Sensitivity and Specificity of Human Papillomavirus (HPV) 16 Early Antigen Serology for HPV-Driven Oropharyngeal Cancer: A Systematic Literature Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Search Strategy and Study Selection
2.3. Data Extraction and Synthesis
2.4. Statistical Analysis
3. Results
3.1. Search and Study Selection
3.2. Study Characteristics
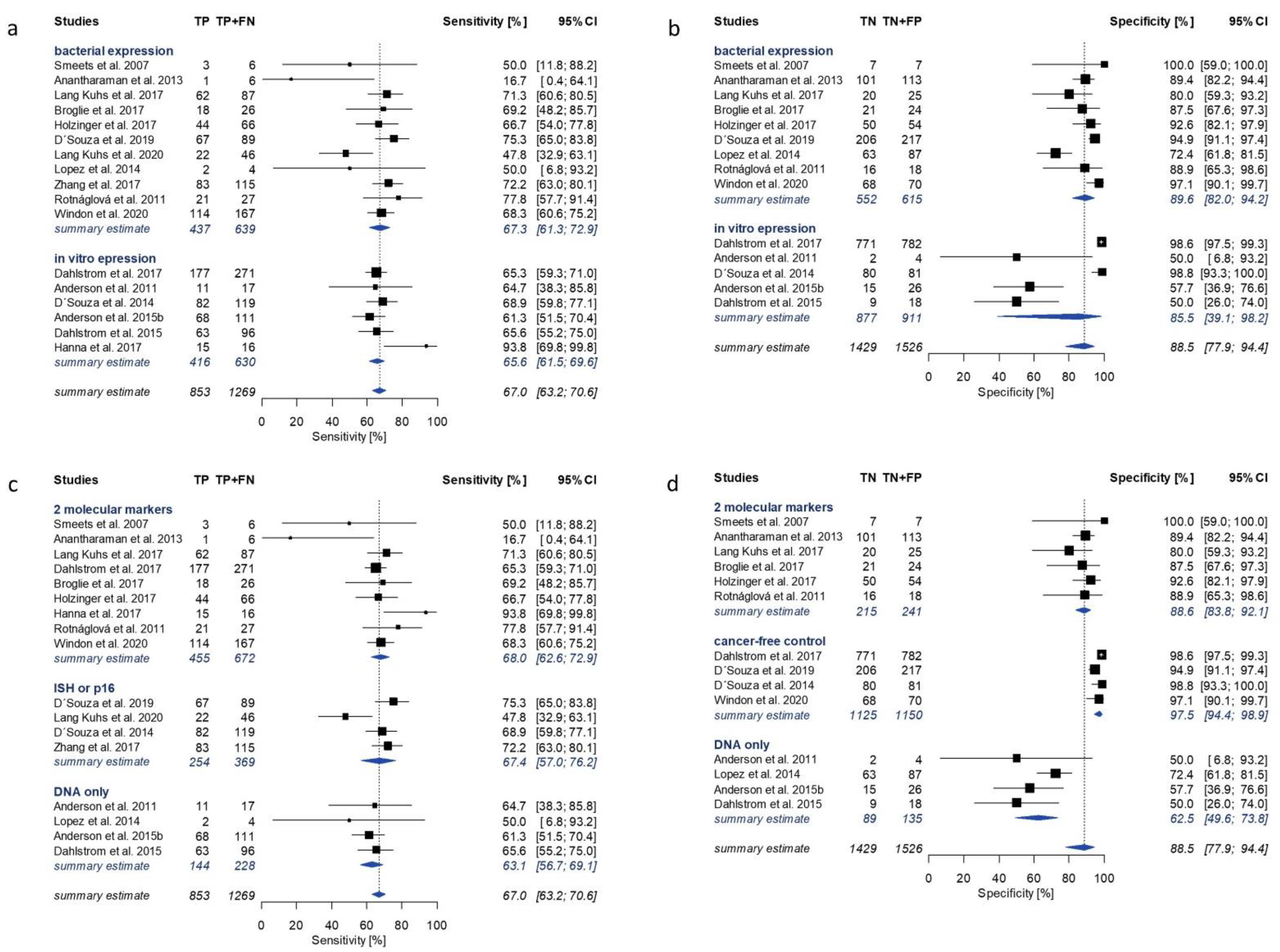
3.3. HPV16 E2, E6 and E7 Serology Summary Estimates
3.4. Subgroup Analysis
3.5. Other HPV16 Proteins and Combinatorial Algorithms
3.6. Risk of Bias Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Castellsagué, X.; Mena, M.; Alemany, L. Epidemiology of HPV-Positive Tumors in Europe and in the World. Recent Results Cancer Res. 2017, 206, 27–35. [Google Scholar] [CrossRef]
- Anantharaman, D.; Abedi-Ardekani, B.; Beachler, D.C.; Gheit, T.; Olshan, A.F.; Wisniewski, K.; Wunsch-Filho, V.; Toporcov, T.N.; Tajara, E.H.; Levi, J.E.; et al. Geographic Heterogeneity in the Prevalence of Human Papillomavirus in Head and Neck Cancer. Int. J. Cancer 2017, 140, 1968–1975. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Castellsagué, X.; Alemany, L.; Quer, M.; Halec, G.; Quirós, B.; Tous, S.; Clavero, O.; Alòs, L.; Biegner, T.; Szafarowski, T.; et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Issaeva, N.; Yarbrough, W.G. HPV-Driven Oropharyngeal Cancer: Current Knowledge of Molecular Biology and Mechanisms of Carcinogenesis. Cancers Head Neck 2018, 3, 12. [Google Scholar] [CrossRef]
- Kimple, R.J.; Smith, M.A.; Blitzer, G.C.; Torres, A.D.; Martin, J.A.; Yang, R.Z.; Peet, C.R.; Lorenz, L.D.; Nickel, K.P.; Klingelhutz, A.J.; et al. Enhanced Radiation Sensitivity in HPV-Positive Head and Neck Cancer. Cancer Res. 2013, 73, 4791–4800. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Rosenberg, A.J.; Vokes, E.E. Optimizing Treatment De-Escalation in Head and Neck Cancer: Current and Future Perspectives. Oncologist 2021, 26, 40–48. [Google Scholar] [CrossRef]
- von Knebel Doeberitz, M. The Causal Role of Human Papillomavirus Infections in Non-Anogenital Cancers. It’s Time to Ask for the Functional Evidence. Int. J. Cancer 2016, 139, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Romagosa, C.; Simonetti, S.; López-Vicente, L.; Mazo, A.; Lleonart, M.E.; Castellvi, J.; Ramon y Cajal, S. P16(Ink4a) Overexpression in Cancer: A Tumor Suppressor Gene Associated with Senescence and High-Grade Tumors. Oncogene 2011, 30, 2087–2097. [Google Scholar] [CrossRef]
- McLaughlin-Drubin, M.E.; Crum, C.P.; Münger, K. Human Papillomavirus E7 Oncoprotein Induces KDM6A and KDM6B Histone Demethylase Expression and Causes Epigenetic Reprogramming. Proc. Natl. Acad. Sci. USA 2011, 108, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Prigge, E.-S.; Arbyn, M.; von Knebel Doeberitz, M.; Reuschenbach, M. Diagnostic Accuracy of P16INK4a Immunohistochemistry in Oropharyngeal Squamous Cell Carcinomas: A Systematic Review and Meta-Analysis. Int. J. Cancer 2017, 140, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.M.; Caughron, S.K.; Dunn, S.T.; Knezetic, J.; Gatalica, Z. Detection of High-Risk HPV in Head and Neck Squamous Cell Carcinomas: Comparison of Chromogenic in Situ Hybridization and a Reverse Line Blot Method. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Hasegawa, M.; Aoki, K.; Matayoshi, S.; Kiyuna, A.; Yamashita, Y.; Uehara, T.; Agena, S.; Maeda, H.; Xie, M.; et al. A Comprehensive Evaluation of Human Papillomavirus Positive Status and P16INK4a Overexpression as a Prognostic Biomarker in Head and Neck Squamous Cell Carcinoma. Int. J. Oncol. 2014, 45, 67–76. [Google Scholar] [CrossRef]
- Walline, H.M.; Komarck, C.; McHugh, J.B.; Byrd, S.A.; Spector, M.E.; Hauff, S.J.; Graham, M.P.; Bellile, E.; Moyer, J.S.; Prince, M.E.; et al. High-Risk Human Papillomavirus Detection in Oropharyngeal, Nasopharyngeal, and Oral Cavity Cancers: Comparison of Multiple Methods. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1320–1327. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred Reporting Items for a Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Liang, C.; Marsit, C.J.; McClean, M.D.; Nelson, H.H.; Christensen, B.C.; Haddad, R.I.; Clark, J.R.; Wein, R.O.; Grillone, G.A.; Houseman, E.A.; et al. Biomarkers of HPV in Head and Neck Squamous Cell Carcinoma. Cancer Res. 2012, 72, 5004–5013. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Sankey, S.S.; Weissfeld, L.A.; Fine, M.J.; Kapoor, W. An Assessment of the Use of the Continuity Correction for Sparse Data in Meta-Analysis. Commun. Stat. Simul. Comput. 1996, 25, 1031–1056. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 9783319242774. [Google Scholar]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Herrero, R.; Castellsagué, X.; Pawlita, M.; Lissowska, J.; Kee, F.; Balaram, P.; Rajkumar, T.; Sridhar, H.; Rose, B.; Pintos, J.; et al. Human Papillomavirus and Oral Cancer: The International Agency for Research on Cancer Multicenter Study. J. Natl. Cancer Inst. 2003, 95, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Smeets, S.J.; Hesselink, A.T.; Speel, E.-J.M.; Haesevoets, A.; Snijders, P.J.F.; Pawlita, M.; Meijer, C.J.L.M.; Braakhuis, B.J.M.; Leemans, C.R.; Brakenhoff, R.H. A Novel Algorithm for Reliable Detection of Human Papillomavirus in Paraffin Embedded Head and Neck Cancer Specimen. Int. J. Cancer 2007, 121, 2465–2472. [Google Scholar] [CrossRef]
- D’Souza, G.; Zhang, H.H.; D’Souza, W.D.; Meyer, R.R.; Gillison, M.L. Moderate Predictive Value of Demographic and Behavioral Characteristics for a Diagnosis of HPV16-Positive and HPV16-Negative Head and Neck Cancer. Oral Oncol. 2010, 46, 100–104. [Google Scholar] [CrossRef]
- Anderson, K.S.; Wong, J.; D’Souza, G.; Riemer, A.B.; Lorch, J.; Haddad, R.; Pai, S.I.; Longtine, J.; McClean, M.; LaBaer, J.; et al. Serum Antibodies to the HPV16 Proteome as Biomarkers for Head and Neck Cancer. Br. J. Cancer 2011, 104, 1896–1905. [Google Scholar] [CrossRef]
- Rotnáglová, E.; Tachezy, R.; Saláková, M.; Procházka, B.; Košl’abová, E.; Veselá, E.; Ludvíková, V.; Hamšíková, E.; Klozar, J. HPV Involvement in Tonsillar Cancer: Prognostic Significance and Clinically Relevant Markers. Int. J. Cancer 2011, 129, 101–110. [Google Scholar] [CrossRef]
- Anantharaman, D.; Gheit, T.; Waterboer, T.; Abedi-Ardekani, B.; Carreira, C.; McKay-Chopin, S.; Gaborieau, V.; Marron, M.; Lagiou, P.; Ahrens, W.; et al. Human Papillomavirus Infections and Upper Aero-Digestive Tract Cancers: The ARCAGE Study. J. Natl. Cancer Inst. 2013, 105, 536–545. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Gross, N.D.; Pai, S.I.; Haddad, R.; Anderson, K.S.; Rajan, S.; Gerber, J.; Gillison, M.L.; Posner, M.R. Oral Human Papillomavirus (HPV) Infection in HPV-Positive Patients with Oropharyngeal Cancer and Their Partners. J. Clin. Oncol. 2014, 32, 2408–2415. [Google Scholar] [CrossRef] [PubMed]
- López, R.V.M.; Levi, J.E.; Eluf-Neto, J.; Koifman, R.J.; Koifman, S.; Curado, M.P.; Michaluart-Junior, P.; Figueiredo, D.L.A.; Saggioro, F.P.; de Carvalho, M.B.; et al. Human Papillomavirus (HPV) 16 and the Prognosis of Head and Neck Cancer in a Geographical Region with a Low Prevalence of HPV Infection. Cancer Causes Control. 2014, 25, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.S.; Gerber, J.E.; D’Souza, G.; Pai, S.I.; Cheng, J.N.; Alam, R.; Kesiraju, S.; Chowell, D.; Gross, N.D.; Haddad, R.; et al. Biologic Predictors of Serologic Responses to HPV in Oropharyngeal Cancer: The HOTSPOT Study. Oral Oncol. 2015, 51, 751–758. [Google Scholar] [CrossRef]
- Anderson, K.S.; Dahlstrom, K.R.; Cheng, J.N.; Alam, R.; Li, G.; Wei, Q.; Gross, N.D.; Chowell, D.; Posner, M.; Sturgis, E.M. HPV16 Antibodies as Risk Factors for Oropharyngeal Cancer and Their Association with Tumor HPV and Smoking Status. Oral Oncol. 2015, 51, 662–667. [Google Scholar] [CrossRef]
- Dahlstrom, K.R.; Anderson, K.S.; Cheng, J.N.; Chowell, D.; Li, G.; Posner, M.; Sturgis, E.M. HPV Serum Antibodies as Predictors of Survival and Disease Progression in Patients with HPV-Positive Squamous Cell Carcinoma of the Oropharynx. Clin. Cancer Res. 2015, 21, 2861–2869. [Google Scholar] [CrossRef]
- Lang Kuhs, K.A.; Pawlita, M.; Gibson, S.P.; Schmitt, N.C.; Trivedi, S.; Argiris, A.; Kreimer, A.R.; Ferris, R.L.; Waterboer, T. Characterization of Human Papillomavirus Antibodies in Individuals with Head and Neck Cancer. Cancer Epidemiol. 2016, 42, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lang Kuhs, K.A.; Kreimer, A.R.; Trivedi, S.; Holzinger, D.; Pawlita, M.; Pfeiffer, R.M.; Gibson, S.P.; Schmitt, N.C.; Hildesheim, A.; Waterboer, T.; et al. Human Papillomavirus 16 E6 Antibodies Are Sensitive for Human Papillomavirus-Driven Oropharyngeal Cancer and Are Associated with Recurrence. Cancer 2017, 123, 4382–4390. [Google Scholar] [CrossRef]
- Dahlstrom, K.R.; Anderson, K.S.; Field, M.S.; Chowell, D.; Ning, J.; Li, N.; Wei, Q.; Li, G.; Sturgis, E.M. Diagnostic Accuracy of Serum Antibodies to Human Papillomavirus Type 16 Early Antigens in the Detection of Human Papillomavirus-Related Oropharyngeal Cancer. Cancer 2017, 123, 4886–4894. [Google Scholar] [CrossRef]
- Broglie, M.A.; Jochum, W.; Michel, A.; Waterboer, T.; Foerbs, D.; Schoenegg, R.; Stoeckli, S.J.; Pawlita, M.; Holzinger, D. Evaluation of Type-Specific Antibodies to High Risk-Human Papillomavirus (HPV) Proteins in Patients with Oropharyngeal Cancer. Oral Oncol. 2017, 70, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Wichmann, G.; Baboci, L.; Michel, A.; Höfler, D.; Wiesenfarth, M.; Schroeder, L.; Boscolo-Rizzo, P.; Herold-Mende, C.; Dyckhoff, G.; et al. Sensitivity and Specificity of Antibodies against HPV16 E6 and Other Early Proteins for the Detection of HPV16-Driven Oropharyngeal Squamous Cell Carcinoma. Int. J. Cancer 2017, 140, 2748–2757. [Google Scholar] [CrossRef]
- Zhang, Y.; Waterboer, T.; Haddad, R.I.; Miles, B.A.; Wentz, A.; Gross, N.D.; Fakhry, C.; Quon, H.; Lorch, J.H.; Gourin, C.G.; et al. Human Papillomavirus (HPV) 16 Antibodies at Diagnosis of HPV-Related Oropharyngeal Cancer and Antibody Trajectories after Treatment. Oral Oncol. 2017, 67, 77–82. [Google Scholar] [CrossRef]
- Hanna, G.J.; Sridharan, V.; Margalit, D.N.; La Follette, S.K.; Chau, N.G.; Rabinowits, G.; Lorch, J.H.; Haddad, R.I.; Tishler, R.B.; Anderson, K.S.; et al. Salivary and Serum HPV Antibody Levels before and after Definitive Treatment in Patients with Oropharyngeal Squamous Cell Carcinoma. Cancer Biomark. 2017, 19, 129–136. [Google Scholar] [CrossRef]
- Anantharaman, D.; Billot, A.; Waterboer, T.; Gheit, T.; Abedi-Ardekani, B.; Lagiou, P.; Lagiou, A.; Ahrens, W.; Holcátová, I.; Merletti, F.; et al. Predictors of Oropharyngeal Cancer Survival in Europe. Oral Oncol. 2018, 81, 89–94. [Google Scholar] [CrossRef]
- D’Souza, G.; Clemens, G.; Troy, T.; Castillo, R.G.; Struijk, L.; Waterboer, T.; Bender, N.; Pierorazio, P.M.; Best, S.R.; Strickler, H.; et al. Evaluating the Utility and Prevalence of HPV Biomarkers in Oral Rinses and Serology for HPV-Related Oropharyngeal Cancer. Cancer Prev. Res. 2019, 12, 689–700. [Google Scholar] [CrossRef]
- Lang Kuhs, K.A.; Wood, C.B.; Wiggleton, J.; Aulino, J.M.; Latimer, B.; Smith, D.K.; Bender, N.; Rohde, S.; Mannion, K.; Kim, Y.; et al. Transcervical Sonography and Human Papillomavirus 16 E6 Antibodies Are Sensitive for the Detection of Oropharyngeal Cancer. Cancer 2020, 126, 2658–2665. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xu, W.; Su, J.; Ren, X.; Cheng, D.; Chen, Z.; Bender, N.; Mirshams, M.; Habbous, S.; de Almeida, J.R.; et al. Multiple Imputation and Clinico-Serological Models to Predict Human Papillomavirus Status in Oropharyngeal Carcinoma: An Alternative When Tissue Is Unavailable. Int. J. Cancer 2020, 146, 2166–2174. [Google Scholar] [CrossRef]
- Fakhry, C.; Waterboer, T.; Westra, W.H.; Rooper, L.M.; Windon, M.; Troy, T.; Koch, W.; Gourin, C.G.; Bender, N.; Yavvari, S.; et al. Distinct Biomarker and Behavioral Profiles of Human Papillomavirus-Related Oropharynx Cancer Patients by Age. Oral Oncol. 2020, 101, 104522. [Google Scholar] [CrossRef] [PubMed]
- Windon, M.J.; D’Souza, G.; Waterboer, T.; Rooper, L.; Westra, W.H.; Troy, T.; Pardoll, D.; Tan, M.; Yavvari, S.; Kiess, A.P.; et al. Risk Factors for Human Papillomavirus-Positive Nonoropharyngeal Squamous Cell Carcinoma. Head Neck 2020, 42, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Shiels, M.S.; Fakhry, C.; Johansson, M.; Pawlita, M.; Brennan, P.; Hildesheim, A.; Waterboer, T. Screening for Human Papillomavirus-Driven Oropharyngeal Cancer: Considerations for Feasibility and Strategies for Research. Cancer 2018, 124, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Waterboer, T.; Brenner, N.; Gallagher, R.; Hillman, R.J.; Jin, F.; Grulich, A.; Poynten, I.M. Early Detection of Human Papillomavirus-Driven Oropharyngeal Cancer Using Serology from the Study of Prevention of Anal Cancer. JAMA Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nominé, Y.; Ristriani, T.; Laurent, C.; Lefèvre, J.F.; Weiss, E.; Travé, G. A Strategy for Optimizing the Monodispersity of Fusion Proteins: Application to Purification of Recombinant HPV E6 Oncoprotein. Protein Eng. 2001, 14, 297–305. [Google Scholar] [CrossRef]
- Coutlée, F.; Gravitt, P.; Kornegay, J.; Hankins, C.; Richardson, H.; Lapointe, N.; Voyer, H.; Franco, E. Use of PGMY Primers in L1 Consensus PCR Improves Detection of Human Papillomavirus DNA in Genital Samples. J. Clin. Microbiol. 2002, 40, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Kleter, B.; van Doorn, L.J.; Schrauwen, L.; Molijn, A.; Sastrowijoto, S.; ter Schegget, J.; Lindeman, J.; ter Harmsel, B.; Burger, M.; Quint, W. Development and Clinical Evaluation of a Highly Sensitive PCR-Reverse Hybridization Line Probe Assay for Detection and Identification of Anogenital Human Papillomavirus. J. Clin. Microbiol. 1999, 37, 2508–2517. [Google Scholar] [CrossRef]
- Gephardt, G.N.; Zarbo, R.J. Extraneous Tissue in Surgical Pathology: A College of American Pathologists Q-Probes Study of 275 Laboratories. Arch. Pathol. Lab. Med. 1996, 120, 1009–1014. [Google Scholar] [PubMed]
- Boscolo-Rizzo, P.; Pawlita, M.; Holzinger, D. From HPV-Positive towards HPV-Driven Oropharyngeal Squamous Cell Carcinomas. Cancer Treat. Rev. 2016, 42, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Brenner, N.; Mentzer, A.J.; Hill, M.; Almond, R.; Allen, N.; Pawlita, M.; Waterboer, T. Characterization of Human Papillomavirus (HPV) 16 E6 Seropositive Individuals without HPV-Associated Malignancies after 10 Years of Follow-up in the UK Biobank. EBioMedicine 2020, 62, 103123. [Google Scholar] [CrossRef]
- Onishi, A.; Sugiyama, D.; Kogata, Y.; Saegusa, J.; Sugimoto, T.; Kawano, S.; Morinobu, A.; Nishimura, K.; Kumagai, S. Diagnostic Accuracy of Serum 1,3-β-D-Glucan for Pneumocystis Jiroveci Pneumonia, Invasive Candidiasis, and Invasive Aspergillosis: Systematic Review and Meta-Analysis. J. Clin. Microbiol. 2012, 50, 7–15. [Google Scholar] [CrossRef]
- Balachandra, S.; Kusin, S.B.; Lee, R.; Blackwell, J.-M.; Tiro, J.A.; Cowell, L.G.; Chiang, C.-M.; Wu, S.-Y.; Varma, S.; Rivera, E.L.; et al. Blood-Based Biomarkers of Human Papillomavirus-Associated Cancers: A Systematic Review and Meta-Analysis. Cancer 2021, 127, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Wuerdemann, N.; Jain, R.; Adams, A.; Speel, E.-J.M.; Wagner, S.; Joosse, S.A.; Klussmann, J.P. Cell-Free HPV-DNA as a Biomarker for Oropharyngeal Squamous Cell Carcinoma—A Step Towards Personalized Medicine? Cancers 2020, 12, 2997. [Google Scholar] [CrossRef] [PubMed]





| First Author | Year | n Total | HPV+ (n) | HPV− (n) | Reference Method(s) | Antigen Expression System | HPV16 Antigens 1 | Country of Origin | Time of Collection 2 |
|---|---|---|---|---|---|---|---|---|---|
| Herrero 3 [26] | 2003 | 137 | 26 | 111 | DNA PCR | bacterial | E6 and/or E7 | Italy, Spain, Ireland, Poland, India, Cuba, Sudan, Canada, Australia | 1996–1999 |
| Smeets [27] | 2007 | 13 | 6 | 7 | RT-PCR and DNA PCR | bacterial | E6, E7 | Netherlands | na |
| D’Souza 3 [28] | 2010 | 119 | 85 | 34 | DNA ISH | bacterial | E6 and/or E7 | USA | 2000–2006 |
| Anderson [29] | 2011 | 21 | 17 | 4 | DNA PCR | in vitro | E7, E1 | USA | na |
| Rotnáglová [30] | 2011 | 45 | 27 | 18 | DNA PCR and RT-PCR | bacterial | E6, E7 | Czech Republic | 2001–2007 |
| Liang [18] | 2012 | 89 | 35 | 54 | p16 IHC | bacterial | E6 | USA | 1999–2003 |
| Anantharaman 4 [31] | 2013 | 119 | 6 | 113 | p16 IHC and DNA PCR | bacterial | E6, E7 | Germany, Greece, Italy, Ireland, UK, Spain, Norway, Czech Republic, Croatia | 2000–2005 |
| D’Souza 4 [32] | 2014 | 200 | 119 | 81 5 | p16 IHC and/or ISH 6 | in vitro | E6, E7 | USA | 2009–2013 |
| Lopez [33] | 2014 | 91 | 4 | 87 | DNA PCR | bacterial | E6, E7 | Brazil | 1998–2008 |
| Anderson 4 [34] | 2015a | 136 | 55 | 81 5 | p16 IHC and/or ISH 6 | in vitro | E6, E7, E1, E2, E4, E5 | USA | 2009–2013 |
| Anderson [35] | 2015b | 137 | 111 | 26 5 | DNA PCR | in vitro | E6, E7, E1, E2, E4, E5 | USA | 2006–2008 |
| Dahlstrom [36] | 2015 | 114 | 96 | 18 | DNA PCR | in vitro | E6, E7, E1, E2, E4, E5 | USA | 2006–2008 |
| Lang Kuhs [37] | 2016 | 10 | 6 | 4 | p16 IHC and ISH | bacterial | E6 | USA | 2003–2006 |
| Lang Kuhs [38] | 2017 | 112 | 87 | 25 | p16 IHC and ISH | bacterial | E6, E7, E1, E2, E4 | USA | 2000–2017 |
| Dahlstrom [39] | 2017 | 1053 | 271 | 782 5 | p16 IHC and ISH | in vitro | E6, E7, E1, E2, E4, E5 | USA | 2003–2013 |
| Broglie [40] | 2017 | 50 | 26 | 24 | p16 IHC and DNA PCR | bacterial | E6, E7, E1, E2, E4 | Switzerland | na |
| Holzinger [41] | 2017 | 120 | 66 | 54 | (NASBA or RT-PCR) and DNA PCR | bacterial | E6, E7, E1, E2 | Italy, Germany | na |
| Zhang [42] | 2017 | 115 | 115 | - 7 | p16 IHC and/or ISH 6 | bacterial | E6, E7, E1, E2, E4 | USA | 2009–2013 |
| Hanna [43] | 2017 | 16 | 16 | - 7 | p16 IHC and (ISH or DNA PCR) | in vitro | E6, E7, E2 | USA | 2013–2015 |
| Anantharaman 4 [44] | 2018 | 153 | 49 | 104 | p16 IHC and DNA PCR | bacterial | E6 | Germany, Greece, Italy, Ireland, UK, Spain, Norway, Czech Republic, Croatia | 2002–2005 |
| D’Souza [45] | 2019 | 306 | 89 | 217 5 | p16 IHC and/or ISH 6 | bacterial | E6, E7, E1, E2 | USA | 2009–2013 |
| Lang Kuhs [46] | 2020 | 46 | 46 | - 7 | p16 IHC | bacterial | E6, E7, E1, E2, E4 | USA | 2017–2018 |
| Ren [47] | 2020 | 696 | 524 | 172 | p16 IHC | bacterial | E6 | Canada | na |
| Fakhry 4 [48] | 2020 | 146 | 146 | - 7 | p16 IHC and RNA ISH | bacterial | E6, E7 | USA | 2013–2018 |
| Windon 4 [49] | 2020 | 220 | 150 | 70 5 | p16 IHC and RNA ISH | bacterial | E6, E7 | USA | 2013–2018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hibbert, J.; Halec, G.; Baaken, D.; Waterboer, T.; Brenner, N. Sensitivity and Specificity of Human Papillomavirus (HPV) 16 Early Antigen Serology for HPV-Driven Oropharyngeal Cancer: A Systematic Literature Review and Meta-Analysis. Cancers 2021, 13, 3010. https://doi.org/10.3390/cancers13123010
Hibbert J, Halec G, Baaken D, Waterboer T, Brenner N. Sensitivity and Specificity of Human Papillomavirus (HPV) 16 Early Antigen Serology for HPV-Driven Oropharyngeal Cancer: A Systematic Literature Review and Meta-Analysis. Cancers. 2021; 13(12):3010. https://doi.org/10.3390/cancers13123010
Chicago/Turabian StyleHibbert, Julia, Gordana Halec, Dan Baaken, Tim Waterboer, and Nicole Brenner. 2021. "Sensitivity and Specificity of Human Papillomavirus (HPV) 16 Early Antigen Serology for HPV-Driven Oropharyngeal Cancer: A Systematic Literature Review and Meta-Analysis" Cancers 13, no. 12: 3010. https://doi.org/10.3390/cancers13123010
APA StyleHibbert, J., Halec, G., Baaken, D., Waterboer, T., & Brenner, N. (2021). Sensitivity and Specificity of Human Papillomavirus (HPV) 16 Early Antigen Serology for HPV-Driven Oropharyngeal Cancer: A Systematic Literature Review and Meta-Analysis. Cancers, 13(12), 3010. https://doi.org/10.3390/cancers13123010






