Salmonella Impacts Tumor-Induced Macrophage Polarization, and Inhibits SNAI1-Mediated Metastasis in Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
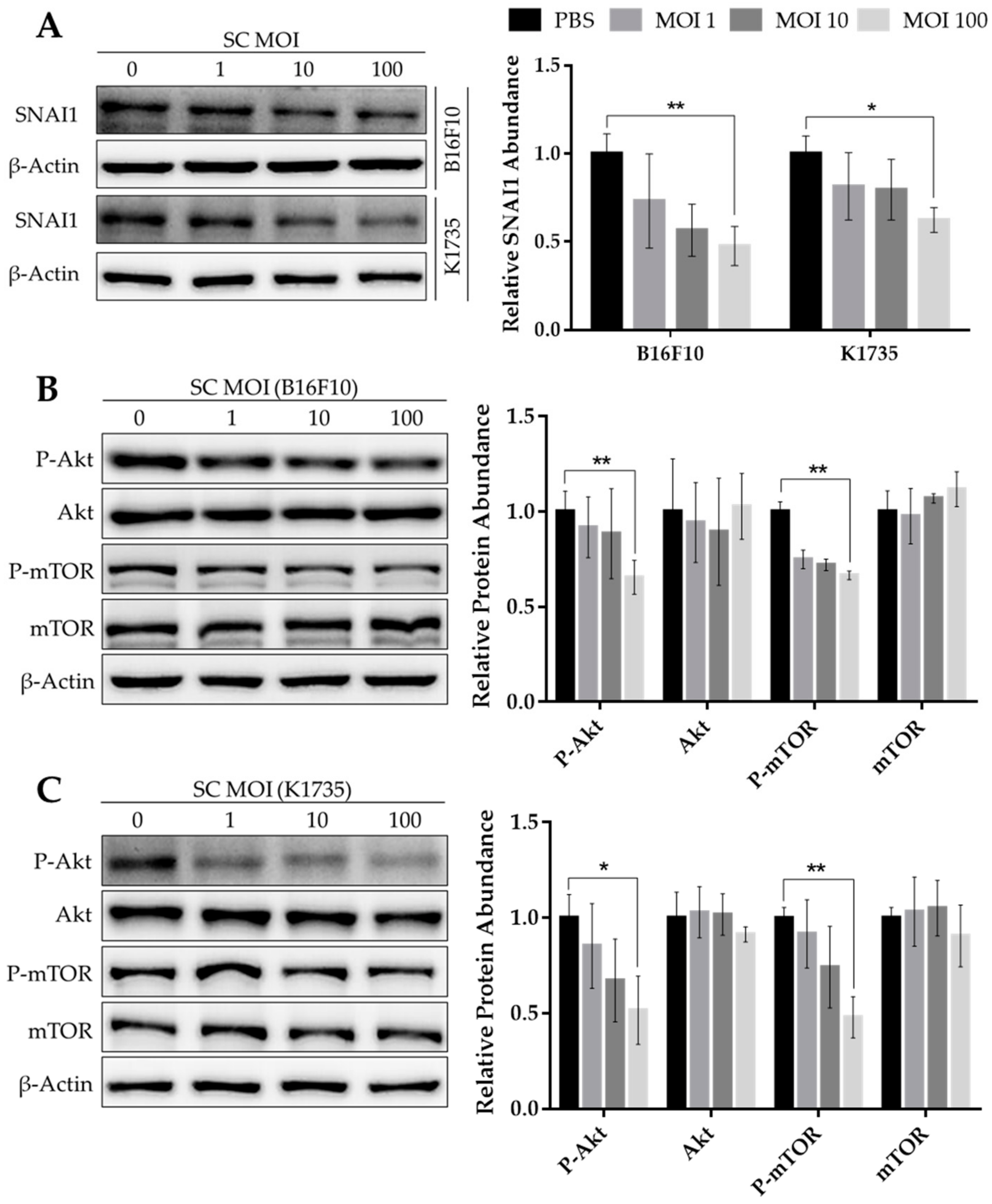
2.1. Salmonella Suppresses Akt/mTOR Activity and SNAI1 Expression in Melanoma
2.2. Salmonella Inhibits Migrative Behavior of Melanoma Cells In Vitro
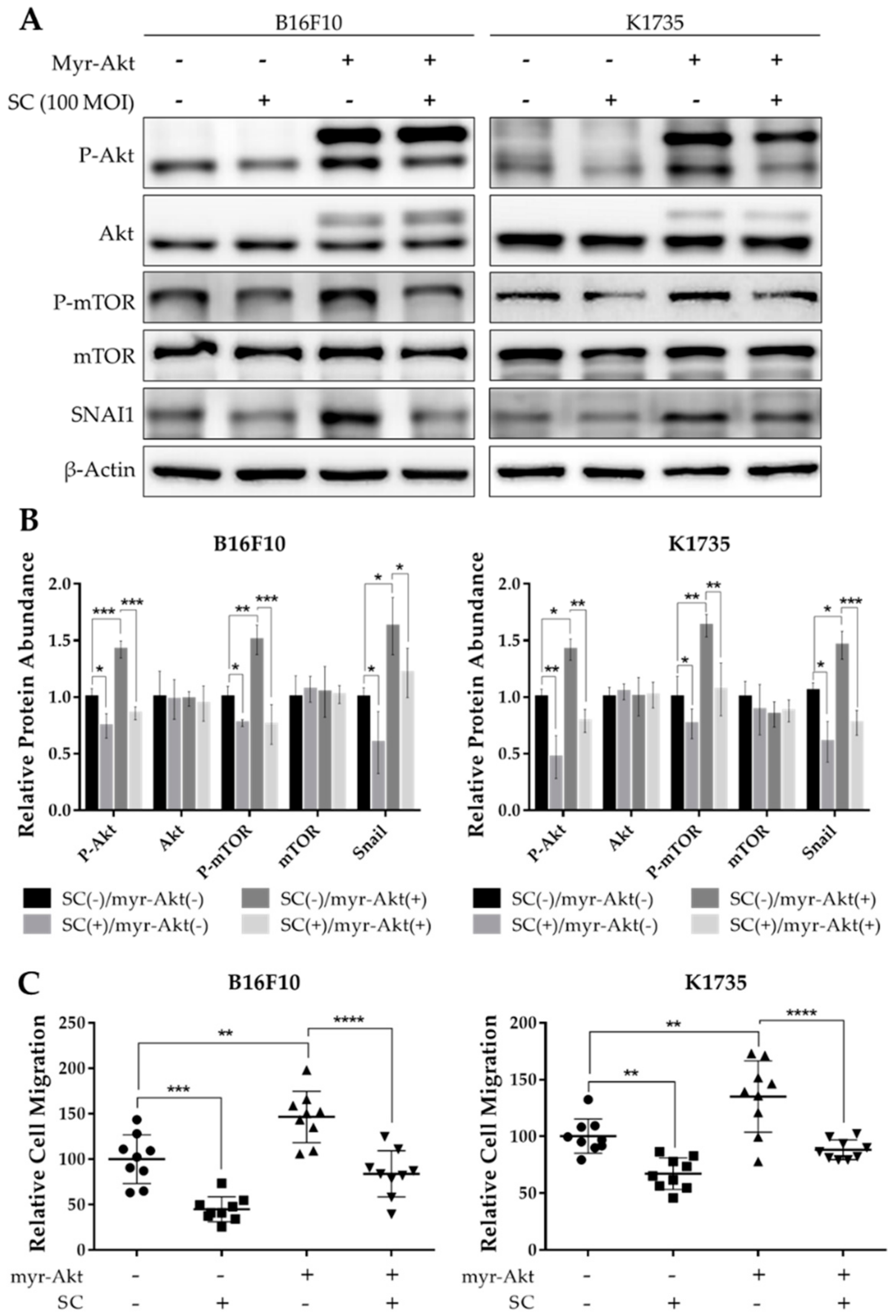
2.3. Salmonella Treatment Reverses the Constitutively Active Akt-Induced SNAI1 Overexpression and Tumor Cell Migration
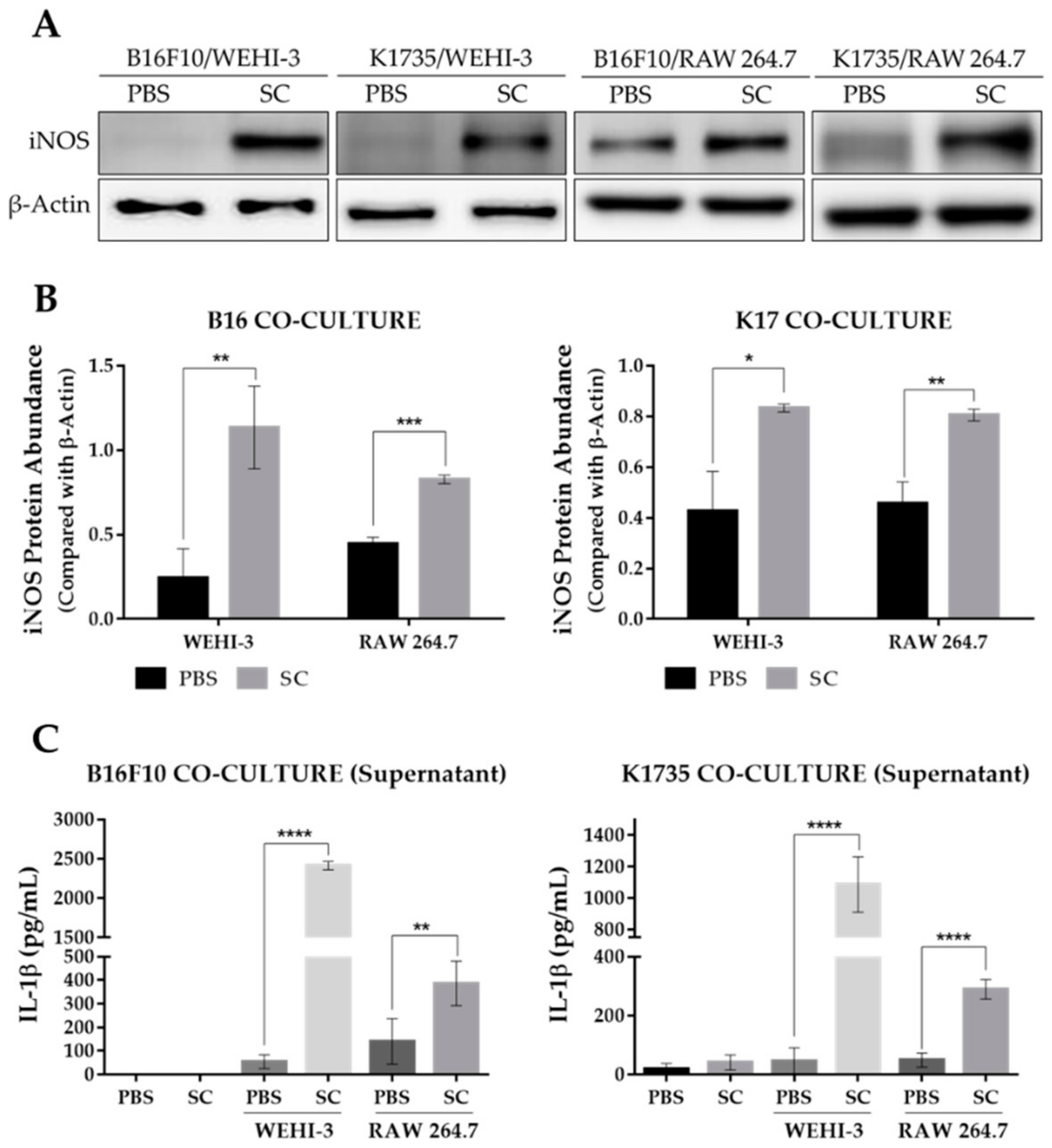
2.4. Salmonella-Treated Melanoma Cells Reprogram Macrophage into M1-Like Phenotype
2.5. Salmonella Increases Tumor-Infiltrating M1-Like Macrophages and Inhibits Lung Metastasis In Vivo
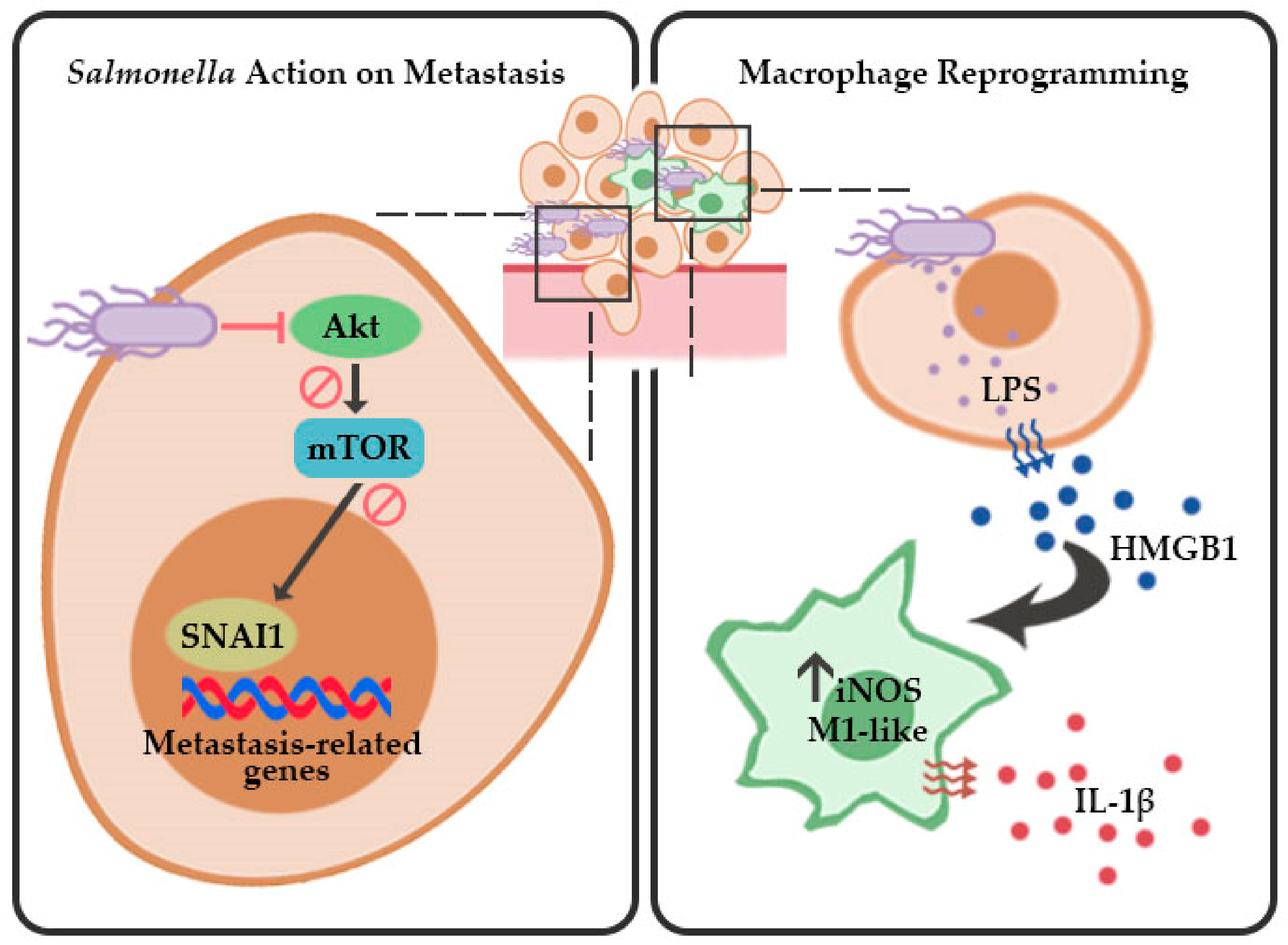
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Bacteria, Plasmid, and Animals
4.2. Western Blot Analysis
4.3. In Vitro Inhibition of Melanoma Cell Migration
4.4. Constitutive Akt Expression
4.5. Macrophage Polarization
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Animal Study
4.7.1. Survival Analysis
4.7.2. Assessment of Lung Metastasis and Macrophage Infiltrations
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, L.E.; Shalinb, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Sig Transduct. Target Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, G.; Kwon, C.I.; Kim, J.W.; Park, P.W.; Hahm, K.B. TWIST1 and SNAI1 as markers of poor prognosis in human colorectal cancer are associated with the expression of ALDH1 and TGF-β1. Oncol. Rep. 2014, 31, 1380–1388. [Google Scholar] [CrossRef]
- Moody, S.E.; Perez, D.; Pan, T.C.; Sarkisian, C.J.; Portocarrero, C.P.; Sterner, C.J.; Notorfrancesco, K.L.; Cardiff, R.D.; Chodosh, L.A. The transcriptional repressor snail promotes mammary tumor recurrence. Cancer Cell 2005, 8, 197–209. [Google Scholar] [CrossRef]
- Tian, Y.; Qi, P.; Niu, Q.; Hu, X. Combined Snail and E-cadherin predicts overall survival of cervical carcinoma patients: Comparison among various epithelial-mesenchymal transition proteins. Front Mol. Biosci. 2020, 7, 22. [Google Scholar] [CrossRef]
- Kuphal, S.; Palm, H.G.; Poser, I.; Bosserhoff, A.K. Snail-regulated genes in malignant melanoma. Melanoma Res. 2005, 15, 305–313. [Google Scholar] [CrossRef]
- Dong, J.; Zhai, B.; Sun, W.; Hu, F.; Cheng, H.; Xu, J. Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced multi-drug resistance to sorafenib in hepatocellular carcinoma cells. PLoS ONE 2017, 12, e0185088. [Google Scholar]
- Li, J.; Xu, H.; Wang, Q.; Wang, S.; Xiong, N. 14-3-3ζ promotes gliomas cells invasion by regulating Snail through the PI3K/AKT signaling. Cancer Med. 2019, 8, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Brenot, A.; Knolhoff, B.L.; DeNardo, D.G.; Longmore, G.D. SNAIL1 action in tumor cells influences macrophage polarization and metastasis in breast cancer through altered GM-CSF secretion. Oncogenesis 2018, 7, 32. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Wang, L.; Zhang, C. Interplay between inflammatory tumor microenvironment and cancer stem cells (Review). Oncol. Lett. 2018, 16, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Porta, C.; Riboldi, E.; Ippolito, A.; Sica, A. Molecular and epigenetic basis of macrophage polarized activation. Semin. Immunol. 2015, 27, 237–248. [Google Scholar] [CrossRef]
- Guerriero, J.L.; Ditsworth, D.; Catanzaro, J.M.; Sabino, G.; Furie, M.B.; Kew, R.R.; Crawford, H.C.; Zong, W.X. DNA Alkylating Therapy Induces Tumor Regression through an HMGB1-Mediated Activation of Innate Immunity. J. Immunol. 2011, 186, 3517–3526. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, P.; Yu, Y.; Lu, H.; Liu, Y.; Ni, P.; Su, X.; Wang, D.; Liu, Y.; Wang, J.; et al. HMGB1 Facilitated Macrophage Reprogramming towards a Proinflammatory M1-like Phenotype in Experimental Autoimmune Myocarditis Development. Sci. Rep. 2016, 6, 21884. [Google Scholar] [CrossRef]
- Lampiasi, N.; Russo, R.; Zito, F. The Alternative Faces of Macrophage Generate Osteoclasts. Biomed. Res. Int. 2016, 2016, 9089610. [Google Scholar] [CrossRef] [PubMed]
- Pangilinan, C.R.; Lee, C.-H. Salmonella-based targeted cancer therapy: Updates on a promising and innovative tumor immunotherapeutic strategy. Biomedicines 2019, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Min, J.J. Salmonella-mediated cancer therapy: Roles and potentials. Nucl. Med. Mol. Imaging 2017, 51, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-W.; Lee, C.-H. Salmonella as an innovative therapeutic antitumor agent. Int. J. Mol. Sci. 2014, 15, 14546–14554. [Google Scholar] [CrossRef]
- Revathidevi, S.; Munirajan, A.K. Akt in cancer: Mediator and more. Semin. Cancer Biol. 2019, 59, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.R.; Schmid, M.C. Macrophages as Key Drivers of Cancer Progression and Metastasis. Mediators Inflamm. 2017, 2017, 9624760. [Google Scholar] [CrossRef]
- Sanchez, L.R.; Borriello, L.; Entenberg, D.; Condeelis, J.S.; Oktay, M.H.; Karagiannis, G.S. The emerging roles of macrophages in cancer metastasis and response to chemotherapy. J. Leukoc. Biol. 2019, 106, 259–274. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef]
- Yang, C.-J.; Chang, W.-W.; Lin, S.-T.; Chen, M.-C.; Lee, C.-H. Salmonella overcomes drug resistance in tumor through P-glycoprotein downregulation. Int. J. Med. Sci. 2018, 15, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Pangilinan, C.R.; Lee, C.-H. Salmonella breaks tumor immune tolerance by downregulating tumor Programmed Death-Ligand 1 expression. Cancers 2020, 12, 57. [Google Scholar] [CrossRef]
- Massoumi, R.; Kuphal, S.; Hellerbrand, C.; Haas, B.; Wild, P.; Spruss, T.; Pfeifer, A.; Fässler, R.; Bosserhoff, A.K. Down-regulation of CYLD expression by Snail promotes tumor progression in malignant melanoma. J. Exp. Med. 2009, 206, 221–232. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, Z.; Sun, Y.; Chen, J.; Zhang, B.; Wu, M.; Li, T.; Hu, L.; Zeng, J. The EMT-related transcription factor snail up-regulates FAPα in malignant melanoma cells. Exp. Cell Res. 2018, 364, 160–167. [Google Scholar] [CrossRef]
- Aladowicz, E.; Ferro, L.; Vitali, G.C.; Venditti, E.; Fornasari, L.; Lanfrancone, L. Molecular networks in melanoma invasion and metastasis. Future Oncol. 2013, 9, 713–726. [Google Scholar] [CrossRef]
- Alonso, S.R.; Tracey, L.; Ortiz, P.; Perez-Gomez, B.; Palacios, J.; Pollan, M.; Linares, J.; Serrano, S.; Saez-Castillo, A.I.; Sanchez, L.; et al. A high-throughput study in melanoma identifies epithelial–mesenchymal transition as a major determinant of metastasis. Cancer Res. 2007, 67, 3450–3460. [Google Scholar] [CrossRef] [PubMed]
- Olmeda, D.; Jordá, M.; Peinado, H.; Fabra, Á.; Cano, A. Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene 2007, 26, 1862–1874. [Google Scholar] [CrossRef]
- Karamanou, K.; Franchi, M.; Proult, I.; Rivet, R.; Vynios, D.; Brézillon, S. Lumican inhibits in vivo melanoma metastasis by altering matrix-effectors and invadopodia markers. Cells 2021, 10, 841. [Google Scholar] [CrossRef]
- Fang, R.; Zhang, G.; Guo, Q.; Ning, F.; Wang, H.; Cai, S.; Du, J. Nodal promotes aggressive phenotype via Snail-mediated epithelial–mesenchymal transition in murine melanoma. Cancer Lett. 2013, 333, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bi, Y.; Li, Y.; Fu, R.; Lv, W.; Jiang, N.; Xu, Y.; Ren, B.; Chen, Y.; Xie, H.; et al. A potent CBP/p300-Snail interaction inhibitor suppresses tumor growth and metastasis in wild-type p53-expressing cancer. Sci. Adv. 2020, 6, eaaw8500. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H. Employment of Salmonella in cancer gene therapy. Methods Mol. Biol. 2016, 1409, 79–83. [Google Scholar] [PubMed]
- Siroy, A.E.; Davies, M.A.; Lazar, A.J. The PI3K-AKT pathway in melanoma. In Genetics of Melanoma. Cancer Genetics; Torres-Cabala, C., Curry, J., Eds.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.H. Akt as therapeutic target for cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Wang, J.; Lu, W.; Tang, X.; Chen, J.; Mou, H.; Chen, Q. Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer. Mol. Ther. Oncolytics 2016, 3, 16018. [Google Scholar] [CrossRef] [PubMed]
- Kaimala, S.; Mohamed, Y.A.; Nader, N.; Isaac, J.; Elkord, E.; Chouaib, S.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. Salmonella-mediated tumor regression involves targeting of tumor myeloid suppressor cells causing a shift to M1-like phenotype and reduction in suppressive capacity. Cancer Immunol. Immunother. 2014, 63, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, R.; Peng, Z.; Hu, B.; Rao, X.; Li, J. HMGB1 participates in LPS-induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF-κB signaling pathways. Int. J. Mol. Med. 2020, 45, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef] [PubMed]
- De Melo, F.M.; Braga, C.J.M.; Pereira, F.V.; Maricato, J.T.; Origassa, C.S.; Souza, M.F.; Melo, A.C.; Silva, P.; Tomaz, S.L.; Gimenes, K.P.; et al. Anti-metastatic immunotherapy based on mucosal administration of flagellin and immunomodulatory P10. Immunol. Cell Biol. 2015, 93, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, A.; Mongini, C.; Gravisaco, M.J.; Canellada, A.; Tesone, A.I.; Goin, J.C.; Waldner, C.I. An oral Salmonella-based vaccine inhibits liver metastases by promoting tumor-specific T-cell-mediated immunity in celiac and portal lymph nodes: A preclinical study. Front. Immunol. 2016, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.H.; Nguyen, V.H.; Jiang, S.N.; Park, S.H.; Tan, W.; Hong, S.H.; Shin, M.G.; Chung, I.J.; Hong, Y.; Bom, H.S.; et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017, 9, eaak9537. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, C.L.; Shiau, A.L. Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis. Clin. Cancer Res. 2008, 14, 1905–1912. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pangilinan, C.R.; Wu, L.-H.; Lee, C.-H. Salmonella Impacts Tumor-Induced Macrophage Polarization, and Inhibits SNAI1-Mediated Metastasis in Melanoma. Cancers 2021, 13, 2894. https://doi.org/10.3390/cancers13122894
Pangilinan CR, Wu L-H, Lee C-H. Salmonella Impacts Tumor-Induced Macrophage Polarization, and Inhibits SNAI1-Mediated Metastasis in Melanoma. Cancers. 2021; 13(12):2894. https://doi.org/10.3390/cancers13122894
Chicago/Turabian StylePangilinan, Christian R., Li-Hsien Wu, and Che-Hsin Lee. 2021. "Salmonella Impacts Tumor-Induced Macrophage Polarization, and Inhibits SNAI1-Mediated Metastasis in Melanoma" Cancers 13, no. 12: 2894. https://doi.org/10.3390/cancers13122894
APA StylePangilinan, C. R., Wu, L.-H., & Lee, C.-H. (2021). Salmonella Impacts Tumor-Induced Macrophage Polarization, and Inhibits SNAI1-Mediated Metastasis in Melanoma. Cancers, 13(12), 2894. https://doi.org/10.3390/cancers13122894





