Nucleolin Targeting by N6L Inhibits Wnt/β-Catenin Pathway Activation in Pancreatic Ductal Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
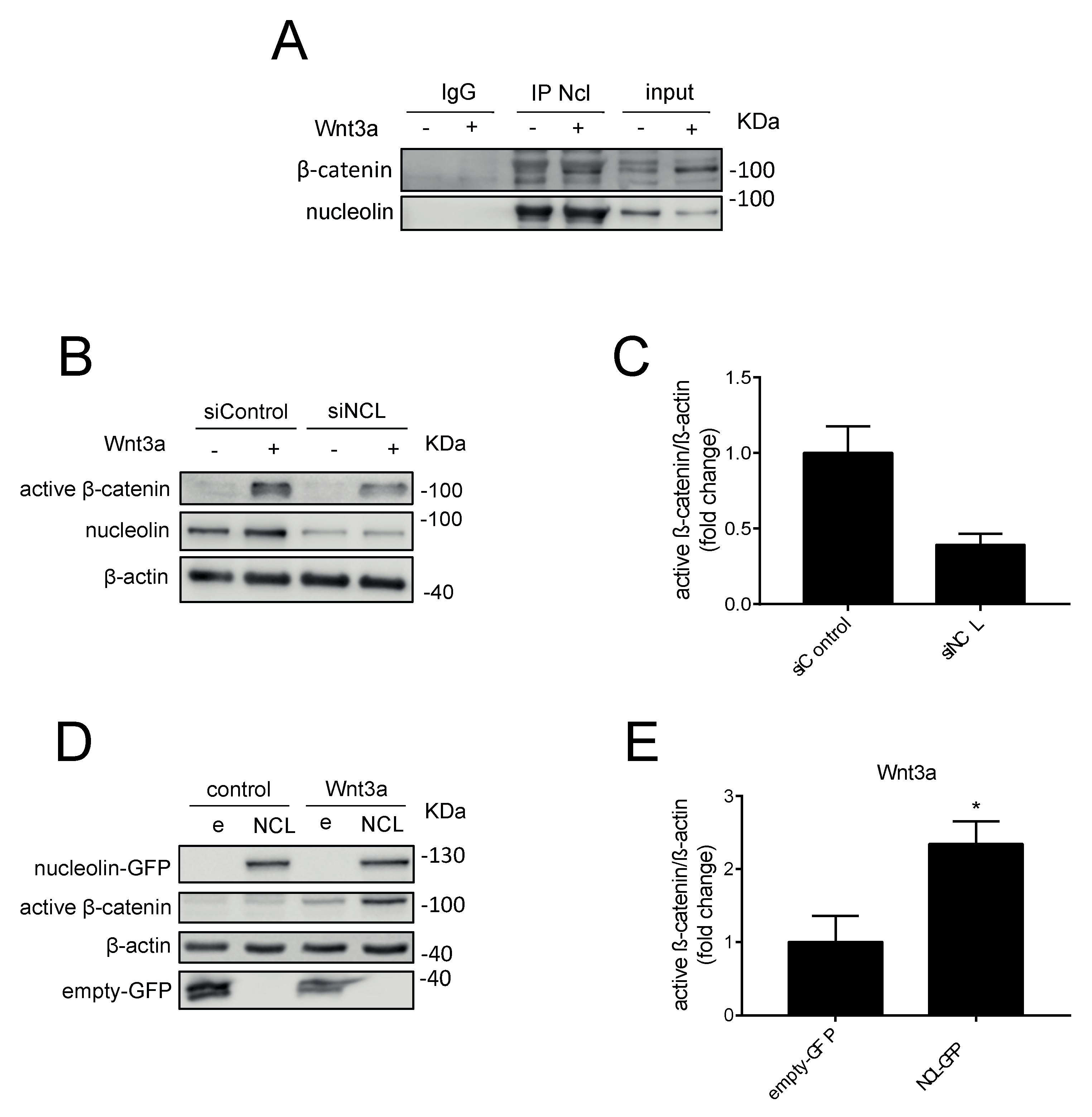
2.1. N6L Interacts with β-Catenin
2.2. N6L Inhibits the Activation of the Wnt/β-Catenin Pathway and Prevents β-Catenin Stabilization
2.3. Nucleolin Promotes β-Catenin Stabilization under Wnt3A Stimulation
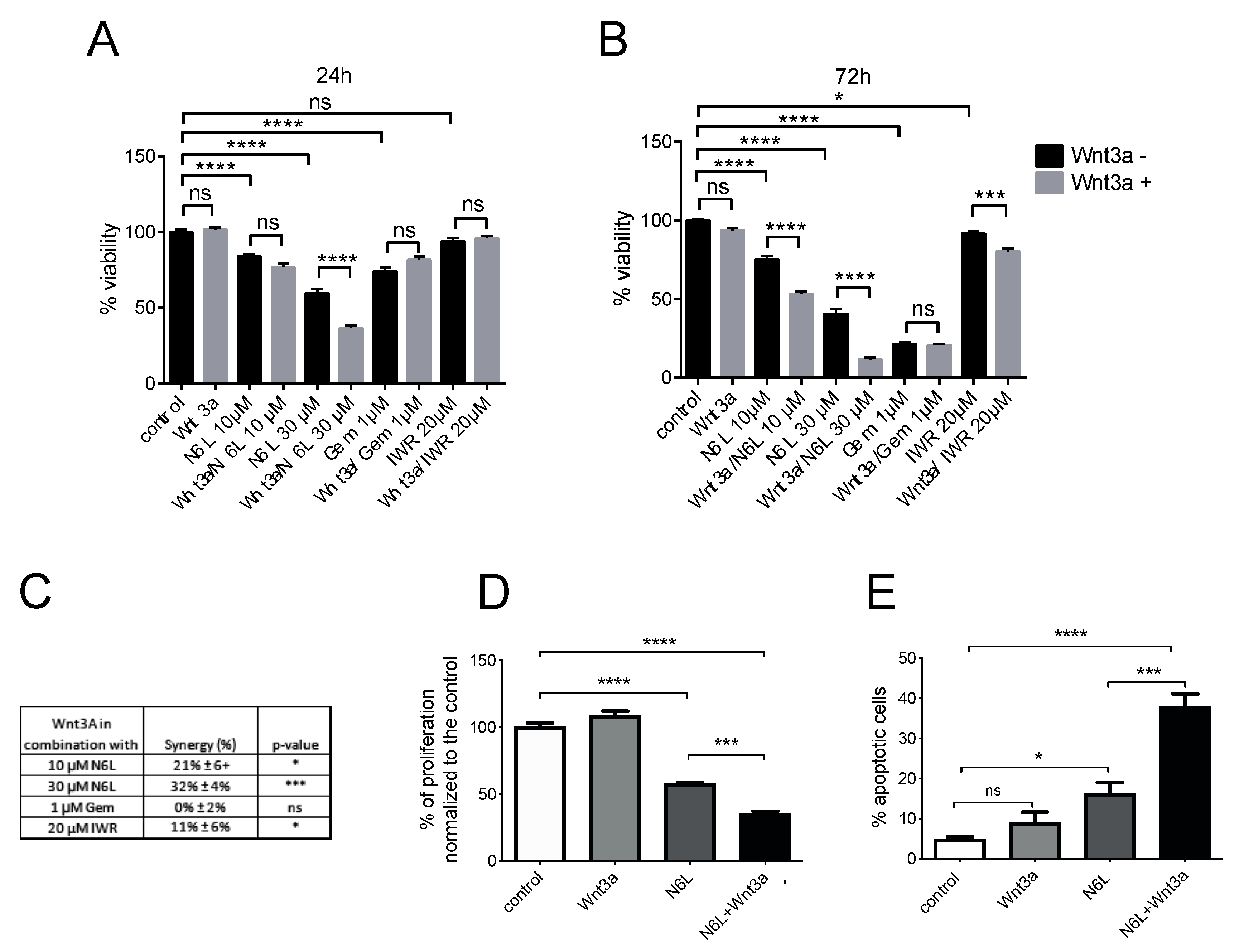
2.4. Wnt/β-Catenin Pathway Activation Specifically Sensitizes Cells to N6L Targeting
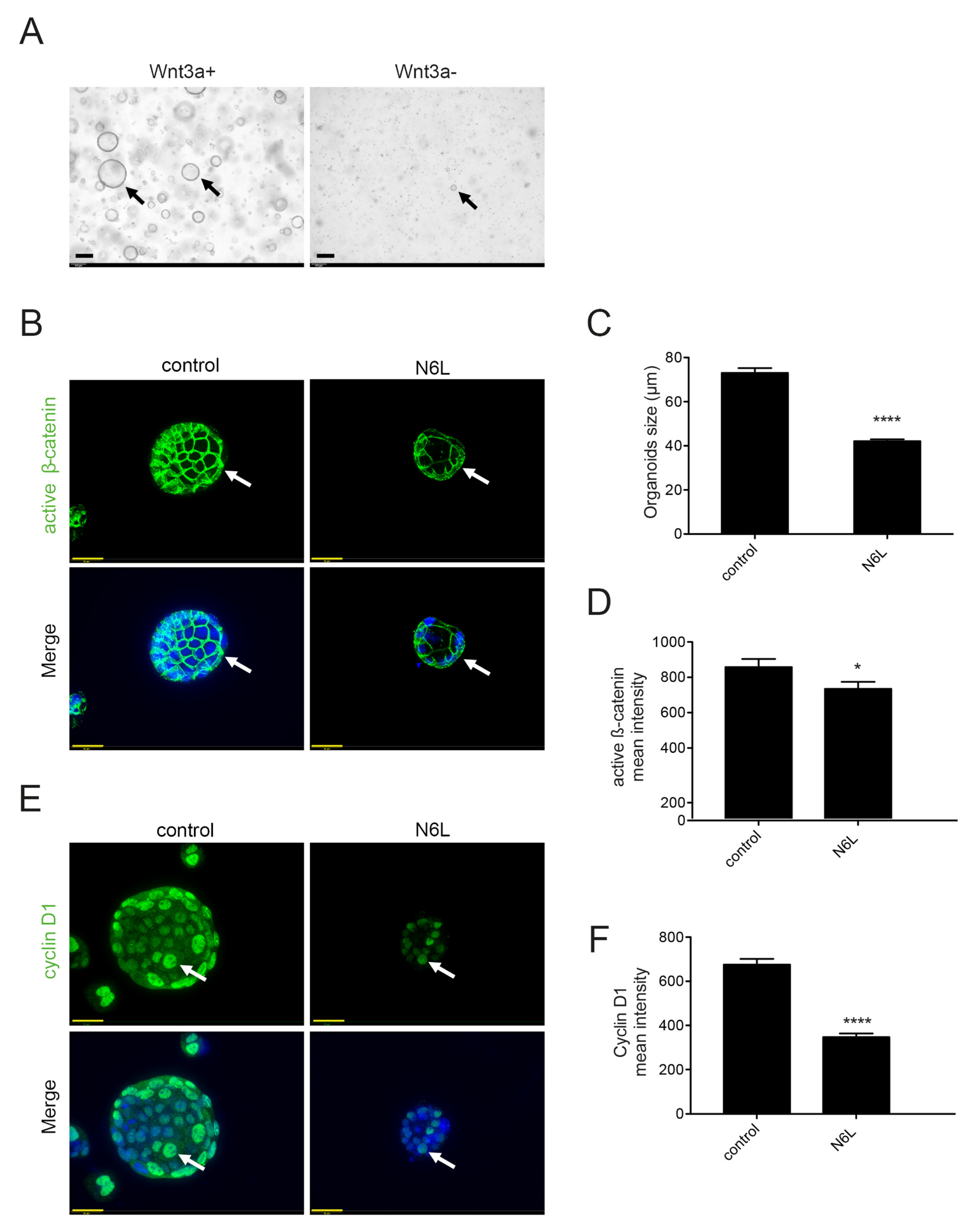
2.5. Wnt/β-Catenin Pathway Activation Is Essential for the Generation and Progression of MIA PaCa-2 Tumor-Derived 3D Culture
2.6. N6L Inhibits the Activation of the Wnt/β-Catenin Pathway and Prevents β-Catenin Stabilization
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Growth Assay
4.3. RT-qPCR
4.4. Cell Transfection
4.5. Dual-Luciferase Reporter Assay
4.6. Immunoprecipitation
4.7. Western Blotting
4.8. Pancreatic Tumor Mouse Models and MIA PaCa-2 Tumour-Derived 3D Culture
4.9. Immunofluorescence and Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Gilles, M.-E.; Maione, F.; Cossutta, M.; Carpentier, G.; Caruana, L.; Di Maria, S.; Houppe, C.; Destouches, D.; Shchors, K.; Prochasson, C.; et al. Nucleolin Targeting Impairs the Progression of Pancreatic Cancer and Promotes the Normalization of Tumor Vasculature. Cancer Res. 2016, 76, 7181–7193. [Google Scholar] [CrossRef]
- Storck, S.; Shukla, M.; Dimitrov, S.; Bouvet, P. Functions of the Histone Chaperone Nucleolin in Diseases. Prokaryotic Cytoskeletons 2007, 41, 125–144. [Google Scholar] [CrossRef]
- Berger, C.M.; Gaume, X.; Bouvet, P. The roles of nucleolin subcellular localization in cancer. Biochimie 2015, 113, 78–85. [Google Scholar] [CrossRef]
- Destouches, D.; Page, N.; Hamma-Kourbali, Y.; Machi, V.; Chaloin, O.; Frechault, S.; Birmpas, C.; Katsoris, P.; Beyrath, J.; Albanese, P.; et al. A Simple Approach to Cancer Therapy Afforded by Multivalent Pseudopeptides That Target Cell-Surface Nucleoproteins. Cancer Res. 2011, 71, 3296–3305. [Google Scholar] [CrossRef]
- Destouches, D.; Sader, M.; Terry, S.; Marchand, C.; Maillé, P.; Soyeux, P.; Carpentier, G.; Semprez, F.; Céraline, J.; Allory, Y.; et al. Implication of NPM1 phosphorylation and preclinical evaluation of the nucleoprotein antagonist N6L in prostate cancer. Oncotarget 2016, 7, 69397–69411. [Google Scholar] [CrossRef] [PubMed]
- De Cola, A.; Franceschini, M.; Di Matteo, A.; Colotti, G.; Celani, R.; Clemente, E.; Ippoliti, R.; Cimini, A.; Dhez, A.; Vallée, B.; et al. N6L pseudopeptide interferes with nucleophosmin protein-protein interactions and sensitizes leukemic cells to chemotherapy. Cancer Lett. 2018, 412, 272–282. [Google Scholar] [CrossRef]
- Ramos, K.S.; Moore, S.; Runge, I.; Tavera-Garcia, M.A.; Cascone, I.; Courty, J.; Reyes-Reyes, E. The Nucleolin Antagonist N6L Inhibits LINE1 Retrotransposon Activity in Non-Small Cell Lung Carcinoma Cells. J. Cancer 2020, 11, 733–740. [Google Scholar] [CrossRef]
- Benedetti, E.; Antonosante, A.; d’Angelo, M.; Cristiano, L.; Galzio, R.; Destouches, D.; Florio, T.M.; Dhez, A.C.; Astarita, C.; Cinque, B.; et al. Nucleolin antagonist triggers au-tophagic cell death in human glioblastoma primary cells and decreased in vivo tumor growth in orthotopic brain tumor model. Oncotarget 2015, 6, 42091–42104. [Google Scholar] [CrossRef]
- Dhez, A.-C.; Benedetti, E.; Antonosante, A.; Panella, G.; Ranieri, B.; Florio, T.M.; Cristiano, L.; Angelucci, F.; Giansanti, F.; Di Leandro, L.; et al. Targeted therapy of human glioblastoma via delivery of a toxin through a peptide directed to cell surface nucleolin. J. Cell. Physiol. 2018, 233, 4091–4105. [Google Scholar] [CrossRef]
- Sanhaji, M.; Göring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Prina-Mello, A.; Stapf, M.; Ludwig, R.; Volkov, Y.; et al. The phenotype of target pancreatic cancer cells influences cell death by magnetic hyperthermia with nanoparticles carrying gemicitabine and the pseudo-peptide NucAnt. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 101983. [Google Scholar] [CrossRef] [PubMed]
- Belbekhouche, S.; Cossutta, M.; Habert, D.; Hamadi, S.; Modjinou, T.; Cascone, I.; Courty, J. N6L-functionalized nanoparticles for targeted and inhibited pancreatic cancer cells. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125461. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; Gilles, M.-E.; Sader, M.; Cossutta, M.; Vallée, B.; Houppe, C.; Habert, D.; Brissault, B.; Leroy, E.; Maione, F.; et al. Multivalent cationic pseudopeptide polyplexes as a tool for cancer therapy. Oncotarget 2017, 8, 90108–90122. [Google Scholar] [CrossRef] [PubMed]
- Cheriyamundath, S.; Ben-Ze’Ev, A. Wnt/β-Catenin Target Genes in Colon Cancer Metastasis: The Special Case of L1CAM. Cancers 2020, 12, 3444. [Google Scholar] [CrossRef] [PubMed]
- Polakis, P. Wnt Signaling in Cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008052. [Google Scholar] [CrossRef]
- Spiegelman, V.S.; Slaga, T.J.; Pagano, M.; Minamoto, T.; Ronai, Z.; Fuchs, S.Y. Wnt/β-Catenin Signaling Induces the Expression and Activity of βTrCP Ubiquitin Ligase Receptor. Mol. Cell 2000, 5, 877–882. [Google Scholar] [CrossRef]
- He, X.; Semenov, M.; Tamai, K.; Zeng, X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling:Arrows point the way. Development 2004, 131, 1663–1677. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Gordon, M.D.; Nusse, R. Wnt Signaling: Multiple Pathways, Multiple Receptors, and Multiple Transcription Factors. J. Biol. Chem. 2006, 281, 22429–22433. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Pan, W. GSK3: A multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 2010, 35, 161–168. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Q.; He, C.; Li, F.; Sheng, H.; Shen, X.; Zhang, X.; Zhu, S.; Chen, H.; Chen, X.; et al. Activation of the Wnt pathway through Wnt2 promotes metastasis in pancreatic cancer. Am. J. Cancer Res. 2014, 4, 537–544. [Google Scholar] [PubMed]
- Modi, S.; Kir, D.; Banerjee, S.; Saluja, A. Control of Apoptosis in Treatment and Biology of Pancreatic Cancer. J. Cell. Biochem. 2016, 117, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.N.; Kim, O.R.; Lee, M.A. β-Catenin expression is associated with cell invasiveness in pancreatic cancer. Korean J. Intern. Med. 2019, 34, 618–625. [Google Scholar] [CrossRef]
- Zhang, Y.; Morris, J.P.; Yan, W.; Schofield, H.K.; Gurney, A.; Simeone, D.M.; Millar, S.E.; Hoey, T.; Hebrok, M.; Di Magliano, M.P. Canonical Wnt Signaling Is Required for Pancreatic Carcinogenesis. Cancer Res. 2013, 73, 4909–4922. [Google Scholar] [CrossRef]
- Callebaut, C.; Jacotot, E.; Guichard, G.; Krust, B.; Rey-Cuille, M.-A.; Cointe, D.; Benkirane, N.; Blanco, J.; Muller, S.; Briand, J.-P.; et al. Inhibition of HIV Infection by Pseudopeptides Blocking Viral Envelope Glycoprotein-Mediated Membrane Fusion and Cell Death. Virology 1996, 218, 181–192. [Google Scholar] [CrossRef]
- Koutsioumpa, M.; Drosou, G.; Mikelis, C.; Theochari, K.; Vourtsis, D.; Katsoris, P.; Giannopoulou, E.; Courty, J.; Petrou, C.; Magafa, V.; et al. Pleiotrophin expression and role in physiological angiogenesis in vivo: Potential involvement of nucleolin. Vasc. Cell 2012, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Makena, M.R.; Gatla, H.; Verlekar, D.; Sukhavasi, S.; Pandey, M.K.; Pramanik, K.C. Wnt/β-Catenin Signaling: The Culprit in Pancreatic Carcinogenesis and Therapeutic Resistance. Int. J. Mol. Sci. 2019, 20, 4242. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Shibamoto, S.; Higano, K.; Takada, R.; Ito, F.; Takeichi, M.; Takada, S. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells 1998, 3, 659–670. [Google Scholar] [CrossRef]
- Toyoda, E.; Doi, R.; Koizumi, M.; Kami, K.; Ito, D.; Mori, T.; Fujimoto, K.; Nakajima, S.; Wada, M.; Imamura, M. Analysis of E-, N-Cadherin, α-, β-, and γ-Catenin Expression in Human Pancreatic Carcinoma Cell Lines. Pancreas 2005, 30, 168–173. [Google Scholar] [CrossRef]
- Sarkar, S.; Mandal, C.; Sangwan, R.; Mandal, C. Coupling G2/M arrest to the Wnt/β-catenin pathway restrains pancreatic adenocarcinoma. Endocr.-Relat. Cancer 2014, 21, 113–125. [Google Scholar] [CrossRef]
- Romero, D.; Iglesias, M.; Vary, C.P.H.; Quintanilla, M. Functional blockade of Smad4 leads to a decrease in β-catenin levels and signaling activity in human pancreatic carcinoma cells. Carcinogenesis 2008, 29, 1070–1076. [Google Scholar] [CrossRef][Green Version]
- Farin, H.F.; Jordens, I.; Mosa, M.H.; Basak, O.; Korving, J.; Tauriello, D.V.F.; De Punder, K.; Angers, S.; Peters, P.J.; Maurice, M.M.; et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 2016, 530, 340–343. [Google Scholar] [CrossRef]
- Chen, B.; E Dodge, M.; Tang, W.; Lu, J.; Ma, Z.; Fan, C.-W.; Wei, S.; Hao, W.; Kilgore, J.; Williams, N.S.; et al. Small molecule–mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009, 5, 100–107. [Google Scholar] [CrossRef]
- Di Veroli, G.Y.; Fornari, C.; Wang, D.; Mollard, S.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. Combenefit: An interactive platform for the analysis and visualization of drug combinations. Bioinformatics 2016, 32, 2866–2868. [Google Scholar] [CrossRef]
- Boj, S.F.; Hwang, C.-I.; Baker, L.A.; Chio, I.I.C.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015, 160, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Tiriac, H.; Belleau, P.; Engle, D.D.; Plenker, D.; Deschênes, A.; Somerville, T.D.D.; Froeling, F.E.M.; Burkhart, R.A.; Denroche, R.E.; Jang, G.H.; et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov. 2018, 8, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Kafri, P.; Hasenson, S.E.; Kanter, I.; Sheinberger, J.; Kinor, N.; Yunger, S.; Shav-Tal, Y. Quantifying β-catenin subcellular dynamics and cyclin D1 mRNA transcription during Wnt signaling in single living cells. Elife 2016, 5, e16748. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Shah, Y.M. In vitro Organoid Culture of Primary Mouse Colon Tumors. J. Vis. Exp. 2013, 17, e50210. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Brown, A.; Papkoff, J.; Scambler, P.; Shackleford, G.; McMahon, A.; Moon, R.; Varmus, H. A new nomenclature for int-1 and related genes: The Wnt gene family. Cell 1991, 64, 231. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Q.; Zheng, L.; Aldarouish, M.; Zhou, Z.-H.; Pan, N.; Liu, J.-Q.; Chen, F.-X.; Wang, L.-X. Wnt pathway activator TWS119 enhances the proliferation and cytolytic activity of human γδT cells against colon cancer. Exp. Cell Res. 2018, 362, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.C.; Fofaria, N.M.; Gupta, P.; Ranjan, A.; Kim, S.-H.; Srivastava, S.K. Inhibition of β-Catenin signaling suppresses pancreatic tumor growth by disrupting nuclear β-Catenin/TCF-1 complex: Critical role of STAT-3. Oncotarget 2015, 6, 11561–11574. [Google Scholar] [CrossRef] [PubMed]
- Reister, S.; Mahotka, C.; van den Höfel, N.; Grinstein, E. Nucleolin promotes Wnt signaling in human hematopoietic stem/progenitor cells. Leukemia 2019, 33, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Seino, T.; Kawasaki, S.; Shimokawa, M.; Tamagawa, H.; Toshimitsu, K.; Fujii, M.; Ohta, Y.; Matano, M.; Nanki, K.; Kawasaki, K.; et al. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell 2018, 22, 454–467.e6. [Google Scholar] [CrossRef]
- Zhong, Z.; Virshup, D.M. Wnt Signaling and Drug Resistance in Cancer. Mol. Pharmacol. 2020, 97, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Germinaro, M.; Micsenyi, A.; Monga, N.K.; Bell, A.; Sood, A.; Malhotra, V.; Sood, N.; Midda, V.; Monga, D.K.; et al. Aberrant Wnt/β-Catenin Signaling in Pancreatic Adenocarcinoma. Neoplasia 2006, 8, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.W.; Olsen, M.; Hao, Y.; Lee, S.P.; Lee, K.T.; Chen, X.; Van De Rijn, M.; Brown, P.O. Gene Expression Patterns in Pancreatic Tumors, Cells and Tissues. PLoS ONE 2007, 2, e323. [Google Scholar] [CrossRef]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.-H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core Signaling Pathways in Human Pancreatic Cancers Revealed by Global Genomic Analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef]
- López-Knowles, E.; Zardawi, S.J.; McNeil, C.M.; Millar, E.K.; Crea, P.; Musgrove, E.A.; Sutherland, R.L.; O’Toole, S.A. Cytoplasmic Localization of β-Catenin is a Marker of Poor Outcome in Breast Cancer Patients. Cancer Epidemiol. Prev. Biomark. 2010, 19, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Pannone, G.; Papagerakis, S.; McGuff, H.S.; Cafarelli, B.; Lepore, S.; De Maria, S.; Rubini, C.; Mattoni, M.; Staibano, S.; et al. Beta-Catenin and Epithelial Tumors: A Study Based on 374 Oropharyngeal Cancers. BioMed Res. Int. 2014, 2014, 948264. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qu, Q.; Guo, Y.; Xiang, Y.; Feng, D. Tankyrases/β-catenin Signaling Pathway as an Anti-proliferation and Anti-metastatic Target in Hepatocarcinoma Cell Lines. J. Cancer 2020, 11, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Talar, B.; Gajos-Michniewicz, A.; Talar, M.; Chouaib, S.; Czyz, M. Pentoxifylline Inhibits WNT Signalling in β-Cateninhigh Patient-Derived Melanoma Cell Populations. PLoS ONE 2016, 11, e0158275. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.G.; Lee, B.L.; Kim, W.H. Distribution of LGR5 + Cells and Associated Implications during the Early Stage of Gastric Tumorigenesis. PLoS ONE 2013, 8, e82390. [Google Scholar] [CrossRef] [PubMed]
- Gurney, A.; Axelrod, F.; Bond, C.J.; Cain, J.; Chartier, C.; Donigan, L.; Fischer, M.; Chaudhari, A.; Ji, M.; Kapoun, A.M.; et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 11717–11722. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.M.; Cancilla, B.; Yeung, V.P.; Cattaruzza, F.; Chartier, C.; Murriel, C.L.; Cain, J.; Tam, R.; Cheng, C.-Y.; Evans, J.W.; et al. WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci. Adv. 2017, 3, e1700090. [Google Scholar] [CrossRef]
- Cao, J.; Ma, J.; Sun, L.; Li, J.; Qin, T.; Zhou, C.; Cheng, L.; Chen, K.; Qian, W.; Duan, W.; et al. Targeting glypican-4 overcomes 5-FU resistance and attenuates stem cell–like properties via suppression of Wnt/β-catenin pathway in pancreatic cancer cells. J. Cell. Biochem. 2018, 119, 9498–9512. [Google Scholar] [CrossRef]
- Wang, B.; Zou, Q.; Sun, M.; Chen, J.; Wang, T.; Bai, Y.; Chen, Z.; Chen, B.; Zhou, M. Reversion of trichostatin A resistance via inhibition of the Wnt signaling pathway in human pancreatic cancer cells. Oncol. Rep. 2014, 32, 2015–2022. [Google Scholar] [CrossRef]
- Cossutta, M.; Darche, M.; Carpentier, G.; Houppe, C.; Ponzo, M.; Raineri, F.; Vallée, B.; Gilles, M.-E.; Villain, D.; Picard, E.; et al. Weibel-Palade Bodies Orchestrate Pericytes During Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1843–1858. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raineri, F.; Bourgoin-Voillard, S.; Cossutta, M.; Habert, D.; Ponzo, M.; Houppe, C.; Vallée, B.; Boniotto, M.; Chalabi-Dchar, M.; Bouvet, P.; et al. Nucleolin Targeting by N6L Inhibits Wnt/β-Catenin Pathway Activation in Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 2986. https://doi.org/10.3390/cancers13122986
Raineri F, Bourgoin-Voillard S, Cossutta M, Habert D, Ponzo M, Houppe C, Vallée B, Boniotto M, Chalabi-Dchar M, Bouvet P, et al. Nucleolin Targeting by N6L Inhibits Wnt/β-Catenin Pathway Activation in Pancreatic Ductal Adenocarcinoma. Cancers. 2021; 13(12):2986. https://doi.org/10.3390/cancers13122986
Chicago/Turabian StyleRaineri, Fabio, Sandrine Bourgoin-Voillard, Mélissande Cossutta, Damien Habert, Matteo Ponzo, Claire Houppe, Benoît Vallée, Michele Boniotto, Mounira Chalabi-Dchar, Philippe Bouvet, and et al. 2021. "Nucleolin Targeting by N6L Inhibits Wnt/β-Catenin Pathway Activation in Pancreatic Ductal Adenocarcinoma" Cancers 13, no. 12: 2986. https://doi.org/10.3390/cancers13122986
APA StyleRaineri, F., Bourgoin-Voillard, S., Cossutta, M., Habert, D., Ponzo, M., Houppe, C., Vallée, B., Boniotto, M., Chalabi-Dchar, M., Bouvet, P., Couvelard, A., Cros, J., Debesset, A., Cohen, J. L., Courty, J., & Cascone, I. (2021). Nucleolin Targeting by N6L Inhibits Wnt/β-Catenin Pathway Activation in Pancreatic Ductal Adenocarcinoma. Cancers, 13(12), 2986. https://doi.org/10.3390/cancers13122986








