Dynamic Physiological Culture of Ex Vivo Human Tissue: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
3.1. Literature Search
3.2. Mechano-Transduction Culture Models
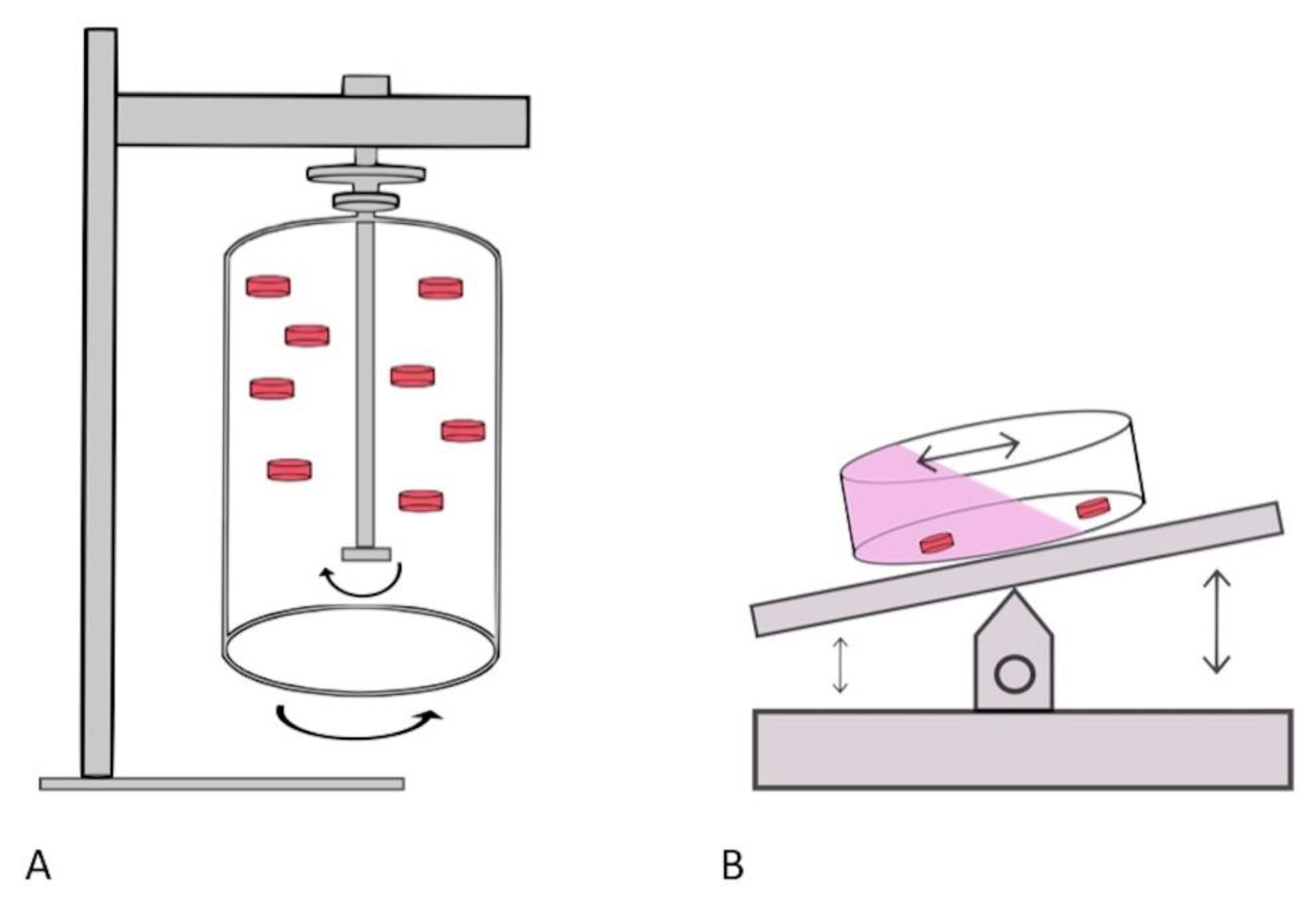
3.3. Dynamic Culture with a Rotational Bioreactor
3.4. Dynamic Perfusion Culture
3.5. Successful Culture of Ex Vivo Human Tissue with Dynamic Culture Techniques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansmann, J.; Egger, D.; Kasper, C. Advanced Dynamic Cell and Tissue Culture. Bioengineering 2018, 5, 65. [Google Scholar] [CrossRef]
- Selden, C.; Fuller, B. Role of Bioreactor Technology in Tissue Engineering for Clinical Use and Therapeutic Target Design. Bioengineering 2018, 5, 32. [Google Scholar] [CrossRef]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80–86. [Google Scholar] [CrossRef]
- Crabbé, A.; Liu, Y.; Sarker, S.F.; Bonenfant, N.R.; Barrila, J.; Borg, Z.D.; Lee, J.J.; Weiss, D.J.; Nickerson, C.A. Recellularization of decellularized lung scaffolds is enhanced by dynamic suspension culture. PLoS ONE 2015, 10, e0126846. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.J.; Domansky, K.; Kaazempur-Mofrad, M.R.; Kalezi, A.; Capitano, A.; Upadhyaya, A.; Kurzawski, P.; Wack, K.E.; Stolz, D.B.; Kamm, R.; et al. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol. Bioeng. 2002, 78, 257–269. [Google Scholar] [CrossRef]
- Carrier, R.L.; Rupnick, M.; Langer, R.; Schoen, F.J.; Freed, L.E.; Vunjak-Novakovic, G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002, 8, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Shekaran, A.; Lam, A.; Sim, E.; Jialing, L.; Jian, L.; Wen, J.T.; Chan, J.K.; Choolani, M.; Reuveny, S.; Birch, W.; et al. Biodegradable ECM-coated PCL microcarriers support scalable human early MSC expansion and in vivo bone formation. Cytotherapy 2016, 18, 1332–1344. [Google Scholar] [CrossRef]
- Ball, O.; Nguyen, B.N.B.; Placone, J.K.; Fisher, J.P. 3D Printed Vascular Networks Enhance Viability in High-Volume Perfusion Bioreactor. Ann. Biomed. Eng. 2016, 44, 3435–3445. [Google Scholar] [CrossRef]
- Meinert, C.; Schrobback, K.; Hutmacher, D.W.; Klein, T.J. A novel bioreactor system for biaxial mechanical loading enhances the properties of tissue-engineered human cartilage. Sci. Rep. 2017, 7, 16997. [Google Scholar] [CrossRef] [PubMed]
- Nietzer, S.; Baur, F.; Sieber, S. Mimicking Metastases Including Tumor Stroma: A New Technique to Generate a Three-Dimensional Colorectal Cancer Model Based on a Biological Decellularized Intestinal Scaffold. Tissue Eng. Part C Methods 2016, 22, 621–635. [Google Scholar] [CrossRef]
- Carlini, M.J.; De Lorenzo, M.S.; Puricelli, L. Cross-talk between tumor cells and the microenvironment at the metastatic niche. Curr. Pharm. Biotechnol. 2011, 12, 1900–1908. [Google Scholar] [CrossRef]
- Vertrees, R.A.; Jordan, J.M.; Solley, T.; Goodwin, T.J. Tissue Culture Models. Basic Concepts Mol. Pathol. 2009, 2, 159–182. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, D.H.; Gawri, R.; Moir, J.; Beckman, L.; Eglin, D.; Steffen, T.; Roughley, P.J.; Ouellet, J.A.; Haglund, L. Dynamic loading, matrix maintenance and cell injection therapy of human intervertebral discs cultured in a bioreactor. Eur. Cells Mater. 2016, 31, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Ladd, M.R.; Lee, S.J.; Atala, A.; Yoo, J.J. Bioreactor maintained living skin matrix. Tissue Eng. Part A 2009, 15, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Aiyangar, A.K.; Vivanco, J.; Au, A.G.; Anderson, P.A.; Smith, E.L.; Ploeg, H.L. Dependence of anisotropy of human lumbar vertebral trabecular bone on quantitative computed tomography-based apparent density. J. Biomech. Eng. 2014, 136, 091003. [Google Scholar] [CrossRef]
- Walter, B.A.; Illien-Junger, S.; Nasser, P.R.; Hecht, A.C.; Iatridis, J. Development and validation of a bioreactor system for dynamic loading and mechanical characterization of whole human intervertebral discs in organ culture. J. Biomech. 2014, 47, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.; Hatfill, S.; Chuaqui, R.; Vocke, C.; Emmert-Buck, M.; Linehan, W.M.; Duray, P.H. Long term organ culture of human prostate tissue in a NASA-designed rotating wall bioreactor. J. Urol. 1999, 161, 290–297. [Google Scholar] [CrossRef]
- Licato, L.; Prieto, G.; Grimm, E. A novel preclinical model of human malignant melanoma utilizing bioreactor rotating-wall vessels. Vitr. Cell. Dev. Biol. Anim. 2001, 37, 121–126. [Google Scholar] [CrossRef]
- Duray, P.H.; Yin, S.R.; Ito, Y.; Bezrukov, L.; Cox, C.; Cho, M.S.; Fitzgerald, W.; Dorward, D.; Zimmerberg, J.; Margolis, L. Invasion of human tissue ex vivo by Borrelia burgdorferi. J. Infect. Dis. 2005, 191, 1747–1754. [Google Scholar] [CrossRef]
- Ferrarini, M.; Steimberg, N.; Ponzoni, M.; Belloni, D.; Berenzi, A.; Girlanda, S.; Caligaris-Cappio, F.; Mazzoleni, G.; Ferrero, E. Ex-vivo dynamic 3-D culture of human tissues in the RCCS™ bioreactor allows the study of Multiple Myeloma biology and response to therapy. PLoS ONE 2013, 8, e71613. [Google Scholar] [CrossRef]
- Drew, J.E.; Farquharson, A.J.; Vase, H.; Carey, F.A.; Steele, R.J.; Ross, R.A.; Bunton, D.C. Molecular Profiling of Multiplexed Gene Markers to Assess Viability of Ex Vivo Human Colon Explant Cultures. Biores. Open Access 2015, 4, 425–430. [Google Scholar] [CrossRef]
- Paish, H.L.; Reed, L.H.; Brown, H.; Bryan, M.C.; Govaere, O.; Leslie, J.; Barksby, B.S.; Garcia Macia, M.; Watson, A.; Xu, X.; et al. A Bioreactor Technology for Modeling Fibrosis in Human and Rodent Precision-Cut Liver Slices. Hepatology 2019, 70, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Surowiec, S.M.; Conklin, B.S.; Li, J.S.; Lin, P.H.; Weiss, V.J.; Lumsden, A.B. A new perfusion culture system used to study human vein. J. Surg. Res. 2000, 88, 34–41. [Google Scholar] [CrossRef]
- Strehl, R.; Tallheden, T.; Sjogren-Jansson, E.; Minuth, W.W.; Lindahl, A. Long-term maintenance of human articular cartilage in culture for biomaterial testing. Biomaterials 2005, 26, 4540–4549. [Google Scholar] [CrossRef]
- Cheah, L.T.; Dou, Y.H.; Seymour, A.M.; Dyer, C.E.; Haswell, S.J.; Wadhawan, J.D.; Greenman, J. Microfluidic perfusion system for maintaining viable heart tissue with real-time electrochemical monitoring of reactive oxygen species. Lab Chip 2010, 10, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Midwoud, P.M.; Merema, M.T.; Verpoorte, E.; Groothuis, G.M. Microfluidics Enables Small-Scale Tissue-Based Drug Metabolism Studies With Scarce Human Tissue. J. Lab. Autom. 2011, 16, 468–476. [Google Scholar] [CrossRef]
- Ataç, B.; Wagner, I.; Horland, R.; Lauster, R.; Marx, U.; Tonevitsky, A.G.; Azar, R.P.; Lindner, G. Skin and hair on-a-chip: In vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip 2013, 13, 3555–3561. [Google Scholar] [CrossRef]
- Astolfi, M.; Péant, B.; Lateef, M.A.; Rousset, N.; Kendall-Dupont, J.; Carmona, E.; Monet, F.; Saad, F.; Provencher, D.; Mes-Masson, A.M.; et al. Micro-dissected tumor tissues on chip: An ex vivo method for drug testing and personalized therapy. Lab Chip 2016, 16, 312–325. [Google Scholar] [CrossRef]
- Perrard, M.H.; Sereni, N.; Schluth-Bolard, C.; Blondet, A.; DEstaing, S.G.; Plotton, I.; Morel-Journel, N.; Lejeune, H.; David, L.; Durand, P. Complete Human and Rat Ex Vivo Spermatogenesis from Fresh or Frozen Testicular Tissue. Biol. Reprod. 2016, 95, 89. [Google Scholar] [CrossRef]
- Muraro, M.G.; Muenst, S.; Mele, V.; Quagliata, L.; Iezzi, G.; Tzankov, A.; Weber, W.P.; Spagnoli, G.C.; Soysal, S.D. Ex-vivo assessment of drug response on breast cancer primary tissue with preserved microenvironments. Oncoimmunology 2017, 6, e1331798. [Google Scholar] [CrossRef] [PubMed]
- Piola, M.; Ruiter, M.; Vismara, R.; Mastrullo, V.; Agrifoglio, M.; Zanobini, M.; Pesce, M.; Soncini, M.; Fiore, G.B. Full Mimicking of Coronary Hemodynamics for Ex-Vivo Stimulation of Human Saphenous Veins. Ann. Biomed. Eng. 2017, 45, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Bower, R.; Green, V.L.; Kuvshinova, E.; Kuvshinov, D.; Karsai, L.; Crank, S.T.; Stafford, N.D.; Greenman, J. Maintenance of head and neck tumor on-chip: Gateway to personalized treatment? Future Sci. OA 2017, 3, FSO174. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.D.; Horowitz, L.F.; Castro, K.; Kenerson, H.; Bhattacharjee, N.; Gandhe, G.; Raman, A.; Monnat, R.J.; Yeung, R.; Rostomily, R.C.; et al. A microfluidic platform for functional testing of cancer drugs on intact tumor slices. Lab Chip 2020, 20, 1658–1675. [Google Scholar] [CrossRef]
- Riley, A.; Green, V.; Cheah, R.; McKenzie, G.; Karsai, L.; England, J.; Greenman, J. A novel microfluidic device capable of maintaining functional thyroid carcinoma specimens ex vivo provides a new drug screening platform. BMC Cancer 2019, 19, 259. [Google Scholar] [CrossRef]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef]
- Ringer, S. Concerning the influence exerted by each of the constituents of the blood on the contraction of the ventricle. J. Physiol. 1882, 3, 380–393. [Google Scholar] [CrossRef]
- Rouwkema, J.; Koopman, B.; Blitterswijk, C.; Dhert, W.; Malda, J. Supply of Nutrients to Cells in Engineered Tissues. Biotechnol. Genet. Eng. Rev. 2009, 26, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; MacArthur, B.; Malda, J.; Pettet, G.; Please, C. Heterogeneous proliferation within engineered cartilaginous tissue: The role of oxygen tension. Biotechnol. Bioeng. 2005, 91, 607–615. [Google Scholar] [CrossRef]
- Malda, J.; Rouwkema, J.; Martens, D.E.; Le Comte, E.P.; Kooy, F.K.; Tramper, J.; van Blitterswijk, C.A.; Riesle, J. Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: Measurement and modeling. Biotechnol. Bioeng. 2004, 86, 9–18. [Google Scholar] [CrossRef]
- Malda, J.; Woodfield, T.B.; van der Vloodt, F.; Kooy, F.K.; Martens, D.E.; Tramper, J.; van Blitterswijk, C.A.; Riesle, J. The effect of PEGT/PBT scaffold architecture on oxygen gradients in tissue engineered cartilaginous constructs. Biomaterials 2004, 25, 5773–5780. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Castillo-León, J. Microfluidics and Lab-on-a-Chip Devices: History and Challenges. In Lab-On-A-Chip Devices and Micro-Total Analysis Systems; Springer: Cham, Switzerland, 2014; pp. 1–15. [Google Scholar] [CrossRef]
- Tsamandouras, N.; Chen, W.L.K.; Edington, C.D.; Stokes, C.L.; Griffith, L.G.; Cirit, M. Integrated Gut and Liver Microphysiological Systems for Quantitative In Vitro Pharmacokinetic Studies. AAPS J. 2017, 19, 1499–1512. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef]
- Edington, C.D.; Chen, W.L.K.; Geishecker, E.; Kassis, T.; Soenksen, L.R.; Bhushan, B.M.; Freake, D.; Kirschner, J.; Maass, C.; Tsamandouras, N.; et al. Interconnected microphysiological systems for quantitative biology and pharmacology studies. Sci. Rep. 2018, 8, 4530. [Google Scholar] [CrossRef]
- Oleaga, C.; Bernabini, C.; Smith, A.S.; Srinivasan, B.; Jackson, M.; McLamb, W.; Platt, V.; Bridges, R.; Cai, Y.; Santhanam, N.; et al. Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci. Rep. 2016, 6, 20030. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef]
- Duzagac, F.; Saorin, G.; Memeo, L.; Canzonieri, V.; Rizzolio, F. Microfluidic Organoids-on-a-Chip: Quantum Leap in Cancer Research. Cancers 2021, 13, 737. [Google Scholar] [CrossRef]
- Schuster, B.; Junkin, M.; Kashaf, S.S.; Romero-Calvo, I.; Kirby, K.; Matthews, J.; Weber, C.R.; Rzhetsky, A.; White, K.P.; Tay, S. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat. Commun. 2020, 11, 5271. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Chen, D.; Meng, Z.; Chen, W.; Huang, W. Protocol for generation of lung adenocarcinoma organoids from clinical samples. STAR Protoc. 2020, 2, 100239. [Google Scholar] [CrossRef]
- Dekkers, J.F.; van Vliet, E.J.; Sachs, N.; Rosenbluth, J.M.; Kopper, O.; Rebel, H.G.; Wehrens, E.J.; Piani, C.; Visvader, J.E.; Verissimo, C.S.; et al. Long-term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat. Protoc. 2021, 16, 1936–1965. [Google Scholar] [CrossRef]
- Velasco, V.; Shariati, S.A.; Esfandyarpour, R. Microtechnology-based methods for organoid models. Microsyst. Nanoeng. 2020, 6, 76. [Google Scholar] [CrossRef]
- Yu, F.; Hunziker, W.; Choudhury, D. Engineering Microfluidic Organoid-on-a-Chip Platforms. Micromachines 2019, 10, 165. [Google Scholar] [CrossRef]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef]
- Garcia-Montero, A.; Vasseur, S.; Mallo, G.V.; Soubeyran, P.; Dagorn, J.C.; Iovanna, J.L. Expression of the stress-induced p8 mRNA is transiently activated after culture medium change. Eur. J. Cell Biol. 2001, 80, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.; Küffer, J.; Ströbel, S.; Dubini, G.; Martin, I.; Wendt, D. Computational evaluation of oxygen and shear stress distributions in 3D perfusion culture systems: Macro-scale and micro-structured models. J. Biomech. 2008, 41, 2918–2925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Mousavi Shaegh, S.A.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef] [PubMed]


| Author | Tissue Cultured | Nature of Physiology Being Replicated | Direction of Load | Loading Weight Applied | Characteristics of Applied Loading Force | Duration of Force Applied | Recovery Time | Number of Samples Cultured per Bioreactor |
|---|---|---|---|---|---|---|---|---|
| Rosenzweig, D. (2000) [15] | Intervertebral Disc | Dynamic cyclic compression | Vertical | Loads were cycled 0.1 MPa and 0.3, 0.6 or 1.2 MPa | Dynamic loading | 2 periods of 2 h per each load | Periods of 6 and 14 h, maintaining a low-static load (0.1 MPa) | One sample fits entirely into one independent bioreactor (1 of 3 units) |
| Ladd. M. (2016) [16] | Juvenile prepuce | Mechanical expansion/stretch | Uniaxial stretch | Expansion of the skin by 20% of its initial length per day for 5 days to double the original length | Dynamic stretching | 5 days. Progressive stretch maintained until target maintained | Maintenance of each stretch for 55 min | One sample per bioreactor unit |
| Aiyangar, A. (2017) [17] | Trabecular bone (L1 vertebrae) | Dynamic cyclic compression | Vertical | 10 N or 20 N | Dynamic loading | NS | Unloading period after each compression | One sample per bioreactor unit |
| Walter, B. (2017) [18] | Intervertebral Disc | Dynamic cyclic compression | Vertical | 0.1 MPa/0.2 MPa | Dynamic and static loading | 12 h for each load for 7, 14 or 21 days | No recovery time | Able to load 6 or 9 samples simultaneously |
| Author | Tissue Cultured | Description of Bioreactor | Rotation Speed | Time Interval for Media Exchange | Volume of Media within a Bioreactor |
|---|---|---|---|---|---|
| Margolis, L. (1999) [19] | Prostatic tissue | Cultured tissue is suspended in culturing liquid medium enclosed by an inner and outer rotating cylinder. The tissue and medium rotate in unison under low shear force. The rotation results in an equilibrium between gravitational-induced sedimentation of the tissue/cells and a centrifugal force. | 30 Rpm | Bioreactor medium was 50% renewed every 7 days | 55 mL |
| Licato, L. (2001) [20] | Melanoma tissue | Rotating-wall vessel bioreactor is completely fluid filled. Specific rotation around a horizontal axis occurs that results in the cultured tissue or cells to be in a state of continuous free fall under low shear stress conditions, designed for mass transfer of nutrients. | 20 Rpm | Media replaced once a week. Bubbles were removed from the vessels daily, so that the chamber remained completely fluid filled | 55 mL |
| Durray, P. (2005) [21] | Tonsillar tissue | Same method and technique as described by Margolis (1999) | 35–40 Rpm | Media sampled every 3 days and replaced. | 200 mL |
| Ferrarini, P. (2013) [22] | Bone marrow | Horizontally rotating bioreactor utilised in order to create a laminar flow (Rotatory Cell Culture System). Cultured 3D tissues suspended in a “free falling” position in order to minimise turbulence and shear forces across the tissue. Gas exchange membrane incorporated in vessels to ensure optimal oxygenation. | Continuously rocked by a rocker device | NS | 10 mL |
| Drew, J. (2015) [23] | Colon tissue | Explant placed on wire mesh on 6-well plates with minimal coverage of media over the explant. Cultured under continuous rocking motion within incubator. | Continuously rocked by a rocker device | Chamber was flushed with 95%O2/5% CO2 for 10 min at each culture point. Cultured for 14 h | 3.5 mL |
| Paish, H. (2019) [24] | Liver tissue | BioR plate used for ex-vivo tissue culture. The BioR plate has 2 wells which are interconnected by a common channel in order to facilitate cross communication between the wells. Plate cultured on a rocking bioreactor platform. Tissue cultured on trans-well support | Continuously rocked by a rocker device | Media replenished daily | 3 mL |
| Author | Tissue Cultured | Perfusion Flow Rate | Timing of Flow | Pump Mechanism | Volume of Chamber | Outflow Management |
|---|---|---|---|---|---|---|
| Surowiec, S. (2000) [25] | Saphenous vein | 100 mL/min | Intermittent | Peristaltic pump | 500 mL | NS |
| Strehl, R. (2005) [26] | Articular cartilage from femoral trochlear region | 1 mL/h | Intermittent | Peristaltic pump | N/A | Passive |
| Cheah, L. (2010) [27] | Heart tissue | 120 mL/min | Intermittent | Peristaltic pump | 400 mL | Active |
| Midwoud, P. (2011) [28] | Liver tissue | 10 mL/min | Continuous | Peristaltic pump | 25 μL | Passive |
| Atac, B. (2013) [29] | Juvenile prepuce | 7–70 mL min | Intermittent | Micropump | 500 mL | Active |
| Astolfi, M. (2016) [30] | Ovarian and prostate tissue | 20 μL/min | Intermittent | Micropipette pump | 500 μL | Active |
| Perrard, M. (2016) [31] | Testicular tissue | NS | NS | NS | 8 mL | Passive |
| Muraro, M. (2017) [32] | Breast tissue | 0.3 mL/min | Continuous | Syringe pump | 8 mL | Passive |
| Piola, M. (2017) [33] | Saphenous vein | 40–240 mL/min | Intermittent | Peristaltic pump | 50 mL | Passive |
| Bower, R. (2017) [34] | Laryngeal, oropharyngeal or oral cavity tissue | 2 μL/min | Intermittent | Syringe pump | NS | Passive |
| Rodriquez, A. (2019) [35] | Rectal tissue | 1.5 mL h−1 | Continuous | Syringe pump | 480 μL | Active |
| Author | Tissue Cultured | Condition of Tissue | Duration Cultured Ex-Vivo | Tissue Acquisition | Duration of Ischaemic Cellular Injury | Tissue Sample Preparation | Tissue Sample Thickness | Viability Assessments | Preservation of Native Function within Tissue | Drug Delivery Assessed | Description | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrointestinal System | Duray, P. (2005) [21] | Tonsillar tissue | Normal | 12 days | Tonsillectomy | Processed within 5 h of surgery | Manual dissection (Scalpel) | 2 mm cubes | Histological assessment | N/A | N/A | Inoculating of tonsils with Borelia burgoferfi with subsequent confirmation of bacterial proliferation with PCR. |
| Midwoud, P. (2011) [28] | Liver tissue | Normal | 24 h | Redundant donor tissue after split-liver transplantation | N/A | Microtome (Krumdieck tissue slicer) | 100 μm-thickness | Bile acid synthesis by CYP71, Trends in transaminase level | Bile acid synthesis | Drug metabolism | Co-cultured of liver with intestinal slice (multi tissue culture) | |
| Paish, H. (2019) [24] | Liver tissue | Normal | 6 days | Hepatectomy | 2 h | Vibratome (Leica VT1200S) | 250-μm-thickness | Albumin secretion | Albumin secretion | Drug induced fibrosis (TGFβ1 + PDGFββ) and anti-fibrsosis drug (ALKi) | Profiling immune cell populations during ex vivo culture. Platform for antifibrosis drug screen | |
| Rodriquez, A. (2019) [35] | Colon tissue | Diseased (rectal cancer) | 3 days | Colectomy | N/A | Vibratome (Leica VT1200S) | 250 μm-thick | Hoechst staining | N/A | Yes -Assessed by apoptosis and proliferation rates | Comprehensive drug screen (3 regimes tested) | |
| Musculoskeletal System | Strehl, R. (2005) [26] | Articular cartilage from femoral trochlear region | Normal | 56 days | Surgical specimens | Processed immediately after surgery | Stainless steel punch (MiltexInstruments) | Full thickness cylindrical explants of 3 mm × 3 mm | Histological assessment of morphology and IHC staining for extracellular matrix. Mitotic index determined. | N/A | N/A | Determining hyaline cartilage composition with sub population changes over time. Proliferation capacity ex vivo |
| Aiyangar, A. (2014) [17] | Trabecular bone (L1 vertebrae) | Normal | N/A | Post mortem acquisition | N/A | Diamond coated band saw | 5 mm in height and 10 mm in diameter | N/A | Mechanical properties (pressure distribution) | N/A | Radiological assessment (CT) of vertebral body volumetry. | |
| Walter, B. (2014) [18] | Intervertebral Disc | Normal | 21 days | Lumbar Cadaveric samples | N/A | Histologic band saw (Exakt310) | N/A | Viability assays (MTT & DAPI staining) | Mechanical properties (Mechanotransduction) | N/A | Comprehensive assessment of pressure application and subsequent tissue response | |
| Rosenzweig, D. (2016) [15] | Intervertebral Disc | Normal | 10 days | Post mortem acquisition | Less than 4 h | High-speed drill (Foredom) | Varying disc height—0.8–1.65 cm | Viability assays (MTT & DAPI staining) | Mechanical properties (Mechanotransduction) | N/A | Detailed assessment of pressure application with a intermittent cycle with a recovery period | |
| Margolis, L. (1999) [19] | Prostatic tissue | Normal | 28 days | Transurethral prostatectomy/needle biopsy | N/A | Manual dissection (dissection) | 1 × 1 mm blocks | Histological assessment | N/A | N/A | Determining PSA expression ex-vivo | |
| Ladd, M. (2009) [16] | Juvenile prepuce | Normal | 6 days | Routine circumcision | N/A | N/A | N/A | Histological and IHC assessments | Mechanical properties of skin (collagen staining) | N/A | Tensile strength assessment of skin | |
| Genito-Urinary System | Atac, B. (2013) [29] | Juvenile prepuce | Normal | 14 days | Routine circumcisions | Immediately after surgery | N/A | N/A | Histological assessment. IF staining for apoptosis and proliferation. | N/A | N/A | Multi organ culture-skin and hair |
| Perrard, M. (2016) [31] | Testicular tissue | Normal | 60 days | Orchidectomy | N/A | N/A | 20 to 50 mm3 of isolated seminiferous tubule segments | Histological assessment for morphology | Spermatogenesis | N/A | Evaluating spermatogenesis ex vivo | |
| Astolfi, M. (2016) [30] | Ovarian and prostate tissue | Diseased (Ovarian and prostate cancer) | 8 days | Surgical resection | Processed within 3 h of surgery | Vibratome | 300 micrometre slices | Staining with liability dyes–CTG and PI | N/A | Yes, carboplatin used. | Personalised drug screen | |
| Cardiovascular System | Surowiec, S. (2000) [25] | Saphenous vein | Normal | 96 h | Segments obtained following coronary artery bypass grafts | Immediately after surgery | Manual dissection (dissection) | Average vessel length of 5 cm was used for these experiments (range 3–10 cm) | Histological assessment of morphology and BrdU staining for proliferation | Dynamic response to stimulus-relaxation and contraction | Yes, arterenol + carbachol | Determining tissue response to external stimuli |
| Cheah, L. (2010) [27] | Heart tissue | Normal | 5 h | Cardiac surgery | Placed in the perfusion chamber within 60 min of surgery | Manual dissection (dissection) | N/A | Viability assays and LDH release | Contractile function | N/A | Response to electrostimulation within cardiac tissue | |
| Piola, M. (2017) [33] | Saphenous vein | Normal | 7 days | Segments obtained following coronary artery bypass grafts | Immediately after surgery | Manual dissection (Dissection) | N/A | Histological assessment for morphology. IHC for proliferation index | Dynamic response to stimuli | N/A | Determining the effects of haemodynamic stimuli on vessel patency | |
| Muraro, M. (2017) [32] | Breast tissue | Diseased (Breast cancer) | 21 days | Surgical resection | Immediately after surgery | Vibratome (McIlwain Tissue Chopper device) | 2 × 2 × 2 mm fragments | Histological assessment of morphology. Proliferation index assessed and immune profiling of cancer. | N/A | Yes, anti-oestrogen therapy | Tumour microenviroment preserved -immune checkpoint blockade therapy trailed | |
| Miscellaneous | Licato, L. (2001) [20] | Melanoma tissue | Diseased | 14 days | Surgical resection | Immediately after surgery | Manual dissection (Scalpel) | 1–2 mm2 | Histological assessment of morphology. IHC and immune profiling of tumour | N/A | N/A | Immune profiling of tumour |
| Ferrarini, P. (2013) [22] | Bone marrow | Diseased (Multiple myeloma) | 24 days | Bone marrow biopsy | N/A | N/A | 2–3 mm3 | Histological assessment of morphology | Analysis of supernatants of tumour secretions | Yes, Bortezomib | Assessment of tumour biology determined by tumour secretions into the media | |
| Bower, R. (2017) [34] | Laryngeal, oropharyngeal or oral cavity tissue | Diseased (Head and neck squamous cell carcinomas tissue) | 48 h | Surgical resection | Within 90 min of excision | Manual dissection (Scalpel) | N/A | Histological assessment of morphology. Viability assay with proliferation and cell death via flow cytometry | N/A | N/A | Determining viability trends ex vivo | |
| Riley, A. (2019) [36] | Thyroid tissue | Diseased (Thyroid cancer) | 24 h | Surgical resection during thyroidectomy | Within 60 min of surgical excision | Vibratome (Leica VT1200S) | 5 mm in diameter | Histological assessment of morphology. Viability assay. IHC for proliferation | Hormone production -thyroxine release | N/A | Determining preservation of endocrine function |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, D.L.; Hughes, A.; Soonawalla, Z.; Mukherjee, S.; O’Neill, E. Dynamic Physiological Culture of Ex Vivo Human Tissue: A Systematic Review. Cancers 2021, 13, 2870. https://doi.org/10.3390/cancers13122870
Hughes DL, Hughes A, Soonawalla Z, Mukherjee S, O’Neill E. Dynamic Physiological Culture of Ex Vivo Human Tissue: A Systematic Review. Cancers. 2021; 13(12):2870. https://doi.org/10.3390/cancers13122870
Chicago/Turabian StyleHughes, Daniel Ll, Aron Hughes, Zahir Soonawalla, Somnath Mukherjee, and Eric O’Neill. 2021. "Dynamic Physiological Culture of Ex Vivo Human Tissue: A Systematic Review" Cancers 13, no. 12: 2870. https://doi.org/10.3390/cancers13122870
APA StyleHughes, D. L., Hughes, A., Soonawalla, Z., Mukherjee, S., & O’Neill, E. (2021). Dynamic Physiological Culture of Ex Vivo Human Tissue: A Systematic Review. Cancers, 13(12), 2870. https://doi.org/10.3390/cancers13122870





