven, S.J.S.; Govaert, K.M.; Rinkes, I.H.M.B.; Kranenburg, O. Downregulation of DNA repair proteins and increased DNA damage in hypoxic colon cancer cells is a therapeutically exploitable vulnerability. Oncotarget 2017, 8, 86296–86311. [Google Scholar] [CrossRef] [Green Version]
- Coebergh Van Den Braak, R.R.J.; Ten Hoorn, S.; Sieuwerts, A.M.; Tuynman, J.B.; Smid, M.; Wilting, S.M.; Martens, J.W.M.; Punt, C.J.A.; Foekens, J.A.; Medema, J.P.; et al. Interconnectivity between molecular subtypes and tumor stage in colorectal cancer. BMC Cancer 2020, 20. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Ou, F.-S.; Venook, A.P.; Hochster, H.S.; Niedzwiecki, D.; Goldberg, R.M.; Mayer, R.J.; Bertagnolli, M.M.; Blanke, C.D.; Zemla, T.; et al. Impact of consensus molecular subtyping (CMS) on overall survival (OS) and progression free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J. Clin. Oncol. 2017, 35, 3511. [Google Scholar] [CrossRef]
- Song, N.; Pogue-Geile, K.L.; Gavin, P.G.; Yothers, G.; Kim, S.R.; Johnson, N.L.; Lipchik, C.; Allegra, C.J.; Petrelli, N.J.; O’Connell, M.J.; et al. Clinical outcome from oxaliplatin treatment in stage II/III colon cancer according to intrinsic subtypes: Secondary analysis of NSABP C-07/NRG oncology randomized clinical trial. JAMA Oncol. 2016, 2, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, Q.; Zhang, L.; Mo, S.; Cai, S.; Huang, D.; Peng, J. Immunohistochemistry-based consensus molecular subtypes as a prognostic and predictive biomarker for adjuvant chemotherapy in patients with stage II colorectal cancer. Oncologist 2020, 25, e1968–e1979. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, S.; Wirapati, P.; Lenz, H.J.; Neureiter, D.; Fischer von Weikersthal, L.; Decker, T.; Kiani, A.; Kaiser, F.; Al-Batran, S.; Heintges, T.; et al. Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial. Ann. Oncol. 2019, 30, 1796–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, Y.W.; Cattoglio, C.; Tjian, R. The intertwined roles of transcription and repair proteins. Mol. Cell 2013, 52, 291–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckelmann, B.J.; Bacolla, A.; Wang, H.; Ye, Z.; Guerrero, E.N.; Jiang, W.; El-Zein, R.; Hegde, M.L.; Tomkinson, A.E.; Tainer, J.A.; et al. XRCC1 promotes replication restart, nascent fork degradation and mutagenic DNA repair in BRCA2-deficient cells. NAR Cancer 2020, 2. [Google Scholar] [CrossRef] [PubMed]
- Negrei, C.; Hudita, A.; Ginghina, O.; Galateanu, B.; Voicu, S.N.; Stan, M.; Costache, M.; Fenga, C.; Drakoulis, N.; Tsatsakis, A.M. Colon cancer cells gene expression signature as response to 5-fluorouracil, oxaliplatin, and folinic acid treatment. Front. Pharmacol. 2016, 7, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyatt, M.D.; Wilson, D.M. Participation of DNA repair in the response to 5-fluorouracil. Cell. Mol. life Sci. 2009, 66, 788–799. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, U.S.; Dyczkowski, J.; Beißbarth, T.; Gaedcke, J.; Mansour, W.Y.; Borgmann, K.; Dobbelstein, M. 5-Fluorouracil sensitizes colorectal tumor cells towards double stranded DNA breaks by interfering with homologous recombination repair. Impact J. 2015, 6, 12574–12586. [Google Scholar] [CrossRef]
- Chválová, K.; Brabec, V.; Kašpárková, J. Mechanism of the formation of DNA-protein cross-links by antitumor cisplatin. Nucleic Acids Res. 2007, 35, 1812–1821. [Google Scholar] [CrossRef] [Green Version]
- Ming, X.; Groehler, A.; Michaelson-Richie, E.D.; Villalta, P.W.; Campbell, C.; Tretyakova, N.Y. Mass spectrometry based proteomics study of cisplatin-induced dna-protein cross-linking in human fibrosarcoma (HT1080) cells. Chem. Res. Toxicol. 2017, 30, 980–995. [Google Scholar] [CrossRef] [Green Version]
- Riddell, I.A.; Lippard, S.J. 7. Medicinal chemistry of gold anticancer metallodrugs. In Metallo-Drugs: Development and Action of Anticancer Agents; De Gruyter: Berlin, Germany, 2018; Volume 18, pp. 199–218. ISBN 9783110470734. [Google Scholar]
- Li, F.; Jiang, T.; Li, Q.; Ling, X. Camptothecin (CPT) and its derivatives are known to target topoisomerase I (Top1) as their mechanism of action: Did we miss something in CPT analogue molecular targets for treating human disease such as cancer? Am. J. Cancer Res. 2017, 7, 2350–2394. [Google Scholar]
- Reilly, N.M.; Novara, L.; Di Nicolantonio, F.; Bardelli, A. Exploiting DNA repair defects in colorectal cancer. Mol. Oncol. 2019, 13, 681–700. [Google Scholar] [CrossRef] [Green Version]
- Heeke, A.L.; Pishvaian, M.J.; Lynce, F.; Xiu, J.; Brody, J.R.; Chen, W.-J.; Baker, T.M.; Marshall, J.L.; Isaacs, C. Prevalence of homologous recombination–related gene mutations across multiple cancer types. JCO Precis. Oncol. 2018, 2, 1–13. [Google Scholar] [CrossRef]
- Muzny, D.M.; Bainbridge, M.N.; Chang, K.; Dinh, H.H.; Drummond, J.A.; Fowler, G.; Kovar, C.L.; Lewis, L.R.; Morgan, M.B.; Newsham, I.F.; et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Lamberti, G.; Andrini, E.; Sisi, M.; Di Federico, A.; Ricciuti, B. Targeting DNA damage response and repair genes to enhance anticancer immunotherapy: Rationale and clinical implication. Future Oncol. 2020, 16, 1751–1766. [Google Scholar] [CrossRef] [PubMed]
- Bianco, J.N.; Bergoglio, V.; Lin, Y.L.; Pillaire, M.J.; Schmitz, A.L.; Gilhodes, J.; Lusque, A.; Mazières, J.; Lacroix-Triki, M.; Roumeliotis, T.I.; et al. Overexpression of claspin and timeless protects cancer cells from replication stress in a checkpoint-independent manner. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randon, G.; Fucà, G.; Rossini, D.; Raimondi, A.; Pagani, F.; Perrone, F.; Tamborini, E.; Busico, A.; Peverelli, G.; Morano, F.; et al. Prognostic impact of ATM mutations in patients with metastatic colorectal cancer. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlDubayan, S.H.; Giannakis, M.; Moore, N.D.; Han, G.C.; Reardon, B.; Hamada, T.; Mu, X.J.; Nishihara, R.; Qian, Z.; Liu, L.; et al. Inherited DNA-repair defects in colorectal cancer. Am. J. Hum. Genet. 2018, 102, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhao, J.; Wang, G.; Zhang, F.; Zhang, Z.; Zhang, F.; Zhang, Y.; Dong, H.; Zhao, X.; Duan, J.; et al. Comutations in DNA damage response pathways serve as potential biomarkers for immune checkpoint blockade. Cancer Res. 2018, 78, 6486–6496. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wang, C.; Zhang, Y.; Xu, L.; Fang, W.; Zhu, Y.; Zheng, Y.; Chen, X.; Xie, X.; Hu, X.; et al. Genomic signatures reveal DNA damage response deficiency in colorectal cancer brain metastases. Nat. Commun. 2019, 10, 3190. [Google Scholar] [CrossRef] [Green Version]
- Chartron, E.; Theillet, C.; Guiu, S.; Jacot, W. Targeting homologous repair deficiency in breast and ovarian cancers: Biological pathways, preclinical and clinical data. Crit. Rev. Oncol. Hematol. 2019, 133, 58–73. [Google Scholar] [CrossRef]
- Lin, P.C.; Yeh, Y.M.; Chan, R.H.; Lin, B.W.; Chen, P.C.; Pan, C.C.; Shen, M.R. Sequential and co-occurring DNA damage response genetic mutations impact survival in stage III colorectal cancer patients receiving adjuvant oxaliplatin-based chemotherapy. BMC Cancer 2021, 21, 217. [Google Scholar] [CrossRef]
- Faraoni, I.; Graziani, G. Role of BRCA mutations in cancer treatment with poly(ADP-ribose) polymerase (PARP) inhibitors. Cancers 2018, 10, 487. [Google Scholar] [CrossRef] [Green Version]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, H.; Lord, C.J.; Tutt, A.H.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 2016, 16, 110–120. [Google Scholar] [CrossRef]
- Laporte, G.A.; Leguisamo, N.M.; Kalil, A.N.; Saffi, J. Clinical importance of DNA repair in sporadic colorectal cancer. Crit. Rev. Oncol. Hematol. 2018, 126, 168–185. [Google Scholar] [CrossRef]
- Koopman, M.; Kortman, G.A.M.; Mekenkamp, L.; Ligtenberg, M.J.L.; Hoogerbrugge, N.; Antonini, N.F.; Punt, C.J.A.; Van Krieken, J.H.J.M. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer 2009, 100, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Hegde, M.; Ferber, M.; Mao, R.; Samowitz, W.; Ganguly, A. ACMG technical standards and guidelines for genetic testing for inherited colorectal cancer (Lynch syndrome, familial adenomatous polyposis, and MYH-associated polyposis). Genet. Med. 2014, 16, 101–116. [Google Scholar] [CrossRef] [Green Version]
- Roth, A.D.; Tejpar, S.; Delorenzi, M.; Yan, P.; Fiocca, R.; Klingbiel, D.; Dietrich, D.; Biesmans, B.; Bodoky, G.; Barone, C.; et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: Results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J. Clin. Oncol. 2010, 28, 466–474. [Google Scholar] [CrossRef]
- Ribic, C.M.; Sargent, D.J.; Moore, M.J.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent-Puig, P.; Gryfe, R.; Shepherd, L.E.; et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2003, 349, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Sargent, D.J.; Marsoni, S.; Monges, G.; Thibodeau, S.N.; Labianca, R.; Hamilton, S.R.; French, A.J.; Kabat, B.; Foster, N.R.; Torri, V.; et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J. Clin. Oncol. 2010, 28, 3219–3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Hong, Y.S.; Kim, H.J.; Kim, K.-P.; Lee, J.L.; Park, S.J.; Lim, S.B.; Park, I.J.; Kim, C.W.; Yoon, Y.S.; et al. Defective mismatch repair status was not associated with DFS and OS in stage II colon cancer treated with adjuvant chemotherapy. Ann. Surg. Oncol. 2015, 22, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, G.; Southward, K.; Handley, K.; Magill, L.; Beaumont, C.; Stahlschmidt, J.; Richman, S.; Chambers, P.; Seymour, M.; Kerr, D.; et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J. Clin. Oncol. 2011, 29, 1261–1270. [Google Scholar] [CrossRef]
- Hou, J.T.; Zhao, L.N.; Zhang, D.J.; Lv, D.Y.; He, W.L.; Chen, B.; Li, H.B.; Li, P.R.; Chen, L.Z.; Chen, X.L. Prognostic value of mismatch repair genes for patients with colorectal cancer: Meta-analysis. Technol. Cancer Res. Treat. 2018, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; Sinicrope, F.A. Prognostic and predictive values of mismatch repair deficiency in non-metastatic colorectal cancer. Cancers 2021, 13, 300. [Google Scholar] [CrossRef]
- Weisenberger, D.J.; Siegmund, K.D.; Campan, M.; Young, J.; Long, T.I.; Faasse, M.A.; Kang, G.H.; Widschwendter, M.; Weener, D.; Buchanan, D.; et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat. Genet. 2006, 38, 787–793. [Google Scholar] [CrossRef]
- Pylayeva-Gupta, Y.; Grabocka, E.; Bar-Sagi, D. RAS oncogenes: Weaving a tumorigenic web. Nat. Rev. Cancer 2011, 11, 761–774. [Google Scholar] [CrossRef] [Green Version]
- Hills, S.A.; Diffley, J.F.X. DNA replication and oncogene-induced replicative stress. Curr. Biol. 2014, 24, R435–R444. [Google Scholar] [CrossRef] [Green Version]
- Primo, L.M.F.; Teixeira, L.K. Dna replication stress: Oncogenes in the spotlight. Genet. Mol. Biol. 2020, 43, 1–14. [Google Scholar] [CrossRef]
- Arai, H.; Elliott, A.; Xiu, J.; Wang, J.; Battaglin, F.; Kawanishi, N.; Soni, S.; Zhang, W.; Millstein, J.; Sohal, D.; et al. The landscape of alterations in DNA damage response pathways in colorectal cancer. Clin. Cancer Res. 2021, 27, 3234–3242. [Google Scholar] [CrossRef]
- Boland, P.M.; Yurgelun, M.B.; Boland, C.R. Recent progress in Lynch syndrome and other familial colorectal cancer syndromes. CA Cancer J. Clin. 2018, 68, 217–231. [Google Scholar] [CrossRef]
- Sinha, S.; Villarreal, D.; Shim, E.Y.; Lee, S.E. Risky business: Microhomology-mediated end joining. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2016, 788, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Leguisamo, N.M.; Gloria, H.C.; Kalil, A.N.; Martins, T.V.; Azambuja, D.B.; Meira, L.B.; Saffi, J. Base excision repair imbalance in colorectal cancer has prognostic value and modulates response to chemotherapy. Oncotarget 2017, 8, 54199–54214. [Google Scholar] [CrossRef]
- Fujimori, H.; Hyodo, M.; Matsuno, Y.; Shimizu, A.; Minakawa, Y.; Atsumi, Y.; Nakatsu, Y.; Tsuzuki, T.; Murakami, Y.; Yoshioka, K. Ichi mismatch repair dependence of replication stress-associated DSB recognition and repair. Heliyon 2019, 5, e03057. [Google Scholar] [CrossRef] [Green Version]
- Matsuno, Y.; Atsumi, Y.; Shimizu, A.; Katayama, K.; Fujimori, H.; Hyodo, M.; Minakawa, Y.; Nakatsu, Y.; Kaneko, S.; Hamamoto, R.; et al. Replication stress triggers microsatellite destabilization and hypermutation leading to clonal expansion in vitro. Nat. Commun. 2019, 10, 3925. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.-M.; Kang, Y.; Park, J.; Sung, Y.; Kim, D.; Seo, Y.; Lee, E.A.; Ra, J.S.; Amarsanaa, E.; Park, Y.-U.; et al. MSH2-MSH3 Promotes DNA end Resection during HR and Blocks TMEJ through Interaction with SMARCAD1 and EXO1. Available online: https://www.biorxiv.org/content/10.1101/2021.04.23.441074v1 (accessed on 11 June 2021).
- Yoshioka, K.-I.; Matsuno, Y. Genomic destabilization and its associated mutagenesis increase with senescence-associated phenotype expression. Cancer Sci. 2021, 112, 515–522. [Google Scholar] [CrossRef]
- Chan, Y.W.; Fugger, K.; West, S.C. Unresolved recombination intermediates lead to ultra-fine anaphase bridges, chromosome breaks and aberrations. Nat. Cell Biol. 2018, 20, 92–103. [Google Scholar] [CrossRef]
- Yoshioka, K.I.; Matsuno, Y.; Hyodo, M.; Fujimori, H. Genomic-destabilization-associated mutagenesis and clonal evolution of cells with mutations in tumor-suppressor genes. Cancers 2019, 11, 1643. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.S.; Shi, C. Molecular Testing in Colorectal Cancer. In Diagnostic Molecular Pathology; Elsevier: Cambridge, MA, USA, 2017; pp. 305–320. [Google Scholar]
- Vilar, E.; Gruber, S.B. Microsatellite instability in colorectal cancerthe stable evidence. Nat. Rev. Clin. Oncol. 2010, 7, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Chubb, D.; Broderick, P.; Dobbins, S.E.; Frampton, M.; Kinnersley, B.; Penegar, S.; Price, A.; Ma, Y.P.; Sherborne, A.L.; Palles, C.; et al. Rare disruptive mutations and their contribution to the heritable risk of colorectal cancer. Nat. Commun. 2016, 7, 11883. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.T.; Zhu, F.; Wu, X.; Yuan, F.; Gao, Y.; Gu, L.; Li, G.M.; Lee, T.H.; Her, C. hMRE11 deficiency leads to microsatellite instability and defective DNA mismatch repair. EMBO Rep. 2005, 6, 438–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavelitz, T.; Renfro, L.; Foster, N.R.; Caracol, A.; Welsch, P.; Lao, V.V.; Grady, W.B.; Niedzwiecki, D.; Saltz, L.B.; Bertagnolli, M.M.; et al. MRE11-deficiency associated with improved long-term disease free survival and overall survival in a subset of stage III colon cancer patients in randomized CALGB 89803 trial. PLoS ONE 2014, 9, e108483. [Google Scholar] [CrossRef] [PubMed]
- Ihara, K.; Yamaguchi, S.; Ueno, N.; Tani, Y.; Shida, Y.; Ogata, H.; Domeki, Y.; Okamoto, K.; Nakajima, M.; Sasaki, K.; et al. Expression of DNA double-strand break repair proteins predicts the response and prognosis of colorectal cancer patients undergoing oxaliplatin-based chemotherapy. Oncol. Rep. 2016, 35, 1349–1355. [Google Scholar] [CrossRef] [Green Version]
- Altan, B.; Yokobori, T.; Ide, M.; Bai, T.; Yanoma, T.; Kimura, A.; Kogure, N.; Suzuki, M.; Bao, P.; Mochiki, E.; et al. High expression of MRE11-RAD50-NBS1 is associated with poor prognosis and chemoresistance in gastric cancer. Int. Inst. Anticancer. Res. 2016, 36, 5237–5247. [Google Scholar]
- Ho, V.; Chung, L.; Singh, A.; Lea, V.; Abubakar, A.; Lim, S.H.; Ng, W.; Lee, M.; De Souza, P.; Shin, J.-S.S.; et al. Overexpression of the MRE11-RAD50-NBS1 (MRN) complex in rectal cancer correlates with poor response to neoadjuvant radiotherapy and prognosis. BMC Cancer 2018, 18, 869. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.; Arbman, G.; Sun, X.F. RAD50/MRE11/NBS1 proteins in relation to tumour development and prognosis in patients with microsatellite stable colorectal cancer. Histol. Histopathol. 2008, 23, 1495–1502. [Google Scholar] [CrossRef]
- Vilar, E.; Bartnik, C.M.; Stenzel, S.L.; Raskin, L.; Ahn, J.; Moreno, V.; Mukherjee, B.; Iniesta, M.D.; Morgan, M.A.; Rennert, G.; et al. MRE11 deficiency increases sensitivity to poly(ADP-ribose) polymerase inhibition in microsatellite unstable colorectal cancers. Cancer Res. 2011, 71, 2632–2642. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.W.; Kopsida, M.; Liu, Y.B.; Zhang, H.; Gao, J.F.; Arbman, G.; Cao, S.Y.W.; Li, Y.; Zhou, Z.G.; Sun, X.F. Prognostic heterogeneity of MRE11 based on the location of primary colorectal cancer is caused by activation of different immune signals. Front. Oncol. 2020, 9, 1465. [Google Scholar] [CrossRef]
- McPherson, L.A.; Shen, Y.; Ford, J.M. Poly (ADP-ribose) polymerase inhibitor LT-626: Sensitivity correlates with MRE11 mutations and synergizes with platinums and irinotecan in colorectal cancer cells. Cancer Lett. 2014, 343, 217–223. [Google Scholar] [CrossRef]
- Kantidze, O.L.; Velichko, A.K.; Luzhin, A.V.; Petrova, N.V.; Razin, S.V. Synthetically lethal interactions of ATM, ATR, and DNA-PKcs. Trends Cancer 2018, 4, 755–768. [Google Scholar] [CrossRef]
- Chanut, P.; Britton, S.; Coates, J.; Jackson, S.P.; Calsou, P. Coordinated nuclease activities counteract Ku at single-ended DNA double-strand breaks. Nat. Commun. 2016, 7, 12889. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Liu, C.; Chen, S.H.; Kassab, M.A.; Hoff, J.D.; Walter, N.G.; Yu, X. Super-resolution imaging identifies PARP1 and the Ku complex acting as DNA double-strand break sensors. Nucleic Acids Res. 2018, 46, 3446–3457. [Google Scholar] [CrossRef]
- Zhou, H.; Kawamura, K.; Yanagihara, H.; Kobayashi, J.; Zhang-Akiyama, Q.-M. NBS1 is regulated by two kind of mechanisms: ATM-dependent complex formation with MRE11 and RAD50, and cell cycle–dependent degradation of protein. J. Radiat. Res. 2017, 58, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Falck, J.; Coates, J.; Jackson, S.P. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 2005, 434, 605–611. [Google Scholar] [CrossRef]
- Buscemi, G.; Perego, P.; Carenini, N.; Nakanishi, M.; Chessa, L.; Chen, J.; Khanna, K.K.; Delia, D. Activation of ATM and Chk2 kinases in relation to the amount of DNA strand breaks. Oncogene 2004, 23, 7691–7700. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, S.; Rotman, G.; Ogawa, A.; Shiloh, Y.; Tamai, K.; Elledge, S.J. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2000, 97, 10389–10394. [Google Scholar] [CrossRef] [Green Version]
- Mak, T.W. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 2000, 287, 1824–1827. [Google Scholar] [CrossRef]
- Shiotani, B.; Zou, L. Single-stranded DNA orchestrates an ATM-to-ATR switch at DNA breaks. Mol. Cell 2009, 33, 547–558. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kumagai, A.; Dunphy, W.G. Positive regulation of Wee1 by Chk1 and 14-3-3 proteins. Mol. Biol. Cell 2001, 12, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Rothblum-Oviatt, C.J.; Ryan, C.E.; Piwnica-Worms, H. 14-3-3 Binding regulates catalytic activity of human Wee1 kinase. Cell Growth Differ. 2001, 12, 581–589. [Google Scholar] [PubMed]
- Shibata, A.; Barton, O.; Noon, A.T.; Dahm, K.; Deckbar, D.; Goodarzi, A.A.; Löbrich, M.; Jeggo, P.A. Role of ATM and the damage response mediator proteins 53BP1 and MDC1 in the maintenance of G2/M checkpoint arrest. Mol. Cell. Biol. 2010, 30, 3371–3383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jette, N.; Lees-Miller, S.P. The DNA-dependent protein kinase: A multifunctional protein kinase with roles in DNA double strand break repair and mitosis. Prog. Biophys. Mol. Biol. 2015, 117, 194–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuki, Y.; Jeggo, P.A.; Uchihara, Y.; Takata, M.; Shibata, A. DNA double-strand break end resection: A critical relay point for determining the pathway of repair and signaling. Genome Instab. Dis. 2020, 1, 155–171. [Google Scholar] [CrossRef]
- Teixeira-Silva, A.; Ait Saada, A.; Hardy, J.; Iraqui, I.; Nocente, M.C.; Fréon, K.; Lambert, S.A.E. The end-joining factor Ku acts in the end-resection of double strand break-free arrested replication forks. Nat. Commun. 2017, 8, 1982. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, R.A.; Myler, L.R.; Soniat, M.M.; Makharashvili, N.; Lee, L.; Lees-Miller, S.P.; Finkelstein, I.J.; Paull, T.T. DNA-dependent protein kinase promotes DNA end processing by MRN and CtIP. Sci. Adv. 2020, 6, eaay0922. [Google Scholar] [CrossRef] [Green Version]
- Shibata, A.; Jeggo, P.A. Roles for the DNA-PK complex and 53BP1 in protecting ends from resection during DNA double-strand break repair. J. Radiat. Res. 2020, 61, 718–726. [Google Scholar] [CrossRef]
- Ceccaldi, R.; Rondinelli, B.; D’Andrea, A.D. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 2016, 26, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Iliakis, G.; Murmann, T.; Soni, A. Alternative end-joining repair pathways are the ultimate backup for abrogated classical non-homologous end-joining and homologous recombination repair: Implications for the formation of chromosome translocations. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2015, 793, 166–175. [Google Scholar] [CrossRef]
- Feng, L.; Li, N.; Li, Y.; Wang, J.; Gao, M.; Wang, W.; Chen, J. Cell cycle-dependent inhibition of 53BP1 signaling by BRCA1. Cell Discov. 2015, 1, 15019. [Google Scholar] [CrossRef]
- Gupta, R.; Somyajit, K.; Narita, T.; Maskey, E.; Stanlie, A.; Kremer, M.; Typas, D.; Lammers, M.; Mailand, N.; Nussenzweig, A.; et al. DNA repair network analysis reveals shieldin as a key regulator of NHEJ and PARP inhibitor sensitivity. Cell 2018, 173, 972–988. [Google Scholar] [CrossRef] [Green Version]
- Escribano-Díaz, C.; Orthwein, A.; Fradet-Turcotte, A.; Xing, M.; Young, J.T.F.; Tkáč, J.; Cook, M.A.; Rosebrock, A.P.; Munro, M.; Canny, M.D.; et al. A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Mol. Cell 2013, 49, 872–883. [Google Scholar] [CrossRef] [Green Version]
- Hoa, N.N.; Kobayashi, J.; Omura, M.; Hirakawa, M.; Yang, S.H.; Komatsu, K.; Paull, T.T.; Takeda, S.; Sasanuma, H. BRCA1 and CtIP are both required to recruit Dna2 at double-strand breaks in homologous recombination. PLoS ONE 2015, 10, e0124495. [Google Scholar] [CrossRef]
- Tomimatsu, N.; Mukherjee, B.; Harris, J.L.; Boffo, F.L.; Hardebeck, M.C.; Potts, P.R.; Khanna, K.K.; Burma, S. DNA-damage-induced degradation of EXO1 exonuclease limits DNA end resection to ensure accurate DNA repair. J. Biol. Chem. 2017, 292, 10779–10790. [Google Scholar] [CrossRef] [Green Version]
- Nimonkar, A.V.; Genschel, J.; Kinoshita, E.; Polaczek, P.; Campbell, J.L.; Wyman, C.; Modrich, P.; Kowalczykowski, S.C. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 2011, 25, 350–362. [Google Scholar] [CrossRef] [Green Version]
- Shibata, A.; Moiani, D.; Arvai, A.S.; Perry, J.; Harding, S.M.; Genois, M.M.; Maity, R.; van Rossum-Fikkert, S.; Kertokalio, A.; Romoli, F.; et al. DNA double-strand break repair pathway choice is directed by distinct MRE11 nuclease activities. Mol. Cell 2014, 53, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Moiani, D.; Ronato, D.A.; Brosey, C.A.; Arvai, A.S.; Syed, A.; Masson, J.-Y.; Petricci, E.; Tainer, J.A. Targeting allostery with avatars to design inhibitors assessed by cell activity: Dissecting mre11 endo- and exonuclease activities. Methods Enzymol. 2018, 601, 205–241. [Google Scholar]
- Zhu, M.; Zhao, H.; Limbo, O.; Russell, P. Mre11 complex links sister chromatids to promote repair of a collapsed replication fork. Proc. Natl. Acad. Sci. USA 2018, 115, 8793–8798. [Google Scholar] [CrossRef] [Green Version]
- Macheret, M.; Halazonetis, T.D. DNA replication stress as a hallmark of cancer. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 425–448. [Google Scholar] [CrossRef] [Green Version]
- Zeman, M.K.; Cimprich, K.A. Causes and consequences of replication stress. Nat. Cell Biol. 2014, 16, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic instability an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Bartkova, J.; Hořejší, Z.; Koed, K.; Krämer, A.; Tort, F.; Zleger, K.; Guldberg, P.; Sehested, M.; Nesland, J.M.; Lukas, C.; et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005, 434, 864–870. [Google Scholar] [CrossRef]
- Gorgoulis, V.G.; Vassiliou, L.V.F.; Karakaidos, P.; Zacharatos, P.; Kotsinas, A.; Liloglou, T.; Venere, M.; DiTullio, R.A.; Kastrinakis, N.G.; Levy, B.; et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 2005, 434, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Bertolin, A.P.; Hoffmann, J.S.; Gottifredi, V. Under-replicated DNA: The byproduct of large genomes? Cancers 2020, 12, 2764. [Google Scholar] [CrossRef] [PubMed]
- Franchet, C.; Hoffmann, J.S. When RAD52 allows mitosis to accept unscheduled DNA synthesis. Cancers 2020, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Sansregret, L.; Patterson, J.O.; Dewhurst, S.; López-García, C.; Koch, A.; McGranahan, N.; Chao, W.C.H.; Barry, D.J.; Rowan, A.; Instrell, R.; et al. APC/C dysfunction limits excessive cancer chromosomal instability. Cancer Discov. 2017, 7, 218–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saldivar, J.C.; Cortez, D.; Cimprich, K.A. The essential kinase ATR: Ensuring faithful duplication of a challenging genome. Nat. Rev. Mol. Cell Biol. 2017, 18, 622–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, L.; Fernandez-Vidal, A.; Bertoli, S.; Grgurevic, S.; Lepage, B.; Deshaies, D.; Prade, N.; Carte, M.; Larrue, C.; Sarry, J.E.; et al. CHK1 as a therapeutic target to bypass chemoresistance in AML. Sci. Signal. 2016, 9, ra90. [Google Scholar] [CrossRef]
- Bhowmick, R.; Minocherhomji, S.; Hickson, I.D. RAD52 facilitates mitotic DNA synthesis following replication stress. Mol. Cell 2016, 64, 1117–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickson, I.D.; Bhowmick, R. The “enemies within”: Regions of the genome that are inherently difficult to replicate. F1000Research 2017, 6, 666. [Google Scholar]
- Massey, A.J. Inhibition of ATR-dependent feedback activation of Chk1 sensitises cancer cells to Chk1 inhibitor monotherapy. Cancer Lett. 2016, 383, 41–52. [Google Scholar] [CrossRef]
- Manic, G.; Signore, M.; Sistigu, A.; Russo, G.; Corradi, F.; Siteni, S.; Musella, M.; Vitale, S.; De Angelis, M.L.; Pallocca, M.; et al. CHK1-targeted therapy to deplete DNA replication-stressed, p53-deficient, hyperdiploid colorectal cancer stem cells. Gut 2018, 67, 903–917. [Google Scholar] [CrossRef]
- Webster, P.J.; Littlejohns, A.T.; Gaunt, H.J.; Young, R.S.; Rode, B.; Ritchie, J.E.; Stead, L.F.; Harrison, S.; Droop, A.; Martin, H.L.; et al. Upregulated WEE1 protects endothelial cells of colorectal cancer liver metastases. Oncotarget 2017, 8, 42288–42299. [Google Scholar] [CrossRef] [Green Version]
- Webster, P.J.; Littlejohns, A.T.; Gaunt, H.J.; Prasad, K.R.; Beech, D.J.; Burke, D.A. AZD1775 induces toxicity through double-stranded DNA breaks independently of chemotherapeutic agents in p53-mutated colorectal cancer cells. Cell Cycle 2017, 16, 2176–2182. [Google Scholar] [CrossRef]
- Agostini, L.C.; Jain, A.; Shupp, A.; Nevler, A.; McCarthy, G.; Bussard, K.M.; Yeo, C.J.; Brody, J.R. Combined targeting of PARG and Wee1 causes decreased cell survival and DNA damage in an S-phase dependent manner. Mol. Cancer Res. 2021, 19, 207–214. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Q.; Tao, R.; Chang, W.; Li, R.; Xie, G.; Liu, W.; Zhang, P.; Tao, K. Wee1 inhibition can suppress tumor proliferation and sensitize p53 mutant colonic cancer cells to the anticancer effect of irinotecan. Mol. Med. Rep. 2018, 17, 3344–3349. [Google Scholar] [CrossRef]
- Kalimutho, M.; Bain, A.L.; Mukherjee, B.; Nag, P.; Nanayakkara, D.M.; Harten, S.K.; Harris, J.L.; Subramanian, G.N.; Sinha, D.; Shirasawa, S.; et al. Enhanced dependency of KRAS-mutant colorectal cancer cells on RAD51-dependent homologous recombination repair identified from genetic interactions in Saccharomyces cerevisiae. Mol. Oncol. 2017, 11, 470–490. [Google Scholar] [CrossRef] [Green Version]
- Iwata, T.; Uchino, T.; Koyama, A.; Johmura, Y.; Koyama, K.; Saito, T.; Ishiguro, S.; Arikawa, T.; Komatsu, S.; Miyachi, M.; et al. The G2 checkpoint inhibitor CBP-93872 increases the sensitivity of colorectal and pancreatic cancer cells to chemotherapy. PLoS ONE 2017, 12, e0178221. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Jette, N.; Moussienko, D.; Bebb, D.G.; Lees-Miller, S.P. ATM-deficient colorectal cancer cells are sensitive to the PARP inhibitor olaparib. Transl. Oncol. 2017, 10, 190–196. [Google Scholar] [CrossRef]
- Greene, J.; Nguyen, A.; Bagby, S.M.; Jones, G.N.; Tai, W.M.; Quackenbush, K.S.; Schreiber, A.; Messersmith, W.A.; Devaraj, K.M.; Blatchford, P.; et al. The novel ATM inhibitor (AZ31) enhances antitumor activity in patient derived xenografts that are resistant to irinotecan monotherapy. Oncotarget 2017, 8, 110904–110913. [Google Scholar] [CrossRef] [Green Version]
- Herrero, A.B.; Gutiérrez, N.C. Targeting ongoing DNA damage in multiple myeloma: Effects of DNA damage response inhibitors on plasma cell survival. Front. Oncol. 2017, 7, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petroni, M.; Sardina, F.; Infante, P.; Bartolazzi, A.; Locatelli, E.; Fabretti, F.; Di Giulio, S.; Capalbo, C.; Cardinali, B.; Coppa, A.; et al. MRE11 inhibition highlights a replication stress-dependent vulnerability of MYCN-driven tumors. Cell Death Dis. 2018, 9, 895. [Google Scholar] [CrossRef]
- Manic, G.; Musella, M.; Corradi, F.; Sistigu, A.; Vitale, S.; Soliman Abdel Rehim, S.; Mattiello, L.; Malacaria, E.; Galassi, C.; Signore, M.; et al. Control of replication stress and mitosis in colorectal cancer stem cells through the interplay of PARP1, MRE11 and RAD51. Cell Death Differ. 2021, 1–23. [Google Scholar] [CrossRef]
- Sullivan-Reed, K.; Bolton-Gillespie, E.; Dasgupta, Y.; Langer, S.; Siciliano, M.; Nieborowska-Skorska, M.; Hanamshet, K.; Belyaeva, E.A.; Bernhardy, A.J.; Lee, J.; et al. Simultaneous targeting of PARP1 and RAD52 triggers dual synthetic lethality in BRCA-deficient tumor cells. Cell Rep. 2018, 23, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gelot, C.; Pantelidou, C.; Li, A.; Yücel, H.; Davis, R.; Farkkila, A.; Kochupurakkal, B.; Syed, A.; Shapiro, G.; et al. Polymerase Theta Inhibition Kills Homologous Recombination Deficient Tumors. Available online: https://www.biorxiv.org/content/10.1101/2020.05.23.111658v1.full (accessed on 22 December 2020).
- Shibata, A.; Jeggo, P. A historical reflection on our understanding of radiation-induced DNA double strand break repair in somatic mammalian cells; interfacing the past with the present. Int. J. Radiat. Biol. 2019, 3002, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jeggo, P.A.; Löbrichf, M. How cancer cells hijack DNA double-strand break repair pathways to gain genomic instability. Biochem. J. 2015, 471, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Her, J.; Bunting, S.F. How cells ensure correct repair of DNA double-strand breaks. J. Biol. Chem. 2018, 293, 10502–10511. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.J.; Gibb, B.; Kwon, Y.; Sung, P.; Greene, E.C. Protein dynamics of human RPA and RAD51 on ssDNA during assembly and disassembly of the RAD51 filament. Nucleic Acids Res. 2016, 45, 749–761. [Google Scholar] [CrossRef] [Green Version]
- Andriuskevicius, T.; Kotenko, O.; Makovets, S. Putting together and taking apart: Assembly and disassembly of the Rad51 nucleoprotein filament in DNA repair and genome stability. Cell Stress 2018, 2, 96–112. [Google Scholar] [CrossRef]
- Giannini, G.; Ristori, E.; Cerignoli, F.; Rinaldi, C.; Zani, M.; Viel, A.; Ottini, L.; Crescenzi, M.; Martinotti, S.; Bignami, M.; et al. Human MRE11 is inactivated in mismatch repair-deficient cancers. EMBO Rep. 2002, 3, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Wen, Q.; Scorah, J.; Phear, G.; Rodgers, G.; Rodgers, S.; Meuth, M. A mutant allele of MRE11 found in mismatch repair-deficient tumor cells suppresses the cellular response to DNA replication fork stress in a dominant negative manner. Mol. Biol. Cell 2008, 19, 1693–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupré, A.; Boyer-Chatenet, L.; Sattler, R.M.; Modi, A.P.; Lee, J.-H.; Nicolette, M.L.; Kopelovich, L.; Jasin, M.; Baer, R.; Paull, T.T.; et al. A forward chemical genetic screen reveals an inhibitor of the Mre11–Rad50–Nbs1 complex. Nat. Chem. Biol. 2008, 4, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Petroni, M.; Sardina, F.; Heil, C.; Sahún-Roncero, M.; Colicchia, V.; Veschi, V.; Albini, S.; Fruci, D.; Ricci, B.; Soriani, A.; et al. The MRN complex is transcriptionally regulated by MYCN during neural cell proliferation to control replication stress. Cell Death Differ. 2016, 23, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Chu, P.; Lingeman, R.; McDaniel, H.; Kechichian, S.; Hickey, R.J.; Liu, Z.; Yuan, Y.C.; Sandoval, J.A.; Fields, G.B.; et al. The Mechanism by Which MYCN amplification confers an enhanced sensitivity to a PCNA-derived cell permeable peptide in neuroblastoma cells. EBioMedicine 2015, 2, 1923–1931. [Google Scholar] [CrossRef] [Green Version]
- Kushner, B.H.; Modak, S.; Kramer, K.; Laquaglia, M.P.; Yataghene, K.; Basu, E.M.; Roberts, S.S.; Cheung, N.K.V. Striking dichotomy in outcome of MYCN-amplified neuroblastoma in the contemporary era. Cancer 2014, 120, 2050–2059. [Google Scholar] [CrossRef] [Green Version]
- Mateos-Gomez, P.A.; Gong, F.; Nair, N.; Miller, K.M.; Lazzerini-Denchi, E.; Sfeir, A. Mammalian polymerase θ promotes alternative NHEJ and suppresses recombination. Nature 2015, 518, 254–257. [Google Scholar] [CrossRef] [Green Version]
- Kent, T.; Chandramouly, G.; Mcdevitt, S.M.; Ozdemir, A.Y.; Pomerantz, R.T. Mechanism of microhomology-mediated end-joining promoted by human DNA polymerase θ. Nat. Struct. Mol. Biol. 2015, 22, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Truong, L.N.; Li, Y.; Shi, L.Z.; Hwang, P.Y.H.; He, J.; Wang, H.; Razavian, N.; Berns, M.W.; Wu, X. Microhomology-mediated end joining and homologous recombination share the initial end resection step to repair DNA double-strand breaks in mammalian cells. Proc. Natl. Acad. Sci. USA 2013, 110, 7720–7725. [Google Scholar] [CrossRef] [Green Version]
- Rahal, E.A.; Henricksen, L.A.; Li, Y.; Williams, R.S.; Tainer, J.A.; Dixon, K. ATM regulates Mre11-dependent DNA end-degradation and microhomology-mediated end joining. Cell Cycle 2010, 9, 2866. [Google Scholar] [CrossRef] [Green Version]
- Rass, E.; Grabarz, A.; Plo, I.; Gautier, J.; Bertrand, P.; Lopez, B.S. Role of Mre11 in chromosomal nonhomologous end joining in mammalian cells. Nat. Struct. Mol. Biol. 2009, 16, 819–824. [Google Scholar] [CrossRef]
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Therap. Adv. Gastroenterol. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Tran, L.; Allen, C.T.; Xiao, R.; Moore, E.; Davis, R.; Park, S.J.; Spielbauer, K.; Van Waes, C.; Schmitt, N.C. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol. Res. 2017, 5, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Ye, L.; Lei, Y.; Wan, J.; Chen, H. Downregulation of FoxM1 sensitizes nasopharyngeal carcinoma cells to cisplatin via inhibition of MRN-ATM-mediated DNA repair. BMB Rep. 2019, 52, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Huang, D.; Ramsey, A.J.; Ig-izevbekhai, K.; Zhang, K.; Lajud, S.A.; O’Malley, B.W.; Li, D. PD-L1 and MRN synergy in platinum-based chemoresistance of head and neck squamous cell carcinoma. Br. J. Cancer 2019, 122, 1–8. [Google Scholar] [CrossRef]
- Sato, H.; Niimi, A.; Yasuhara, T.; Permata, T.B.M.; Hagiwara, Y.; Isono, M.; Nuryadi, E.; Sekine, R.; Oike, T.; Kakoti, S.; et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Teo, M.Y.; Seier, K.; Ostrovnaya, I.; Regazzi, A.M.; Kania, B.E.; Moran, M.M.; Cipolla, C.K.; Bluth, M.J.; Chaim, J.; Al-Ahmadie, H.; et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J. Clin. Oncol. 2018, 36, 1685–1694. [Google Scholar] [CrossRef]
- Shim, J.H.; Kim, H.S.; Cha, H.; Kim, S.; Kim, T.M.; Anagnostou, V.; Choi, Y.L.; Jung, H.A.; Sun, J.M.; Ahn, J.S.; et al. HLA-corrected tumor mutation burden and homologous recombination deficiency for the prediction of response to PD-(L)1 blockade in advanced non-small-cell lung cancer patients. Ann. Oncol. 2020, 31, 902–911. [Google Scholar] [CrossRef]
- Sato, H.; Jeggo, P.A.; Shibata, A. Regulation of programmed death-ligand 1 expression in response to DNA damage in cancer cells: Implications for precision medicine. Cancer Sci. 2019, 110, 3415–3423. [Google Scholar] [CrossRef] [Green Version]
- Chae, Y.K.; Anker, J.F.; Bais, P.; Namburi, S.; Giles, F.J.; Chuang, J.H. Mutations in DNA repair genes are associated with increased neo-antigen load and activated T cell infiltration in lung adenocarcinoma. Oncotarget 2018, 9, 7949–7960. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Mand, M.R.; Deshpande, R.A.; Kinoshita, E.; Yang, S.-H.; Wyman, C.; Paull, T.T. Ataxia telangiectasia-mutated (ATM) kinase activity is regulated by atp-driven conformational changes in the Mre11/Rad50/Nbs1 (MRN) complex. J. Biol. Chem. 2013, 288, 12840–12851. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Shimomuki, M.; Katsuki, Y.; Takahashi, D.; Kobayashi, W.; Ishiai, M.; Miyoshi, H.; Takata, M.; Kurumizaka, H. FANCI-FANCD2 stabilizes the RAD51-DNA complex by binding RAD51 and protects the 5′-DNA end. Nucleic Acids Res. 2016, 44, 10758–10771. [Google Scholar] [CrossRef] [Green Version]
- Mason, J.M.; Chan, Y.L.; Weichselbaum, R.W.; Bishop, D.K. Non-enzymatic roles of human RAD51 at stalled replication forks. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Somyajit, K.; Saxena, S.; Babu, S.; Mishra, A.; Nagaraju, G. Mammalian RAD51 paralogs protect nascent DNA at stalled forks and mediate replication restart. Nucleic Acids Res. 2015, 43, 9835–9855. [Google Scholar] [CrossRef]
- Bugreev, D.V.; Rossi, M.J.; Mazin, A. V Cooperation of RAD51 and RAD54 in regression of a model replication fork. Nucleic Acids Res. 2011, 39, 2153–2164. [Google Scholar] [CrossRef] [Green Version]
- Bhat, K.P.; Cortez, D. RPA and RAD51: Fork reversal, fork protection, and genome stability. Nat. Struct. Mol. Biol. 2018, 25, 446–453. [Google Scholar] [CrossRef]
- Slade, D. Maneuvers on PCNA rings during DNA replication and repair. Genes 2018, 9, 416. [Google Scholar] [CrossRef] [Green Version]
- Leung, W.; Baxley, R.M.; Moldovan, G.L.; Bielinsky, A.K. Mechanisms of DNA damage tolerance: Post-translational regulation of PCNA. Genes 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budke, B.; Tueckmantel, W.; Miles, K.; Kozikowski, A.P.; Connell, P.P. Optimization of drug candidates that inhibit the D-loop activity of RAD51 HHS public access. ChemMedChem 2019, 14, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Bae, A.-N.; Jung, S.-J. Clinicopathological and prognostic characteristics of RAD51 in colorectal cancer. Medicina 2020, 56, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, R.; Liu, T.C.; Ruzinova, M.B. High frequency of KRAS mutation in early onset colorectal adenocarcinoma: Implications for pathogenesis. Hum. Pathol. 2016, 56, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Sallmyr, A.; Tomkinson, A.E. Repair of DNA double-strand breaks by mammalian alternative end-joining pathways. J. Biol. Chem. 2018, 293, 10536–10549. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.S.; Majumdar, R.; Moore, G.M.; Narang, H.; Buechelmaier, E.S.; Bazil, M.J.; Ravindran, P.T.; Leeman, J.E.; Li, Y.; Jalan, M.; et al. Measuring nonhomologous end-joining, homologous recombination and alternative end-joining simultaneously at an endogenous locus in any transfectable human cell. Nucleic Acids Res. 2021. [Google Scholar] [CrossRef]
- Hanscom, T.; McVey, M. Regulation of error-prone DNA double-strand break repair and its impact on genome evolution. Cells 2020, 9, 1657. [Google Scholar] [CrossRef]
- Xiong, X.; Du, Z.; Wang, Y.; Feng, Z.; Fan, P.; Yan, C.; Willers, H.; Zhang, J. 53BP1 promotes microhomology-mediated end-joining in G1-phase cells. Nucleic Acids Res. 2015, 43, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Bakr, A.; Köcher, S.; Volquardsen, J.; Petersen, C.; Borgmann, K.; Dikomey, E.; Rothkamm, K.; Mansour, W.Y. Impaired 53BP1/RIF1 DSB mediated end-protection stimulates CtIP-dependent end resection and switches the repair to PARP1-dependent end joining in G1. Oncotarget 2016, 7, 57679–57693. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Hwang, T.; Reh, S.; Dunbayev, Y.; Zhong, Y.; Takata, Y.; Shen, J.; McBride, K.M.; Murnane, J.P.; Bhak, J.; Lee, S.; et al. Defining the mutation signatures of DNA polymerase θ in cancer genomes. NAR Cancer 2020, 2, zcaa017. [Google Scholar] [CrossRef]
- Maiorano, D.; Etri, J.E.; Franchet, C.; Hoffmann, J.S. Translesion synthesis or repair by specialized dna polymerases limits excessive genomic instability upon replication stress. Int. J. Mol. Sci. 2021, 22, 3924. [Google Scholar] [CrossRef]
- Seol, J.-H.; Shim, E.Y.; Lee, S.E. Microhomology-mediated end joining: Good, bad and ugly. Mutat. Res. Mol. Mech. Mutagen. 2018, 809, 81–87. [Google Scholar] [CrossRef]
- Zahn, K.E.; Jensen, R.B.; Wood, R.D.; Doublié, S. Human DNA polymerase θ harbors DNA end-trimming activity critical for DNA repair. Mol. Cell 2021, 81. [Google Scholar] [CrossRef]
- Mateos-Gomez, P.A.; Kent, T.; Deng, S.K.; Mcdevitt, S.; Kashkina, E.; Hoang, T.M.; Pomerantz, R.T.; Sfeir, A. The helicase domain of Polθ counteracts RPA to promote alt-NHEJ. Nat. Struct. Mol. Biol. 2017, 24, 1116–1123. [Google Scholar] [CrossRef]
- Lemée, F.; Bergoglio, V.; Fernandez-Vidal, A.; Machado-Silva, A.; Pillaire, M.J.; Bieth, A.; Gentil, C.; Baker, L.; Martin, A.L.; Leduc, C.; et al. DNA polymerase θ up-regulation is associated with poor survival in breast cancer, perturbs DNA replication, and promotes genetic instability. Proc. Natl. Acad. Sci. USA 2010, 107, 13390–13395. [Google Scholar] [CrossRef] [Green Version]
- Ceccaldi, R.; Liu, J.C.; Amunugama, R.; Hajdu, I.; Primack, B.; Petalcorin, M.I.R.; O’Connor, K.W.; Konstantinopoulos, P.A.; Elledge, S.J.; Boulton, S.J.; et al. Homologous-recombination-deficient tumours are dependent on Polθ -mediated repair. Nature 2015, 518, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Patterson-Fortin, J.; D’Andrea, A.D. Exploiting the microhomology-mediated end-joining pathway in cancer therapy. Cancer Res. 2020, 80, 4593–4600. [Google Scholar] [CrossRef]
- Ahrabi, S.; Sarkar, S.; Pfister, S.X.; Pirovano, G.; Higgins, G.S.; Porter, A.C.G.; Humphrey, T.C. A role for human homologous recombination factors in suppressing microhomology-mediated end joining. Nucleic Acids Res. 2016, 44, 5743–5757. [Google Scholar] [CrossRef] [Green Version]
- Schlacher, K.; Christ, N.; Siaud, N.; Egashira, A.; Wu, H.; Jasin, M. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell 2011, 145, 529–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvajal-Garcia, J.; Cho, J.E.; Carvajal-Garcia, P.; Feng, W.; Wood, R.D.; Sekelsky, J.; Gupta, G.P.; Roberts, S.A.; Ramsden, D.A. Mechanistic basis for microhomology identification and genome scarring by polymerase theta. Proc. Natl. Acad. Sci. USA 2020, 117, 8476–8485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goullet De Rugy, T.; Bashkurov, M.; Datti, A.; Betous, R.; Guitton-Sert, L.; Cazaux, C.; Durocher, D.; Hoffmann, J.S. Excess Polθ functions in response to replicative stress in homologous recombination-proficient cancer cells. Biol. Open 2016, 5, 1485–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, W.; Simpson, D.A.; Carvajal-Garcia, J.; Price, B.A.; Kumar, R.J.; Mose, L.E.; Wood, R.D.; Rashid, N.; Purvis, J.E.; Parker, J.S.; et al. Genetic determinants of cellular addiction to DNA polymerase theta. Nat. Commun. 2019, 10, 4286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Song, Y.; Li, S.; Kurian, S.; Xiang, R.; Chiba, T.; Wu, X. DNA polymerase (POLQ) is important for repair of DNA double-strand breaks caused by fork collapse. J. Biol. Chem. 2019, 294, 3909–3919. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Wyatt, D.W.; Takata, K.-i.; Mu, Y.; Hensley, S.C.; Tomida, J.; Bylund, G.O.; Doublié, S.; Johansson, E.; Ramsden, D.A.; et al. Mechanism of suppression of chromosomal instability by DNA polymerase POLQ. PLoS Genet. 2014, 10, e1004654. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.A.; Cooper, C.D.O.; Aitkenhead, H.; Gileadi, O. Structure of the helicase domain of DNA polymerase theta reveals a possible role in the microhomology-mediated end-joining pathway. Structure 2015, 23, 2319–2330. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, D.W.; Feng, W.; Conlin, M.P.; Yousefzadeh, M.J.; Roberts, S.A.; Mieczkowski, P.; Wood, R.D.; Gupta, G.P.; Ramsden, D.A. Essential roles for polymerase θ-mediated end joining in the repair of chromosome breaks. Mol. Cell 2016, 63, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, K.; Bahar, R.; Seimiya, M.; Chiyo, M.; Wada, A.; Okada, S.; Hatano, M.; Tokuhisa, T.; Kimura, H.; Watanabe, S.; et al. DNA polymerase θ is preferentially expressed in lymphoid tissues and upregulated in human cancers. Int. J. Cancer 2004, 109, 9–16. [Google Scholar] [CrossRef]
- Pillaire, M.J.; Selves, J.; Gordien, K.; Gouraud, P.A.; Gentil, C.; Danjoux, M.; Do, C.; Negre, V.; Bieth, A.; Guimbaud, R.; et al. A DNA replication signature of progression and negative outcome in colorectal cancer. Oncogene 2010, 29, 876–887. [Google Scholar] [CrossRef]
- Stephens, P.J.; McBride, D.J.; Lin, M.L.; Varela, I.; Pleasance, E.D.; Simpson, J.T.; Stebbings, L.A.; Leroy, C.; Edkins, S.; Mudie, L.J.; et al. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature 2009, 462, 1005–1010. [Google Scholar] [CrossRef] [Green Version]
- Bass, A.J.; Lawrence, M.S.; Brace, L.E.; Ramos, A.H.; Drier, Y.; Cibulskis, K.; Sougnez, C.; Voet, D.; Saksena, G.; Sivachenko, A.; et al. Genomic sequencing of colorectal adenocarcinomas identifies a recurrent VTI1A-TCF7L2 fusion. Nat. Genet. 2011, 43, 964–970. [Google Scholar] [CrossRef]
- Li, Y.; Roberts, N.D.; Wala, J.A.; Shapira, O.; Schumacher, S.E.; Kumar, K.; Khurana, E.; Waszak, S.; Korbel, J.O.; Haber, J.E.; et al. Patterns of somatic structural variation in human cancer genomes. Nature 2020, 578, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Ottaviani, D.; LeCain, M.; Sheer, D. The role of microhomology in genomic structural variation. Trends Genet. 2014, 30, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Bentley, J.; L’Hôte, C.; Platt, F.; Hurst, C.D.; Lowery, J.; Taylor, C.; Sak, S.C.; Harnden, P.; Knowles, M.A.; Kiltie, A.E. Papillary and muscle invasive bladder tumors with distinct genomic stability profiles have different DNA repair fidelity and KU DNA-binding activities. Genes Chromosom. Cancer 2009, 48, 310–321. [Google Scholar] [CrossRef]
- Higgins, G.S.; Prevo, R.; Lee, Y.F.; Helleday, T.; Muschel, R.J.; Taylor, S.; Yoshimura, M.; Hickson, I.D.; Bernhard, E.J.; McKenna, W.G. A small interfering RNA screen of genes involved in DNA repair identifies tumor-specific radiosensitization by POLQ knockdown. Cancer Res. 2010, 70, 2984–2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrempf, A.; Slyskova, J.; Loizou, J.I. Targeting the DNA Repair Enzyme Polymerase θ in Cancer Therapy. Trends Cancer 2021, 7, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.S.; Boulton, S.J. Beyond PARP—POLu as an anticancer target. Science 2018, 359, 1217–1218. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Onyango, D.O.; Stark, J.M. Regulation of single-strand annealing and its role in genome maintenance. Trends Genet. 2016, 32, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Elliott, B.; Richardson, C.; Jasin, M. Chromosomal translocation mechanisms at intronic Alu elements in mammalian cells. Mol. Cell 2005, 17, 885–894. [Google Scholar] [CrossRef]
- Malacaria, E.; Honda, M.; Franchitto, A.; Spies, M.; Pichierri, P. Physiological and pathological roles of RAD52 at DNA replication forks. Cancers 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Scott, S.P.; Bussen, W.; Sharma, G.G.; Guo, G.; Pandita, T.K.; Powell, S.N. Rad52 inactivation is synthetically lethal with BRCA2 deficiency. Proc. Natl. Acad. Sci. USA 2011, 108, 686–691. [Google Scholar] [CrossRef] [Green Version]
- Lok, B.H.; Carley, A.C.; Tchang, B.; Powell, S.N. RAD52 inactivation is synthetically lethal with deficiencies in BRCA1 and PALB2 in addition to BRCA2 through RAD51-mediated homologous recombination. Oncogene 2013, 32, 3552–3558. [Google Scholar] [CrossRef] [Green Version]
- Ho, V.; Chung, L.; Singh, A.; Lea, V.; Abubakar, A.; Lim, S.H.; Chua, W.; Ng, W.; Lee, M.; Roberts, T.L.; et al. Aberrant expression of RAD52, its prognostic impact in rectal cancer and association with poor survival of patients. Int. J. Mol. Sci. 2020, 21, 1768. [Google Scholar] [CrossRef] [Green Version]
- Hanamshet, K.; Mazina, O.M.; Mazin, A.V. Reappearance from obscurity: Mammalian Rad52 in homologous recombination. Genes 2016, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Hanamshet, K.; Mazin, A.V. The function of RAD52 N-terminal domain is essential for viability of BRCA-deficient cells. Nucleic Acids Res. 2020, 48, 12778–12791. [Google Scholar] [CrossRef]
- Sotiriou, S.K.; Kamileri, I.; Lugli, N.; Evangelou, K.; Da-Ré, C.; Huber, F.; Padayachy, L.; Tardy, S.; Nicati, N.L.; Barriot, S.; et al. Mammalian RAD52 functions in break-induced replication repair of collapsed DNA replication forks. Mol. Cell 2016, 64, 1127–1134. [Google Scholar] [CrossRef] [Green Version]
- Jalan, M.; Olsen, K.S.; Powell, S.N. Emerging roles of RAD52 in genome maintenance. Cancers 2019, 11, 1038. [Google Scholar] [CrossRef] [Green Version]
- Nickoloff, J.A.; Jones, D.; Lee, S.H.; Williamson, E.A.; Hromas, R. Drugging the cancers addicted to DNA repair. J. Natl. Cancer Inst. 2017, 109, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kelso, A.A.; Lopezcolorado, F.W.; Bhargava, R.; Stark, J.M. Distinct roles of RAD52 and POLQ in chromosomal break repair and replication stress response. PLoS Genet. 2019, 15, e1008319. [Google Scholar] [CrossRef] [Green Version]
- Toma, M.; Sullivan-Reed, K.; Śliwiński, T.; Skorski, T. RAD52 as a potential target for synthetic lethality-based anticancer therapies. Cancers 2019, 11, 1561. [Google Scholar] [CrossRef] [Green Version]
- Bunz, F.; Dutriaux, A.; Lengauer, C.; Waldman, T.; Zhou, S.; Brown, J.P.; Sedivy, J.M.; Kinzler, K.W.; Vogelstein, B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 1998, 282, 1497–1501. [Google Scholar] [CrossRef]
- Aljarbou, F.; Almousa, N.; Bazzi, M.; Aldaihan, S.; Alanazi, M.; Alharbi, O.; Almadi, M.; Aljebreen, A.M.; Azzam, N.A.; Arafa, M.; et al. The expression of telomere-related proteins and DNA damage response and their association with telomere length in colorectal cancer in Saudi patients. PLoS ONE 2018, 13, e0197154. [Google Scholar] [CrossRef] [Green Version]
- Mattiello, L.; Rehim, S.S.A.; Musella, M.; Sistigu, A.; Guarracino, A.; Vitale, S.; Corradi, F.; Galassi, C.; Sperati, F.; Manic, G.; et al. The targeting of mre11 or rad51 sensitizes colorectal cancer stem cells to chk1 inhibition. Cancers 2021, 13, 1957. [Google Scholar] [CrossRef]
- Jette, N.R.; Kumar, M.; Radhamani, S.; Arthur, G.; Goutam, S.; Yip, S.; Kolinsky, M.; Williams, G.J.; Bose, P.; Lees-Miller, S.P. ATM-deficient cancers provide new opportunities for precision oncology. Cancers 2020, 12, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilad, S.; Khosravi, R.; Shkedy, D.; Uziel, T.; Ziv, Y.; Savitsky, K.; Rotman, G.; Smith, S.; Chessa, L.; Jorgensen, T.J.; et al. Predominance of null mutations in ataxia-telangiectasia. Hum. Mol. Genet. 1996, 5, 433–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, J.P.Y.; Ma, H.T.; Poon, R.Y.C. Synergism between ATM and PARP1 inhibition involves DNA damage and abrogating the G 2 DNA damage checkpoint. Mol. Cancer Ther. 2019, 19, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundar, R.; Miranda, S.; Rodrigues, D.N.; Chénard-Poirier, M.; Dolling, D.; Clarke, M.; Figueiredo, I.; Bertan, C.; Yuan, W.; Ferreira, A.; et al. Ataxia telangiectasia mutated protein loss and benefit from oxaliplatin-based chemotherapy in colorectal cancer. Clin. Colorectal Cancer 2018, 17, 280–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, X.C.; Wu, F.; Li, W.T.; Zhu, X.J.; Liu, J.W.; Wang, B.L. Upregulation of WEE1 is a potential prognostic biomarker for patients with colorectal cancer. Oncol. Lett. 2017, 13, 4341–4348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egeland, E.V.; Flatmark, K.; Nesland, J.M.; Flørenes, V.A.; Mælandsmo, G.M.; Boye, K. Expression and clinical significance of Wee1 in colorectal cancer. Tumor Biol. 2016, 37, 12133–12140. [Google Scholar] [CrossRef]
- Bendell, J.C.; Bischoff, H.G.; Hwang, J.; Reinhardt, H.C.; Zander, T.; Wang, X.; Hynes, S.; Pitou, C.; Campbell, R.; Iversen, P.; et al. A phase 1 dose-escalation study of checkpoint kinase 1 (CHK1) inhibitor prexasertib in combination with p38 mitogen-activated protein kinase (p38 MAPK) inhibitor ralimetinib in patients with advanced or metastatic cancer. Investig. New Drugs 2020, 38, 1145–1155. [Google Scholar] [CrossRef]
- Chu, Q.S.; Jonker, D.J.; Provencher, D.M.; Miller, W.H.; Bouganim, N.; Shields, A.F.; Shapiro, G.; Sawyer, M.B.; Lheureux, S.; Samouelian, V.; et al. A phase Ib study of oral Chk1 inhibitor LY2880070 in combination with gemcitabine in patients with advanced or metastatic cancer. J. Clin. Oncol. 2020, 38, 3581. [Google Scholar] [CrossRef]
- Plummer, E.R.; Kristeleit, R.S.; Cojocaru, E.; Haris, N.M.; Carter, L.; Jones, R.H.; Blagden, S.P.; Evans, T.R.J.; Arkenau, H.-T.; Sarker, D.; et al. A first-in-human phase I/II trial of SRA737 (a Chk1 Inhibitor) in subjects with advanced cancer. J. Clin. Oncol. 2019, 37, 3094. [Google Scholar] [CrossRef]
- Abida, W.; Bang, Y.J.; Carter, L.; Azaro, A.; Krebs, M.; Im, S.-A.; Chen, Y.; Buil-Bruna, N.; Li, Y.; Eaton, D.; et al. Abstract A094: Phase I Modular Study of AZD0156, a First-in-Class Oral Selective Inhibitor of Ataxia Telangiectasia Mutated Protein Kinase (ATM), in Combination with Olaparib (AToM Study, Module 1). Available online: https://mct.aacrjournals.org/content/17/1_Supplement/A094 (accessed on 22 April 2021).
- De Bono, J.S.; Tan, D.S.P.; Caldwell, R.; Terbuch, A.; Goh, B.C.; Heong, V.; Haris, N.M.; Bashir, S.; Hong, D.S.; Meric-Bernstam, F.; et al. First-in-human trial of the oral ataxia telangiectasia and Rad3-related (ATR) inhibitor BAY 1895344 in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2019, 37, 3007. [Google Scholar] [CrossRef]
- Terranova, N.; Jansen, M.; Falk, M.; Hendriks, B.S. Population pharmacokinetics of ATR inhibitor berzosertib in phase I studies for different cancer types. Cancer Chemother. Pharmacol. 2021, 87, 185–196. [Google Scholar] [CrossRef]
- Yap, T.A.; O’Carrigan, B.; Penney, M.S.; Lim, J.S.; Brown, J.S.; De Miguel Luken, M.J.; Tunariu, N.; Perez-Lopez, R.; Rodrigues, D.N.; Riisnaes, R.; et al. Phase i trial of first-in-class ATR inhibitor M6620 (VX-970) as monotherapy or in combination with carboplatin in patients with advanced solid tumors. J. Clin. Oncol. 2020, 38, 3195–3204. [Google Scholar] [CrossRef]
- Cohen, D.J.; Grabocka, E.; Bar-Sagi, D.; Godin, R.; Leichman, L.P. A Phase Ib Study Combining Irinotecan with AZD1775, a Selective WEE 1 Kinase Inhibitor, in RAS/RAF Mutated Metastatic Colorectal Cancer Patients Who Progressed on First Line Therapy. Available online: http://ascopubs.org/doi/10.1200/JCO.2017.35.15_suppl.TPS3627 (accessed on 22 April 2021).
- Jhaveri, K.L.; Makker, V.; Wang, X.V.; Chen, A.P.; Flaherty, K.; Conley, B.A.; O’Dwyer, P.J.; Williams, P.M.; Hamilton, S.R.; Harris, L.; et al. Ado-trastuzumab emtansine (T-DM1) in patients (pts) with HER2 amplified (amp) tumors excluding breast and gastric/gastro-esophageal junction (GEJ) adenocarcinomas: Results from the national cancer institute (NCI) molecular analysis for therapy choice (MATCH) trial. J. Clin. Oncol. 2018, 36, 100. [Google Scholar] [CrossRef]
- Cole, K.A.; Pal, S.; Kudgus, R.A.; Ijaz, H.; Liu, X.; Minard, C.G.; Pawel, B.R.; Maris, J.M.; Haas-Kogan, D.A.; Voss, S.D.; et al. Phase I clinical trial of the WEe1 inhibitor adavosertib (AZD1775) with irinotecan in children with relapsed solid tumors: A COG phase I consortium report (ADVL1312). Clin. Cancer Res. 2020, 26, 1213–1219. [Google Scholar] [CrossRef]
- Leijen, S.; Van Geel, R.M.J.M.; Pavlick, A.C.; Tibes, R.; Rosen, L.; Razak, A.R.A.; Lam, R.; Demuth, T.; Rose, S.; Lee, M.A.; et al. Phase I study evaluating WEE1 inhibitor AZD1775 as monotherapy and in combination with gemcitabine, cisplatin, or carboplatin in patients with advanced solid tumors. J. Clin. Oncol. 2016, 34, 4371–4380. [Google Scholar] [CrossRef]
- Tolcher, A.; Mamdani, H.; Chalasani, P.; Meric-Bernstam, F.; Gazdoiu, M.; Makris, L.; Pultar, P.; Voliotis, D. Clinical Activity of Single-Agent ZN-c3, an Oral WEE1 Inhibitor, in a Phase 1 Dose-Escalation Trial in Patients with Advanced Solid Tumors. Available online: https://www.abstractsonline.com/pp8/#!/9325/presentation/5146 (accessed on 21 April 2021).
- Tokunaga, R.; Xiu, J.; Goldberg, R.M.; Philip, P.A.; Seeber, A.; Battaglin, F.; Arai, H.; Lo, J.H.; Naseem, M.; Puccini, A.; et al. The impact of ARID1A mutation on molecular characteristics in colorectal cancer. Eur. J. Cancer 2020, 140, 119–129. [Google Scholar] [CrossRef]
- Wei, X.L.; Wang, D.S.; Xi, S.Y.; Wu, W.J.; Chen, D.L.; Zeng, Z.L.; Wang, R.Y.; Huang, Y.X.; Jin, Y.; Wang, F.; et al. Clinicopathologic and prognostic relevance of ARID1A protein loss in colorectal cancer. World J. Gastroenterol. 2014, 20, 18404–18412. [Google Scholar] [CrossRef]
- Park, Y.; Chui, M.H.; Rahmanto, Y.S.; Yu, Z.C.; Shamanna, R.A.; Bellani, M.A.; Gaillard, S.; Ayhan, A.; Viswanathan, A.; Seidman, M.M.; et al. Loss of ARID1A in tumor cells renders selective vulnerability to combined ionizing radiation and PARP inhibitor therapy. Clin. Cancer Res. 2019, 25, 5584–5593. [Google Scholar] [CrossRef]
- Sen, M.; Wang, X.; Hamdan, F.H.; Rapp, J.; Eggert, J.; Kosinsky, R.L.; Wegwitz, F.; Kutschat, A.P.; Younesi, F.S.; Gaedcke, J.; et al. ARID1A facilitates KRAS signaling-regulated enhancer activity in an AP1-dependent manner in colorectal cancer cells. Clin. Epigenetics 2019, 11. [Google Scholar] [CrossRef]
- Niedermaier, B.; Sak, A.; Zernickel, E.; Xu, S.; Groneberg, M.; Stuschke, M. Targeting ARID1A-mutant colorectal cancer: Depletion of ARID1B increases radiosensitivity and modulates DNA damage response. Sci. Rep. 2019, 9, 18207. [Google Scholar] [CrossRef]
- Gorecki, L.; Andrs, M.; Korabecny, J. Clinical candidates targeting the atr–chk1–wee1 axis in cancer. Cancers 2021, 13, 795. [Google Scholar] [CrossRef]
- Parseghian, C.M.; Napolitano, S.; Loree, J.M.; Kopetz, S. Mechanisms of innate and acquired resistance to anti-EGFR therapy: A review of current knowledge with a focus on rechallenge therapies. Clin. Cancer Res. 2019, 25, 6899–6908. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Crisafulli, G.; Sogari, A.; Reilly, N.M.; Arena, S.; Lamba, S.; Bartolini, A.; Amodio, V.; Magrì, A.; Novara, L.; et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science 2019, 366, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Arena, S.; Siena, S.; Bardelli, A.; Sartore-Bianchi, A. The DNA damage response pathway as a land of therapeutic opportunities for colorectal cancer. Ann. Oncol. 2020, 31, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Leichman, L.; Groshen, S.; O’Neil, B.H.; Messersmith, W.; Berlin, J.; Chan, E.; Leichman, C.G.; Cohen, S.J.; Cohen, D.; Lenz, H.; et al. Phase II study of olaparib (AZD-2281) after standard systemic therapies for disseminated colorectal cancer. Oncologist 2016, 21, 172–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Castro e Gloria, H.; Jesuíno Nogueira, L.; Bencke Grudzinski, P.; da Costa Ghignatti, P.V.; Guecheva, T.N.; Motta Leguisamo, N.; Saffi, J. Olaparib-mediated enhancement of 5-fluorouracil cytotoxicity in mismatch repair deficient colorectal cancer cells. BMC Cancer 2021, 21, 448. [Google Scholar] [CrossRef] [PubMed]
- Arena, S.; Corti, G.; Durinikova, E.; Montone, M.; Reilly, N.M.; Russo, M.; Lorenzato, A.; Arcella, P.; Lazzari, L.; Rospo, G.; et al. A subset of colorectal cancers with cross-sensitivity to olaparib and oxaliplatin. Clin. Cancer Res. 2020, 26, 1372–1384. [Google Scholar] [CrossRef]
- Smeby, J.; Kryeziu, K.; Berg, K.C.G.; Eilertsen, I.A.; Eide, P.W.; Johannessen, B.; Guren, M.G.; Nesbakken, A.; Bruun, J.; Lothe, R.A.; et al. Molecular correlates of sensitivity to PARP inhibition beyond homologous recombination deficiency in pre-clinical models of colorectal cancer point to wild-type TP53 activity. EBioMedicine 2020, 59. [Google Scholar] [CrossRef]
- Serra Elizalde, V.; Llop-Guevara, A.; Pearson, A.; Cruz, C.; Castroviejo-Bermejo, M.; Chopra, N.; Tovey, H.; Toms, C.; Kriplani, D.; Gevensleben, H.; et al. 1O Detection of homologous recombination repair deficiency (HRD) in treatment-naive early triple-negative breast cancer (TNBC) by RAD51 foci and comparison with DNA-based tests. Ann. Oncol. 2021, 32, S21–S22. [Google Scholar] [CrossRef]
- Llop-Guevara, A.; Vladimirova, V.; Schneeweiss, A.; Villacampa, G.; Karn, T.; Zahm, D.-M.; Herencia-Ropero, A.; Jank, P.; van Mackelenbergh, M.; Fasching, P.A.; et al. 2O Association of RAD51 with homologous recombination deficiency (HRD) and clinical outcomes in untreated triple-negative breast cancer (TNBC): Analysis of the GeparSixto randomized clinical trial. Ann. Oncol. 2021, 32, S22. [Google Scholar] [CrossRef]
- Melinda, L.T.; Kirsten, M.T.; Julia, R.; Bryan, H.; Gordon, B.M.; Kristin, C.J.; Zoltan, S.; William, T.B.; Eric, P.W.; Nadine, M.T.; et al. Homologous recombination deficiency (hrd) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin. Cancer Res. 2016, 22, 3764–3773. [Google Scholar] [CrossRef] [Green Version]
- Shiro Takamatsu, A.; Brown, J.; Yamaguchi, K.; Hamanishi, J.; Yamanoi, K.; Takaya, H.; Kaneyasu, T.; Mori, S.; Mandai, M.; Matsumura, N. The utility of homologous recombination deficiency biomarkers across cancer types. medRxiv 2021. [Google Scholar] [CrossRef]
| Inhibitor | Biomarkers | Combined Therapy or Deficiency | Model of Study | Results | Biological Explanation | Reference |
|---|---|---|---|---|---|---|
| Inhibitors in perspective for clinical trial in CRC (inhibitors already tested in clinical trial for another tumor type or condition) | ||||||
| PARPi (ABT-888) | Presence of MSI | MRE11 deficiency | Homozygous MRE11 mutant CRC cell lines (HCT116, LoVo, RKO, SW48) and wild type cell lines (HCT15, SW403, HT29, SW620) | Cell lines with homozygous MRE11 mutation had increased sensitivity to PARPi. | PARPi induces DSB during replication, which is repaired mainly by HR repair. However, the presence of MSI is associated with HR impairment by MRE11 deficiency, which sensitizes cells to PARPi. | [101] |
| ATRi (VX-970) | - | CHK1i (V158411) | CRC cell line (HT29 and U2OS) | Both inhibitors induced loss of viability and the combination of them in low doses increased sensitivity. | Both proteins act on the same cell cycle control axis-repair of DNA DSB forming during S and G2 phases. The inhibition of both reinforces the blocking of the axis, generating replicative stress. | [144] |
| CHK1i (LY2606368) | TP53 mutation and RS markers | - | Primary CRC enriched for cancer stem cells | Sensitive cells displayed signs of replicative stress (P-RPA32, γ-H2AX, and P-ATM) and the effect did not depend on RAS mutation status. | Cells that lack p53 depend on the ATR-CHK1 axis to promote cell cycle arrest when DSB is formed. Therefore, the inhibition of CHK1 kinase in these cells sensitizes them to DSB. Replicative stress induces DSB, therefore cells ongoing RS are sensitive to CHK1i. | [145] |
| WEE1i (AZD1775) | WEE1 overexpression | - | Primary CRC liver metastases endothelial cells | Induction of apoptosis, increase in DNA DSB markers and inhibition of tube formation. | The WEE1 protein is upregulated in CRC liver metastases compared to endothelial cells of the normal adjacent liver and has functional importance by protecting against DNA DSB. The inhibition of WEE1 makes cells vulnerable to DSB. | [146] |
| WEE1i (AZD1775) | TP53 mutation | 5-FU | CRC cell line (HT29) | The WEE1i single treatment had cytotoxic effects by induction of DNA DSB. The co-treatment with 5-FU increased DSB marker (γ-H2AX) and apoptosis compared to the 5-FU single treatment. | The lack of functional p53 prevents G1 arrest, so the cell depends on the G2 checkpoint to repair DNA damage. WEE1 is a kinase that regulates the G2 checkpoint to repair DNA damage, so targeting WEE1 increases DNA damage sensitivity. | [147] |
| WEE1i (AZD1775) | - | PARGi (PDDX-004/PDD00017272) | CRC cell line (HCT116) | The co-treatment increased DNA DSB marker (γ-H2AX) in S-phase dependent manner. | WEE1i induces replication stress and DNA damage while PARGi delays the replication fork restart during replication stress. The combination of both increases DNA damage. | [148] |
| WEE1i (MK1775) | TP53 mutation | Irinotecan | CRC cell line (HT29 and SW480) | The co-treatment increased DNA DSB marker (γ-H2AX) and apoptosis compared to single treatments. | The lack of functional p53 prevents G1 arrest, so the cell depends on the G2 checkpoint to repair DNA damage. WEE1 is a kinase that regulates the G2 checkpoint to repair DNA damage, so targeting WEE1 increases DNA damage sensitivity. | [149] |
| Inhibitors in progress in CRC in vitro studies | ||||||
| PARPi (LT-626) | Presence of MSI | MRE11 deficiency | CRC cell line with a biallelic mutation in MRE11 (HCT116, HCT116/CS, HCT116/C3, RKO, SW48, and LoVo), with monoallelic mutation (DLD1), and wild type (SW837, HT29, and SW480) | Cell lines with a biallelic mutation in MRE11 showed higher sensitivity to PARPi and the knocked-down of MRE11 increased sensitivity to PARPi. | PARPi induces DNA DSB during replication, which is repaired mainly by HR repair. However, the presence of MSI is associated with HR impairment by MRE11 deficiency, which sensitizes cells to PARPi. | [103] |
| RAD51i (RI-1) | KRAS mutation | - | CRC cell line (HCT116, HKe-3) | KRAS-mutant cell (HCT116) was more sensitive to RI-1 than the isogenic wild type HKe-3, which showed a limited response. | KRAS-mutated CRC cells show stalling of the replication fork, which increases HR repair signaling. This occurs because of the hyperactivation of c-MYC. Targeting RAD51, a key protein in HR repair, thus may sensitize KRAS-mutated CRC cells. | [150] |
| RAD51i (B02) | - | - | CRC cell line (SW480) | Induction of apoptosis. | RAD51 expression levels are upregulated in biopsy samples of CRC and may thus support cancer progression. | [48] |
| ATRi (CBP-93872) | TP53 mutation | Oxaliplatin, cisplatin, and 5-FU | CRC cell line (HT29) | ATRi single treatment had no effect, however, the combination with compounds that induce DNA damage as DNA DSB (oxaliplatin and cisplatin) or replication fork arrest (5-FU) induced an increase in apoptosis compared to single therapies. | The lack of functional p53 prevents G1 arrest, so the cell depends on the G2 checkpoint to repair DNA damage. ATR activation regulates the G2 checkpoint to repair DNA damage, so targeting ATR activation may increase DNA damage sensitivity by suppressing the maintenance of the G2 checkpoint. | [151] |
| ATMi (KU55933) | - | PARPi (Olaparib) | CRC cell line (HCT116) | ATMi sensitizes cells to PAPRi and the deletion of p53 increased the co-treatment effect. | PARP1 induces DNA DSB during replication, which activates ATM and so ATR promotes cell cycle arrest and DNA repair. The sensitivity to PARPi increases when the cell lacks ATM levels. | [152] |
| ATMi (AZ31) | - | Irinotecan | CRC cell line (HCT15, HCT116, RKO, LoVo, LS132, and Caco2) and patient-derived xenografts (PDX) | Three (HCT15, HCT116, and RKO) of the six cell lines presented combinational sensitivity to AZ31 and irinotecan compared to single treatments. In CRC PDX models, the co-treatment was effective only in irinotecan-resistant tumors. | Irinotecan induces DNA DSB, which activates ATM to promote cell cycle arrest and DNA repair. The inhibition of ATM may sensitize cells to DNA DSB. | [153] |
| Inhibitors in perspective for in vitro analysis in CRC | ||||||
| MRE11i (mirin) | RS markers | - | Human myeloma cell lines (MM1S, RPMI-8226, JJN3, U266) and B-cell (LINF903). | Only cells presenting RS markers (RAD51 and γ-H2AX signaling) showed sensitivity to mirin. | Human myeloma cell lines presenting DNA DSB signaling depend on DSB repair pathways for survival. To inhibit DSB repair may sensitize them. | [154] |
| MRE11i (mirin) | MYCN amplified | - | Neuroblastoma cell line with MYCN-amplification (SHEP, GIMEN, and SK-N-SH) and MYCN single copy (LAN5, IMR32, and KELLY) and LAN5 xenograft in mice. | Only the cell lines with MYCN-amplification showed increased mRNA levels of MRE11 and sensitivity to mirin treatment. Mirin suppressed tumor growth in xenografted mice. | MRE11 is required to restrain replication stress induced by MYCN-amplification. MRE11 inhibition may trigger intolerable levels of RS. | [155] |
| MRE11i (mirin) | PARP1 upregulation | RAD51i (B02) | CRC-stem cells with upregulation of PARP1, generated by CHK1i (prexasertib) treatment until acquired resistance. | The single treatments with Mirin or B02 were ineffective against CHK1i-resistant cells, but co-treatment killed the cells by induction of mitotic catastrophe and apoptosis. | CHK1i-resistant cells upregulate PARP1 to modulate fork speed and decrease RS levels. MRE11 and RAD51 may cooperate with PARP1 to deal with RS. | [156] |
| RAD52i (F79 aptamer, D-I03) | BRCA1 deficiency | PARPi (talazoparib) | BRCA1-deficient primary acute myeloid leukemia (AML) xenograft in NSG mice, and BRCA1-deficient solid tumor growth in nude mice. | The combination of PARP inhibitor with RAD52 inhibitors selectively reduced BRCA1-deficient tumor growth. | PARPi induces DNA DSB during replication, which is repaired mainly by HR repair. However, BRCA-deficient cells have HR repair impairment, and single-strand annealing (SSA) is a backup pathway that may support survival. The RAD52 inhibition may sensitize cells to PARPi by the accumulation of DNA DSB in BRCA-deficient tumor cells. | [157] |
| RAD52i (F79 aptamer, 6-OH-Dopa, D-I03) | BRCA deficiency | PARPi (olaparib, talazoparib) | Several human tumor cell lines | The combination of PARP inhibitors with RAD52 inhibitors selectively killed BRCA-deficient cells. | See description above. | [157] |
| Polθi (Novobiocin) | BRCA deficiency | PARPi (Olaparib) | Xenograft mice derived from patients with germline BRCA1 mutation and acquired PARPi resistance, and HR-proficient PDX model. | Olaparib single treatment did not reduce tumor growth, while Polθi single treatment reduced tumor growth and the combined therapy was even more efficient. BRCA1 wild type PDX model was resistant to both single and co-treatment. Polθi toxicity depends on the accumulation of RAD51 foci. | PARPi induces DNA DSB during replication, which is repaired mainly by HR repair, but also by MMEJ repair. BRCA-deficient cells have HR repair impairment and respond to PARPi. However, MMEJ has emerged as a backup pathway in PARPi resistant cells. The Polθ inhibition may prevent MMEJ repair and increase PARPi sensitivity in BRCA-deficient tumor cells. | [158] |
| Inhibitor | NCT Number | Conditions | Primary and Secondary Endpoints | Intervention/Treatment for CRC | Phase(s) | Status | Reference | |
|---|---|---|---|---|---|---|---|---|
| Chk1 Inhibitor | Prexasertib (LY2606368) | NCT02860780 | Advanced/metastatic cancer, including CRC with KRAS and/or BRAF mutations | MTD | Prexasertib + ralimetinib | Phase 1 | Completed | [250] |
| NCT02124148 | Advanced/metastatic cancer, including KRAS wild type CRC, which has failed to oxaliplatin- and irinotecan-based chemotherapy or is intolerant of irinotecan or oxaliplatin | Prexasertib + cetuximab | Phase 1b | Completed | - | |||
| LY2880070 | NCT02632448 | Solid tumors, including CRC | MTD | LY2880070 ± gemcitabine | Phase 1b/2a | Recruiting | [251] | |
| SRA737 | NCT02797964 | Advanced solid tumors (including CRC) and non-Hodgkin’s lymphoma | Subjects with TRAE, MTD, recommended Phase 2 dose, ORR | SRA737 | Phase 1/2 | Completed | [252] | |
| ATM Inhibitor | AZD0156 | NCT02588105 | Advanced solid tumors, including CRC | Subjects with TRAE | AZD0156 + irinotecan/ FOLFIRI | Phase 1 | Active, not recruiting | [253] |
| ATR Inhibitor | Ceralasertib (AZD6738) | NCT04704661 | Advanced solid tumors, including CRC that have a change (mutation) in the HER2 gene or protein | RP2D, PD profile of tumor tissues between Top1 inhibition and Top1 + ATR dual inhibition | Ceralasertib + trastuzumab deruxtecan | Phase1/1b | Not yet recruiting | - |
| Elimusertib (BAY 1895344) | NCT04535401 | Advanced or metastatic cancers of the stomach and intestines, including CRC, which have previously progressed on irinotecan with and without DDR defects | MTD | Elimusertib + FOLFIRI | Phase 1 | Not yet recruiting | [254] | |
| Berzosertib (M6620, VX-970) | NCT02157792 | Advanced solid tumors, including CRC harboring molecular aberrations, including ATM loss and an ARID1A mutation, achieved complete response, and maintained this response, with a progression-free survival of 29 months at last assessment | Safety (AE, laboratory values, ECG), ORR | M6620 + carboplatin | Phase 1 | Completed | [255,256] | |
| WEE1 inhibitor | Adavosertib (AZD1775, MK-1755) | NCT02906059 | Metastatic CRC with RAS (KRAS or NRAS) or BRAF mutated | DLT and TREA | AZD1775 + irinotecan | Phase Ib | Completed | [257] |
| NCT02465060 | Advanced refractory solid tumors (including CRC), lymphomas, or multiple myeloma | ORR | Adavosertib + targeted therapy according to mutational status | Phase II | Recruiting | [258,259] | ||
| NCT00648648 | Advanced solid tumors, including CRC | DLT, best ORR | Adavosertib + gemcitabine + cisplatin or carboplatin | Phase 1 | Completed | [260] | ||
| ZN-c3 | NCT04158336 | Solid tumors, including CRC | MTD, RP2D, DLR, ORR | ZN-c3 | Phase I/II | Recruiting | [261] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomasini, P.P.; Guecheva, T.N.; Leguisamo, N.M.; Péricart, S.; Brunac, A.-C.; Hoffmann, J.S.; Saffi, J. Analyzing the Opportunities to Target DNA Double-Strand Breaks Repair and Replicative Stress Responses to Improve Therapeutic Index of Colorectal Cancer. Cancers 2021, 13, 3130. https://doi.org/10.3390/cancers13133130
Tomasini PP, Guecheva TN, Leguisamo NM, Péricart S, Brunac A-C, Hoffmann JS, Saffi J. Analyzing the Opportunities to Target DNA Double-Strand Breaks Repair and Replicative Stress Responses to Improve Therapeutic Index of Colorectal Cancer. Cancers. 2021; 13(13):3130. https://doi.org/10.3390/cancers13133130
Chicago/Turabian StyleTomasini, Paula Pellenz, Temenouga Nikolova Guecheva, Natalia Motta Leguisamo, Sarah Péricart, Anne-Cécile Brunac, Jean Sébastien Hoffmann, and Jenifer Saffi. 2021. "Analyzing the Opportunities to Target DNA Double-Strand Breaks Repair and Replicative Stress Responses to Improve Therapeutic Index of Colorectal Cancer" Cancers 13, no. 13: 3130. https://doi.org/10.3390/cancers13133130
APA StyleTomasini, P. P., Guecheva, T. N., Leguisamo, N. M., Péricart, S., Brunac, A.-C., Hoffmann, J. S., & Saffi, J. (2021). Analyzing the Opportunities to Target DNA Double-Strand Breaks Repair and Replicative Stress Responses to Improve Therapeutic Index of Colorectal Cancer. Cancers, 13(13), 3130. https://doi.org/10.3390/cancers13133130








