Targeted Therapies and Immune Checkpoint Inhibitors in Primary CNS Lymphoma
Abstract
:Simple Summary
Abstract
1. Background
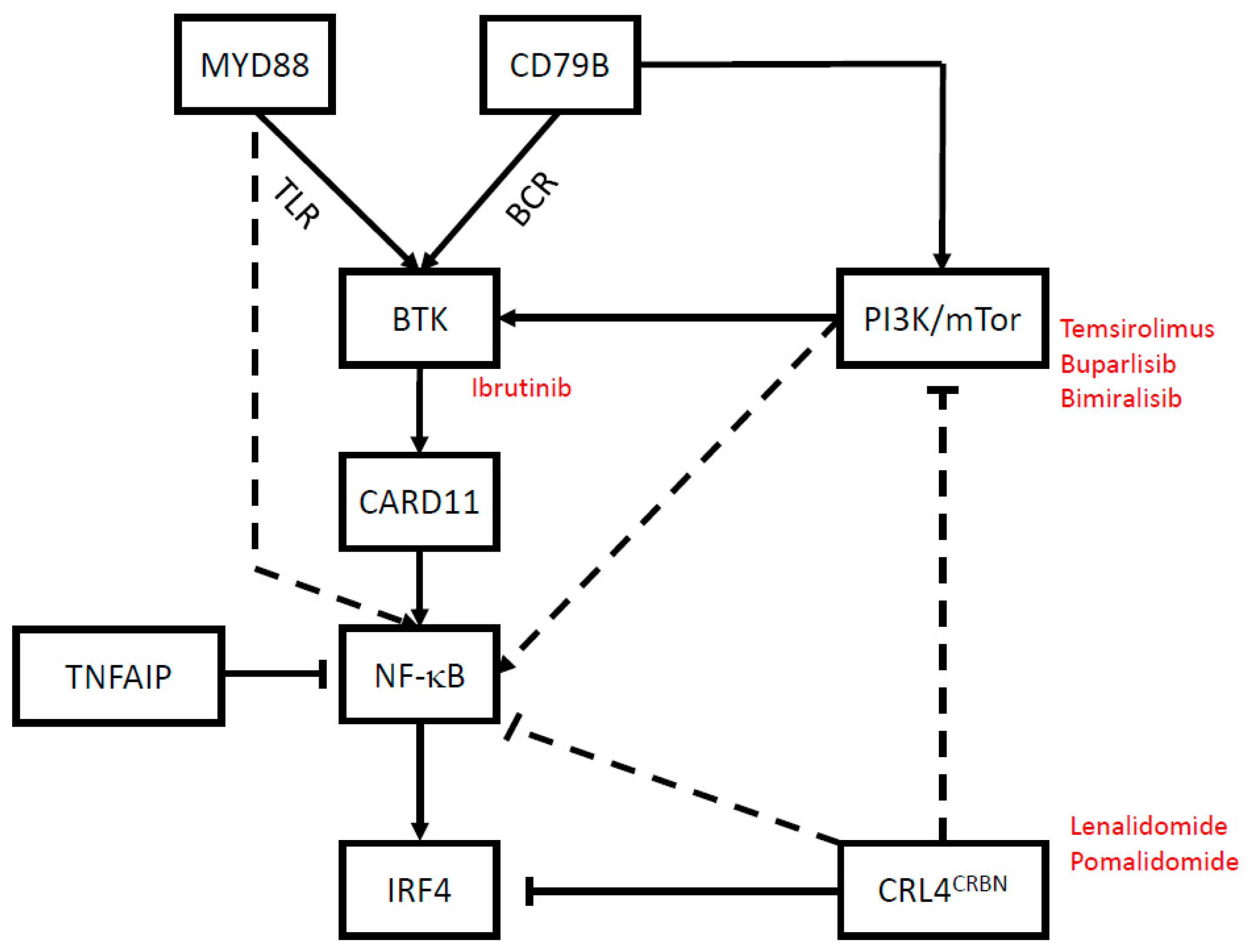
2. Targeted Therapies
2.1. Bruton’s Tyrosine Kinase: Ibrutinib
2.2. PI3K/mTor: Temsirolimus, Buparlisib and Bimiralisib
2.3. Immune Modulatory Small Molecules: Lenalidomide and Pomalidomide
2.4. Other Molecularly Targeted Agents
3. Immune Checkpoint Inhibitors
3.1. Background
3.2. The Immune Signature of PCNSL
3.3. Efficacy of PD-1 Blockade in Preclinical PCNSL Models
3.4. Clinical Data on Immune Checkpoint Inhibitors in PCNSL
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro. Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- Mrugala, M.M.; Rubenstein, J.L.; Ponzoni, M.; Batchelor, T.T. Insights into the biology of primary central nervous system lymphoma. Curr. Oncol. Rep. 2009, 11, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrlinger, U.; Schaefer, N.; Fimmers, R.; Griesinger, F.; Rauch, M.; Kirchen, H.; Roth, P.; Glas, M.; Bamberg, M.; Martus, P.; et al. Early whole brain radiotherapy in primary CNS lymphoma: Negative impact on quality of life in the randomized G-PCNSL-SG1 trial. J. Cancer Res. Clin. Oncol. 2017, 143, 1815–1821. [Google Scholar] [CrossRef] [Green Version]
- Ferreri, A.J.; Reni, M.; Foppoli, M.; Martelli, M.; Pangalis, G.A.; Frezzato, M.; Cabras, M.G.; Fabbri, A.; Corazzelli, G.; Ilariucci, F.; et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: A randomised phase 2 trial. Lancet 2009, 374, 1512–1520. [Google Scholar] [CrossRef]
- Rubenstein, J.L.; Hsi, E.D.; Johnson, J.L.; Jung, S.H.; Nakashima, M.O.; Grant, B.; Cheson, B.D.; Kaplan, L.D. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J. Clin. Oncol. 2013, 31, 3061–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Du, H.; Ye, X.; Zhang, L.; Xiao, H. High-dose methotrexate-based regimens and post-remission consolidation for treatment of newly diagnosed primary cns lymphoma: Meta-analysis of clinical trials. Sci. Rep. 2021, 11, 2125. [Google Scholar] [CrossRef] [PubMed]
- Korfel, A.; Thiel, E.; Martus, P.; Moehle, R.; Griesinger, F.; Rauch, M.; Roeth, A.; Hertenstein, B.; Fischer, T.; Hundsberger, T.; et al. Randomized phase III study of whole-brain radiotherapy for primary CNS lymphoma. Neurology 2015, 84, 1242–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiel, E.; Korfel, A.; Martus, P.; Kanz, L.; Griesinger, F.; Rauch, M.; Roth, A.; Hertenstein, B.; von Toll, T.; Hundsberger, T.; et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010, 11, 1036–1047. [Google Scholar] [CrossRef] [Green Version]
- Ferreri, A.J.M.; Cwynarski, K.; Pulczynski, E.; Fox, C.P.; Schorb, E.; La Rosee, P.; Binder, M.; Fabbri, A.; Torri, V.; Minacapelli, E.; et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: Results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017, 4, e510–e523. [Google Scholar] [CrossRef] [Green Version]
- Illerhaus, G.; Kasenda, B.; Ihorst, G.; Egerer, G.; Lamprecht, M.; Keller, U.; Wolf, H.H.; Hirt, C.; Stilgenbauer, S.; Binder, M.; et al. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: A prospective, single-arm, phase 2 trial. Lancet Haematol. 2016, 3, e388–e397. [Google Scholar] [CrossRef]
- Holdhoff, M.; Ambady, P.; Abdelaziz, A.; Sarai, G.; Bonekamp, D.; Blakeley, J.; Grossman, S.A.; Ye, X. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology 2014, 83, 235–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreri, A.J.; Cwynarski, K.; Pulczynski, E.; Ponzoni, M.; Deckert, M.; Politi, L.S.; Torri, V.; Fox, C.P.; Rosee, P.L.; Schorb, E.; et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016, 3, e217–e227. [Google Scholar] [CrossRef] [Green Version]
- Bromberg, J.E.C.; Issa, S.; Bakunina, K.; Minnema, M.C.; Seute, T.; Durian, M.; Cull, G.; Schouten, H.C.; Stevens, W.B.C.; Zijlstra, J.M.; et al. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): A randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2019, 20, 216–228. [Google Scholar] [CrossRef]
- Van Dijck, R.; Doorduijn, J.K.; Bromberg, J.E.C. The role of rituximab in the treatment of primary central nervous system lymphoma. Cancers 2021, 13, 1920. [Google Scholar] [CrossRef]
- Roth, P.; Hoang-Xuan, K. Challenges in the treatment of elderly patients with primary central nervous system lymphoma. Curr. Opin. Neurol. 2014, 27, 697–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Camilleri-Broet, S.; Criniere, E.; Broet, P.; Delwail, V.; Mokhtari, K.; Moreau, A.; Kujas, M.; Raphael, M.; Iraqi, W.; Sautes-Fridman, C.; et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: Analysis of 83 cases. Blood 2006, 107, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Tun, H.W.; Personett, D.; Baskerville, K.A.; Menke, D.M.; Jaeckle, K.A.; Kreinest, P.; Edenfield, B.; Zubair, A.C.; O’Neill, B.P.; Lai, W.R.; et al. Pathway analysis of primary central nervous system lymphoma. Blood 2008, 111, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Grommes, C.; Nayak, L.; Tun, H.W.; Batchelor, T.T. Introduction of novel agents in the treatment of primary CNS lymphoma. Neuro Oncol 2019, 21, 306–313. [Google Scholar] [CrossRef]
- Braggio, E.; Van Wier, S.; Ojha, J.; McPhail, E.; Asmann, Y.W.; Egan, J.; da Silva, J.A.; Schiff, D.; Lopes, M.B.; Decker, P.A.; et al. Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin. Cancer Res. 2015, 21, 3986–3994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grommes, C.; Pastore, A.; Palaskas, N.; Tang, S.S.; Campos, C.; Schartz, D.; Codega, P.; Nichol, D.; Clark, O.; Hsieh, W.Y.; et al. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017, 7, 1018–1029. [Google Scholar] [CrossRef] [Green Version]
- Montesinos-Rongen, M.; Schafer, E.; Siebert, R.; Deckert, M. Genes regulating the B cell receptor pathway are recurrently mutated in primary central nervous system lymphoma. Acta Neuropathol. 2012, 124, 905–906. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Tateishi, K.; Niwa, T.; Matsushita, Y.; Tamura, K.; Kinoshita, M.; Tanaka, K.; Fukushima, S.; Takami, H.; Arita, H.; et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol. Appl. Neurobiol. 2016, 42, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Vater, I.; Montesinos-Rongen, M.; Schlesner, M.; Haake, A.; Purschke, F.; Sprute, R.; Mettenmeyer, N.; Nazzal, I.; Nagel, I.; Gutwein, J.; et al. The mutational pattern of primary lymphoma of the central nervous system determined by whole-exome sequencing. Leukemia 2015, 29, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.K.; Hoang, T.; Law, S.C.; Brosda, S.; O’Rourke, K.; Tobin, J.W.D.; Vari, F.; Murigneux, V.; Fink, L.; Gunawardana, J.; et al. EBV-associated primary CNS lymphoma occurring after immunosuppression is a distinct immunobiological entity. Blood 2021, 137, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, K.; Kawazu, M.; Kojima, S.; Ueno, T.; Sai, E.; Soda, M.; Ueda, H.; Yasuda, T.; Yamaguchi, H.; Lee, J.; et al. Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol. 2016, 131, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Nishizaki, T.; Kubota, H.; Harada, K.; Suzuki, M.; Sasaki, K. Distinct primary central nervous system lymphoma defined by comparative genomic hybridization and laser scanning cytometry. Cancer Genet. Cytogenet. 2001, 125, 147–150. [Google Scholar] [CrossRef]
- Rickert, C.H.; Dockhorn-Dworniczak, B.; Simon, R.; Paulus, W. Chromosomal imbalances in primary lymphomas of the central nervous system. Am. J. Pathol. 1999, 155, 1445–1451. [Google Scholar] [CrossRef] [Green Version]
- Weber, T.; Weber, R.G.; Kaulich, K.; Actor, B.; Meyer-Puttlitz, B.; Lampel, S.; Buschges, R.; Weigel, R.; Deckert-Schluter, M.; Schmiedek, P.; et al. Characteristic chromosomal imbalances in primary central nervous system lymphomas of the diffuse large B-cell type. Brain Pathol. 2000, 10, 73–84. [Google Scholar] [CrossRef]
- Schwindt, H.; Vater, I.; Kreuz, M.; Montesinos-Rongen, M.; Brunn, A.; Richter, J.; Gesk, S.; Ammerpohl, O.; Wiestler, O.D.; Hasenclever, D.; et al. Chromosomal imbalances and partial uniparental disomies in primary central nervous system lymphoma. Leukemia 2009, 23, 1875–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriks, R.W.; Yuvaraj, S.; Kil, L.P. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat. Rev. Cancer 2014, 14, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Grommes, C.; Tang, S.S.; Wolfe, J.; Kaley, T.J.; Daras, M.; Pentsova, E.I.; Piotrowski, A.F.; Stone, J.; Lin, A.; Nolan, C.P.; et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood 2019, 133, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Soussain, C.; Choquet, S.; Blonski, M.; Leclercq, D.; Houillier, C.; Rezai, K.; Bijou, F.; Houot, R.; Boyle, E.; Gressin, R.; et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II “proof-of-concept” iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur. J. Cancer 2019, 117, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Dunleavy, K.; Roschewski, M.; Widemann, B.C.; Butman, J.A.; Schmitz, R.; Yang, Y.; Cole, D.E.; Melani, C.; Higham, C.S.; et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell 2017, 31, 833–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korfel, A.; Schlegel, U.; Herrlinger, U.; Dreyling, M.; Schmidt, C.; von Baumgarten, L.; Pezzutto, A.; Grobosch, T.; Kebir, S.; Thiel, E.; et al. Phase II Trial of temsirolimus for relapsed/refractory primary CNS lymphoma. J. Clin. Oncol. 2016, 34, 1757–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grommes, C.; Pentsova, E.; Nolan, C.; Wolfe, J.; Mellinghoff, I.K.; Deangelis, L. Phase II study of single agent buparlisib in recurrent/refractory primary (PCNSL) and secondary CNS lymphoma (SCNSL). Ann. Oncol. 2016, 27, VI106. [Google Scholar] [CrossRef] [Green Version]
- Beaufils, F.; Cmiljanovic, N.; Cmiljanovic, V.; Bohnacker, T.; Melone, A.; Marone, R.; Jackson, E.; Zhang, X.; Sele, A.; Borsari, C.; et al. 5-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl) pyridin-2-amine (PQR309), a potent, brain-penetrant, orally bioavailable, pan-class I PI3K/mTOR inhibitor as clinical candidate in oncology. J. Med. Chem. 2017, 60, 7524–7538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohnacker, T.; Prota, A.E.; Beaufils, F.; Burke, J.E.; Melone, A.; Inglis, A.J.; Rageot, D.; Sele, A.M.; Cmiljanovic, V.; Cmiljanovic, N.; et al. Deconvolution of Buparlisib’s mechanism of action defines specific PI3K and tubulin inhibitors for therapeutic intervention. Nat. Commun. 2017, 8, 14683. [Google Scholar] [CrossRef] [Green Version]
- Tarantelli, C.; Gaudio, E.; Arribas, A.J.; Kwee, I.; Hillmann, P.; Rinaldi, A.; Cascione, L.; Spriano, F.; Bernasconi, E.; Guidetti, F.; et al. PQR309 is a novel dual PI3K/mTOR inhibitor with preclinical antitumor activity in lymphomas as a single agent and in combination therapy. Clin. Cancer Res. 2018, 24, 120–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Girona, A.; Mendy, D.; Ito, T.; Miller, K.; Gandhi, A.K.; Kang, J.; Karasawa, S.; Carmel, G.; Jackson, P.; Abbasian, M.; et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012, 26, 2326–2335. [Google Scholar] [CrossRef] [PubMed]
- Kronke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, G.; Middleton, R.E.; Sun, H.; Naniong, M.; Ott, C.J.; Mitsiades, C.S.; Wong, K.K.; Bradner, J.E.; Kaelin, W.G., Jr. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014, 343, 305–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliva, E.N.; Cuzzola, M.; Aloe Spiriti, M.A.; Poloni, A.; Lagana, C.; Rigolino, C.; Morabito, F.; Galimberti, S.; Ghio, R.; Cortelezzi, A.; et al. Biological activity of lenalidomide in myelodysplastic syndromes with del5q: Results of gene expression profiling from a multicenter phase II study. Ann. Hematol. 2013, 92, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Dredge, K.; Horsfall, R.; Robinson, S.P.; Zhang, L.H.; Lu, L.; Tang, Y.; Shirley, M.A.; Muller, G.; Schafer, P.; Stirling, D.; et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc. Res. 2005, 69, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shaffer, A.L., 3rd; Emre, N.C.; Ceribelli, M.; Zhang, M.; Wright, G.; Xiao, W.; Powell, J.; Platig, J.; Kohlhammer, H.; et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 2012, 21, 723–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjorklund, C.C.; Lu, L.; Kang, J.; Hagner, P.R.; Havens, C.G.; Amatangelo, M.; Wang, M.; Ren, Y.; Couto, S.; Breider, M.; et al. Rate of CRL4(CRBN) substrate Ikaros and Aiolos degradation underlies differential activity of lenalidomide and pomalidomide in multiple myeloma cells by regulation of c-Myc and IRF4. Blood Cancer J. 2015, 5, e354. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qiu, Y.; Personett, D.; Huang, P.; Edenfield, B.; Katz, J.; Babusis, D.; Tang, Y.; Shirely, M.A.; Moghaddam, M.F.; et al. Pomalidomide shows significant therapeutic activity against CNS lymphoma with a major impact on the tumor microenvironment in murine models. PLoS ONE 2013, 8, e71754. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.; Geng, H.; Fraser, E.J.; Formaker, P.; Chen, L.; Sharma, J.; Killea, P.; Choi, K.; Ventura, J.; Kurhanewicz, J.; et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018, 2, 1595–1607. [Google Scholar] [CrossRef] [Green Version]
- Platten, M.; Friedrich, M.; Wainwright, D.A.; Panitz, V.; Opitz, C.A. Tryptophan metabolism in brain tumors-IDO and beyond. Curr. Opin. Immunol. 2021, 70, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ghesquieres, H.; Chevrier, M.; Laadhari, M.; Chinot, O.; Choquet, S.; Molucon-Chabrot, C.; Beauchesne, P.; Gressin, R.; Morschhauser, F.; Schmitt, A.; et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: A multicenter prospective “proof of concept” phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA) dagger. Ann. Oncol. 2019, 30, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Tun, H.W.; Johnston, P.B.; DeAngelis, L.M.; Atherton, P.J.; Pederson, L.D.; Koenig, P.A.; Reeder, C.B.; Omuro, A.M.P.; Schiff, D.; O’Neill, B.; et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood 2018, 132, 2240–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braaten, K.M.; Betensky, R.A.; de Leval, L.; Okada, Y.; Hochberg, F.H.; Louis, D.N.; Harris, N.L.; Batchelor, T.T. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin. Cancer Res. 2003, 9, 1063–1069. [Google Scholar] [PubMed]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.H.; Badawi, M.A.; Place, A.E.; Palenski, T.L.; Arrendale, R.; Kim, S.Y.; Federico, S.; Cooper, T.M.; Menon, R. Venetoclax Crosses the blood brain barrier: A pharmacokinetic analysis of the cerebrospinal fluid in pediatric leukemia patients. Blood 2020, 136, 30–31. [Google Scholar] [CrossRef]
- Roth, P.; Mason, W.P.; Richardson, P.G.; Weller, M. Proteasome inhibition for the treatment of glioblastoma. Expert Opin. Investig. Drugs 2020, 29, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Mondello, P.; Brea, E.J.; De Stanchina, E.; Toska, E.; Chang, A.Y.; Fennell, M.; Seshan, V.; Garippa, R.; Scheinberg, D.A.; Baselga, J.; et al. Panobinostat acts synergistically with ibrutinib in diffuse large B cell lymphoma cells with MyD88 L265P mutations. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Wilson, W.H.; Young, R.M.; Schmitz, R.; Yang, Y.; Pittaluga, S.; Wright, G.; Lih, C.J.; Williams, P.M.; Shaffer, A.L.; Gerecitano, J.; et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015, 21, 922–926. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bahr, O.; et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: The checkmate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Chapuy, B.; Roemer, M.G.; Stewart, C.; Tan, Y.; Abo, R.P.; Zhang, L.; Dunford, A.J.; Meredith, D.M.; Thorner, A.R.; Jordanova, E.S.; et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 2016, 127, 869–881. [Google Scholar] [CrossRef] [Green Version]
- Takashima, Y.; Kawaguchi, A.; Sato, R.; Yoshida, K.; Hayano, A.; Homma, J.; Fukai, J.; Iwadate, Y.; Kajiwara, K.; Ishizawa, S.; et al. Differential expression of individual transcript variants of PD-1 and PD-L2 genes on Th-1/Th-2 status is guaranteed for prognosis prediction in PCNSL. Sci. Rep. 2019, 9, 10004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berghoff, A.S.; Ricken, G.; Widhalm, G.; Rajky, O.; Hainfellner, J.A.; Birner, P.; Raderer, M.; Preusser, M. PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL). Clin. Neuropathol. 2014, 33, 42–49. [Google Scholar] [CrossRef]
- Hayano, A.; Komohara, Y.; Takashima, Y.; Takeya, H.; Homma, J.; Fukai, J.; Iwadate, Y.; Kajiwara, K.; Ishizawa, S.; Hondoh, H.; et al. Programmed cell death ligand 1 expression in primary central nervous system lymphomas: A clinicopathological study. Anticancer. Res. 2017, 37, 5655–5666. [Google Scholar] [CrossRef] [PubMed]
- Miyasato, Y.; Takashima, Y.; Takeya, H.; Yano, H.; Hayano, A.; Nakagawa, T.; Makino, K.; Takeya, M.; Yamanaka, R.; Komohara, Y. The expression of PD-1 ligands and IDO1 by macrophage/microglia in primary central nervous system lymphoma. J. Clin. Exp. Hematop. 2018, 58, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, A.; Sumrall, A.; Phuphanich, S.; Spetzler, D.; Gatalica, Z.; Xiu, J.; Michelhaugh, S.; Brenner, A.; Pandey, M.; Kesari, S.; et al. Primary CNS lymphoma commonly expresses immune response biomarkers. Neurooncol. Adv. 2020, 2, vdaa018. [Google Scholar] [CrossRef]
- Monabati, A.; Nematollahi, P.; Dehghanian, A.; Safaei, A.; Sadeghipour, A.; Movahedinia, S.; Mokhtari, M. Immune checkpoint molecules in primary diffuse large B-cell lymphoma of the central nervous system. Basic Clin. Neurosci. 2020, 11, 491–498. [Google Scholar] [CrossRef]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Cho, I.; Lee, H.; Yoon, S.E.; Ryu, K.J.; Ko, Y.H.; Kim, W.S.; Kim, S.J. Serum levels of soluble programmed death-ligand 1 (sPD-L1) in patients with primary central nervous system diffuse large B-cell lymphoma. BMC Cancer 2020, 20, 120. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Li, Z.; Pouzoulet, F.; Vishnu, P.; Copland, J.A., 3rd; Knutson, K.L.; Soussain, C.; Tun, H.W. Immune checkpoint inhibition by anti-PDCD1 (anti-PD1) monoclonal antibody has significant therapeutic activity against central nervous system lymphoma in an immunocompetent preclinical model. Br. J. Haematol. 2018, 183, 674–678. [Google Scholar] [CrossRef] [Green Version]
- Hoang-Xuan, K.; Bessell, E.; Bromberg, J.; Hottinger, A.F.; Preusser, M.; Ruda, R.; Schlegel, U.; Siegal, T.; Soussain, C.; Abacioglu, U.; et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: Guidelines from the european association for neuro-oncology. Lancet Oncol. 2015, 16, e322–e332. [Google Scholar] [CrossRef] [Green Version]
- Nayak, L.; Iwamoto, F.M.; LaCasce, A.; Mukundan, S.; Roemer, M.G.M.; Chapuy, B.; Armand, P.; Rodig, S.J.; Shipp, M.A. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood 2017, 129, 3071–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terziev, D.; Hutter, B.; Klink, B.; Stenzinger, A.; Stogbauer, F.; Glimm, H.; Frohling, S.; Wickenhauser, C.; Jordan, K.; Hurtz, H.J.; et al. Nivolumab maintenance after salvage autologous stem cell transplantation results in long-term remission in multiple relapsed primary CNS lymphoma. Eur. J. Haematol. 2018, 101, 115–118. [Google Scholar] [CrossRef]
- Ambady, P.; Szidonya, L.; Firkins, J.; James, J.; Johansson, K.; White, T.; Jezierski, C.; Doolittle, N.D.; Neuwelt, E.A. Combination immunotherapy as a non-chemotherapy alternative for refractory or recurrent CNS lymphoma. Leuk. Lymphoma 2019, 60, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Nonoguchi, N.; Omura, N.; Shirahata, M.; Iwasaki, K.; Inui, T.; Kuroiwa, T.; Kuwabara, H.; Miyatake, S.I. Immunotherapy of nivolumab with dendritic cell vaccination is effective against intractable recurrent primary central nervous system lymphoma: A case report. Neurol. Med. Chir. 2017, 57, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Graber, J.J.; Plato, B.; Mawad, R.; Moore, D.J. Pembrolizumab immunotherapy for relapsed CNS Lymphoma. Leuk. Lymphoma 2020, 61, 1766–1768. [Google Scholar] [CrossRef]
- de-la-Fuente, C.; Nunez, F.; Cortes-Romera, M.; Franch-Sarto, M.; Ribera, J.M.; Sancho, J.M. Pembrolizumab for refractory primary mediastinal B-cell lymphoma with central nervous system involvement. Hematol. Oncol. 2020. [Google Scholar] [CrossRef]
- Pertz, M.; Kowalski, T.; Thoma, P.; Schlegel, U. What is on your mind? Impaired social cognition in primary central nervous system lymphoma patients despite ongoing complete remission. Cancers 2021, 13, 943. [Google Scholar] [CrossRef] [PubMed]
| Study | Setting | Phase | Identifier |
|---|---|---|---|
| Randomized clinical trials | |||
| Rituximab plus MTX with versus without lenalidomide | First line | 2 | NCT04481815 |
| Maintenance lenalidomide versus procarbazine following induction therapy with MTX plus rituximab and procarbazine | First line, age ≥ 70 years | 2 | NCT03495960 |
| R-MPV (MTX, rituximab, procarbazine, and vincristin) plus ibrutinib versus lenalidomide | First line | 2 | NCT04446962 |
| MRE (MTX, rituximab, and etoposide) plus ibrutinib versus lenalidomide | Recurrent or refractory | 2 | NCT04129710 |
| Uncontrolled studies | |||
| Acalabrutinib plus durvalumab | Recurrent or refractory | 1 | NCT04462328 |
| Rituximab plus ibrutinib plus lenalidomide | Recurrent or refractory | 1 | NCT03703167 |
| MTX and rituximab plus lenalidomide plus nivolumab | First line | 1 | NCT04609046 |
| TEDD (temozolomide, etoposide, doxil, dexamethasone) plus intrathecal cytarabine plus ibrutinib | Recurrent or refractory | 1 | NCT02203526 |
| Venetoclax plus obinutuzumab | Recurrent or refractory | 1 | NCT04073147 |
| MTX and rituximab plus lenalidomide | First line | 1/2 | NCT04120350 |
| MTX and rituximab plus ibrutinib | Recurrent or refractory | 1/2 | NCT02315326 |
| Copanlisib plus ibrutinib | Recurrent or refractory | 1/2 | NCT03581942 |
| Rituximab plus pemprolizumab plus ibrutinib | Recurrent or refractory | 1/2 | NCT04421560 |
| Ibrutinib maintenance following induction with MTX and rituximab | First line, age 60–85 | 2 | NCT02623010 |
| Rituximab plus lenalidomide | First line | 2 | NCT04627753 |
| MTX, rituximab and temozolomide plus lenalidomide | First line | 2 | NCT04737889 |
| Nivolumab plus ibrutinib | Recurrent or refractory | 2 | NCT03770416 |
| Orelabrutinib | Recurrent or refractory | 2 | NCT04438044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirsching, H.-G.; Weller, M.; Balabanov, S.; Roth, P. Targeted Therapies and Immune Checkpoint Inhibitors in Primary CNS Lymphoma. Cancers 2021, 13, 3073. https://doi.org/10.3390/cancers13123073
Wirsching H-G, Weller M, Balabanov S, Roth P. Targeted Therapies and Immune Checkpoint Inhibitors in Primary CNS Lymphoma. Cancers. 2021; 13(12):3073. https://doi.org/10.3390/cancers13123073
Chicago/Turabian StyleWirsching, Hans-Georg, Michael Weller, Stefan Balabanov, and Patrick Roth. 2021. "Targeted Therapies and Immune Checkpoint Inhibitors in Primary CNS Lymphoma" Cancers 13, no. 12: 3073. https://doi.org/10.3390/cancers13123073
APA StyleWirsching, H.-G., Weller, M., Balabanov, S., & Roth, P. (2021). Targeted Therapies and Immune Checkpoint Inhibitors in Primary CNS Lymphoma. Cancers, 13(12), 3073. https://doi.org/10.3390/cancers13123073






