Near Infrared Photoimmunotherapy; A Review of Targets for Cancer Therapy
Abstract
Simple Summary
Abstract
1. Introduction
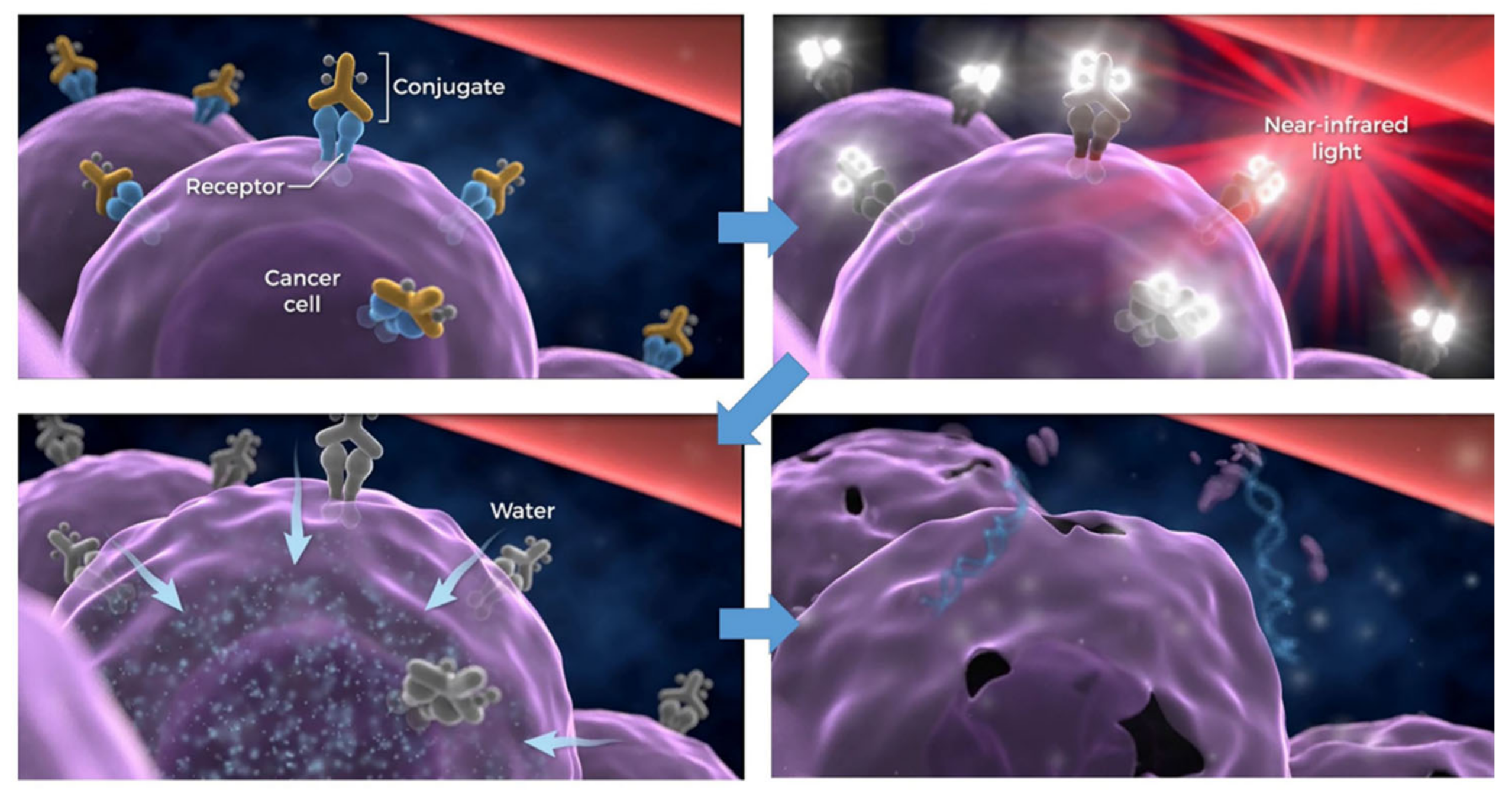
2. NIR-PIT
3. NIR-PIT Targeting Cancer Cells
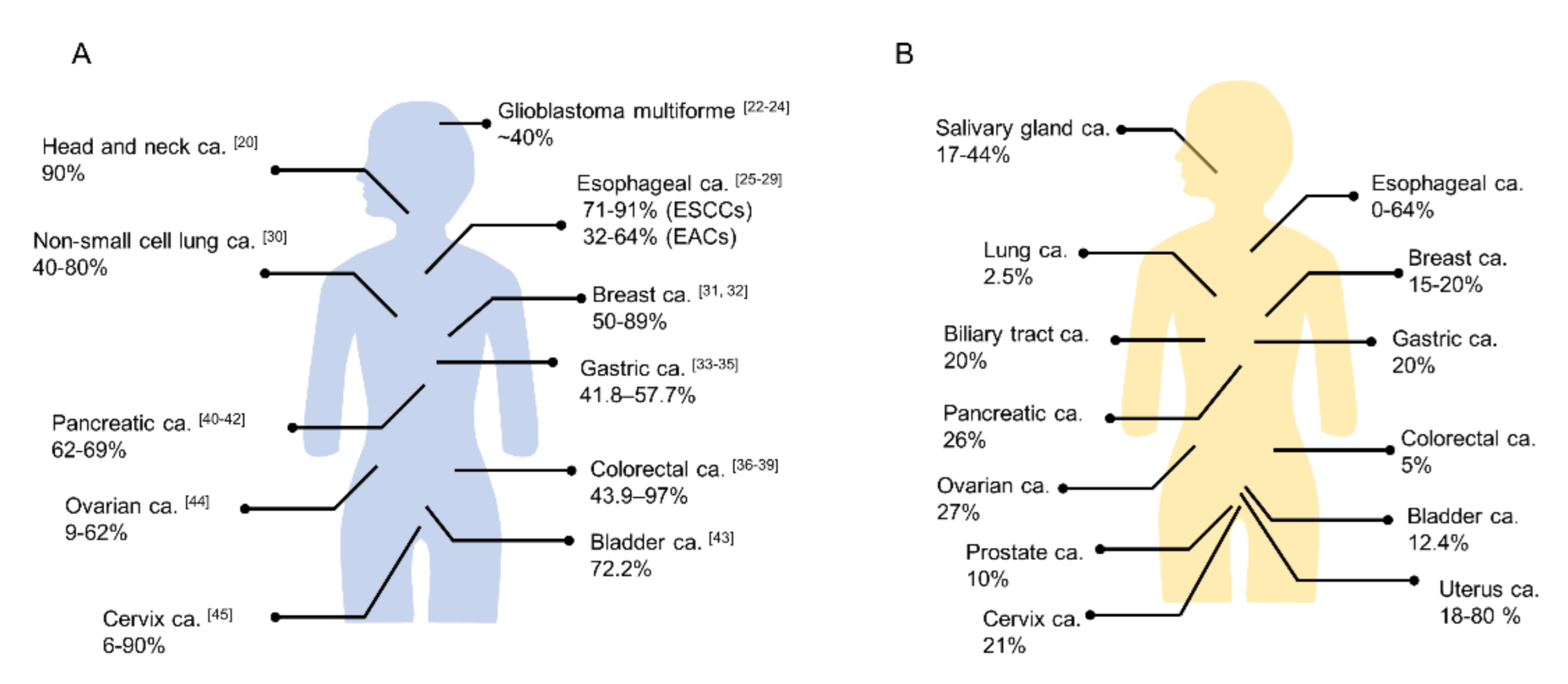
3.1. Epidermal Growth Factor Receptor
3.2. Human Epidermal Growth Factor Receptor 2
3.3. Cancer Stem Cell Markers
3.4. Prostate-Specific Membrane Antigen
3.5. Carcinoembryonic Antigen
3.6. Podoplanin
3.7. Mesothelin
3.8. Glycoprotein A33 Antigen
3.9. Tumor-Associated Calcium Signal Transducer 2
3.10. Cadherin-17
3.11. Delta-Like Protein 3
3.12. Glypican-3
3.13. c-KIT
3.14. CD20
3.15. Cutaneous Lymphocyte Antigen and CD25
3.16. CD146
3.17. H-Type Lectin/β-D-Galactose Receptors
3.18. Programmed Death-Ligand 1
4. NIR-PIT Targeting Non-Cancer Cells
4.1. CD25
4.2. CTLA4
4.3. CD206
4.4. Fibroblast Activation Protein
4.5. CD47
4.6. Vascular Endothelial Growth Factor Receptor
5. NIR-PIT Targeting Forced Expression of New Specific Transmembrane Antigens
6. Combination Therapy of NIR-PIT
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef]
- Sato, K.; Sato, N.; Xu, B.; Nakamura, Y.; Nagaya, T.; Choyke, P.L.; Hasegawa, Y.; Kobayashi, H. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci. Transl. Med. 2016, 8, 352ra110. [Google Scholar] [CrossRef]
- Mew, D.; Wat, C.K.; Towers, G.H.; Levy, J.G. Photoimmunotherapy: Treatment of animal tumors with tumor-specific mono-clonal antibody-hematoporphyrin conjugates. J. Immunol. 1983, 130, 1473–1477. [Google Scholar]
- Lin, C.W.; Amano, T.; Rutledge, A.R.; Shulok, J.R.; Prout, G.R. Photodynamic effect in an experimental bladder tumor treated with intratumor injection of hematoporphyrin derivative. Cancer Res. 1988, 48, 6115–6120. [Google Scholar] [PubMed]
- Sobolev, A.S.; Jans, D.A.; Rosenkranz, A. Targeted intracellular delivery of photosensitizers. Prog. Biophys. Mol. Biol. 2000, 73, 51–90. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Turkbey, B.; Watanabe, R.; Choyke, P.L. Cancer Drug Delivery: Considerations in the Rational Design of Nanosized Bioconjugates. Bioconjugate Chem. 2014, 25, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjugate Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Central Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Choyke, P.L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef]
- Mitsunaga, M.; Nakajima, T.; Sano, K.; Choyke, P.L.; Kobayashi, H. Near-infrared Theranostic Photoimmunotherapy (PIT): Repeated Exposure of Light Enhances the Effect of Immunoconjugate. Bioconjugate Chem. 2012, 23, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Sato, K.; Hanaoka, H.; Watanabe, R.; Harada, T.; Choyke, P.L.; Kobayashi, H. The effects of conjugate and light dose on photo-immunotherapy induced cytotoxicity. BMC Cancer 2014, 14, 389. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; O’Hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal therapy. J. Control. Release 2020, 325, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Tomita, Y.; Nakamura, Y.; Lee, M.-J.; Lee, S.; Tomita, S.; Nagaya, T.; Sato, K.; Yamauchi, T.; Iwai, H.; et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 2017, 8, 10425–10436. [Google Scholar] [CrossRef]
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer: A new approach that kills cancer cells and enhances anti-cancer host immunity. Int. Immunol. 2021, 33, 7–15. [Google Scholar] [CrossRef]
- Green, D.R.; Ferguson, T.; Zitvogel, L.; Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009, 9, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Nagaya, T.; Friedman, J.; Maruoka, Y.; Ogata, F.; Okuyama, S.; Clavijo, P.E.; Choyke, P.L.; Allen, C.; Kobayashi, H. Host Immunity Following Near-Infrared Photoimmunotherapy is Enhanced with PD-1 Checkpoint Blockade to Eradicate Established Antigenic Tumors. Cancer Immunol. Res. 2019, 7, 401–413. [Google Scholar] [CrossRef]
- Roskoski, R. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Xu, M.J.; Johnson, D.E.; Grandis, J.R. EGFR-targeted therapies in the post-genomic era. Cancer Metastasis Rev. 2017, 36, 463–473. [Google Scholar] [CrossRef]
- Nicholson, R.; Gee, J.; Harper, M. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37, 9–15. [Google Scholar] [CrossRef]
- Nagaya, T.; Sato, K.; Harada, T.; Nakamura, Y.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy Targeting EGFR Positive Triple Negative Breast Cancer: Optimizing the Conjugate-Light Regimen. PLoS ONE 2015, 10, e0136829. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ohler, Z.W.; Householder, D.; Nagaya, T.; Sato, K.; Okuyama, S.; Ogata, F.; Daar, D.; Hoa, T.; Choyke, P.L.; et al. Near Infrared Photoimmunotherapy in a Transgenic Mouse Model of Spontaneous Epidermal Growth Factor Receptor (EGFR)-expressing Lung Cancer. Mol. Cancer Ther. 2016, 16, 408–414. [Google Scholar] [CrossRef]
- Nagaya, T.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Knapp, D.W.; Karagiannis, S.N.; Fazekas-Singer, J.; Choyke, P.L.; Leblanc, A.K.; Jensen-Jarolim, E.; et al. Near infrared photoimmunotherapy targeting bladder cancer with a canine anti-epidermal growth factor receptor (EGFR) antibody. Oncotarget 2018, 9, 19026–19038. [Google Scholar] [CrossRef][Green Version]
- Siddiqui, M.R.; Railkar, R.; Sanford, T.; Crooks, D.R.; Eckhaus, M.A.; Haines, D.; Choyke, P.L.; Kobayashi, H.; Agarwal, P.K. Targeting Epidermal Growth Factor Receptor (EGFR) and Human Epidermal Growth Factor Receptor 2 (HER2) Expressing Bladder Cancer Using Combination Photoimmunotherapy (PIT). Sci. Rep. 2019, 9, 2084. [Google Scholar] [CrossRef]
- Burley, T.A.; Mączyńska, J.; Shah, A.; Szopa, W.; Harrington, K.J.; Boult, J.K.; Mrozek-Wilczkiewicz, A.; Vinci, M.; Bamber, J.C.; Kaspera, W.; et al. Near-infrared photoimmunotherapy targeting EGFR-Shedding new light on glioblastoma treatment. Int. J. Cancer 2018, 142, 2363–2374. [Google Scholar] [CrossRef]
- Kalyankrishna, S.; Grandis, J.R. Epidermal Growth Factor Receptor Biology in Head and Neck Cancer. J. Clin. Oncol. 2006, 24, 2666–2672. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study of RM-1929 and Photoimmunotherapy in Patients with Recurrent Head and Neck Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02422979 (accessed on 26 February 2021).
- Libermann, T.A.; Razon, N.; Bartal, A.D.; Yarden, Y.; Schlessinger, J.; Soreq, H. Expression of epidermal growth factor receptors in human brain tumors. Cancer Res. 1984, 44, 753–760. [Google Scholar]
- Libermann, T.A.; Nusbaum, H.R.; Razon, N.; Kris, R.; Lax, I.; Soreq, H.; Whittle, N.; Waterfield, M.D.; Ullrich, A.; Schlessinger, J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nat. Cell Biol. 1985, 313, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Shinojima, N.; Tada, K.; Shiraishi, S.; Kamiryo, T.; Kochi, M.; Nakamura, H.; Makino, K.; Saya, H.; Hirano, H.; Kuratsu, J.-I.; et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003, 63, 6962–6970. [Google Scholar] [PubMed]
- Mukaida, H.; Toi, M.; Hirai, T.; Yamashita, Y.; Toge, T. Clinical significance of the expression of epidermal growth factor and its receptor in esophageal cancer. Cancer 1991, 68, 142–148. [Google Scholar] [CrossRef]
- Itakura, Y.; Sasano, H.; Shiga, C.; Furukawa, Y.; Shiga, K.; Mori, S.; Nagura, H. Epidermal growth factor receptor overexpression in esophageal carcinoma. An immunohistochemical study correlated with clinicopathologic findings and DNA amplification. Cancer 1994, 74, 795–804. [Google Scholar] [CrossRef]
- Wang, K.L.; Wu, T.-T.; Choi, I.S.; Wang, H.; Resetkova, E.; Correa, A.M.; Hofstetter, W.L.; Swisher, S.G.; Ajani, J.A.; Rashid, A.; et al. Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas. Cancer 2007, 109, 658–667. [Google Scholar] [CrossRef]
- Navarini, D.; Gurski, R.R.; Madalosso, C.A.; Aita, L.; Meurer, L.; Fornari, F. Epidermal Growth Factor Receptor Expression in Esophageal Adenocarcinoma: Relationship with Tumor Stage and Survival after Esophagectomy. Gastroenterol. Res. Pract. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Yacoub, L.; Goldman, H.; Odze, R.D. Transforming growth factor-alpha, epidermal growth factor receptor, and MiB-1 expression in Barrett’s-associated neoplasia: Correlation with prognosis. Mod. Pathol. 1997, 10, 105–112. [Google Scholar]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. 2004, 59, 21–26. [Google Scholar] [CrossRef]
- Burness, M.L.; Grushko, T.A.; Olopade, O.I. Epidermal Growth Factor Receptor in Triple-Negative and Basal-Like Breast Cancer: Promising Clinical Target or Only a Marker? Cancer J. 2010, 16, 23–32. [Google Scholar] [CrossRef]
- Changavi, A.A.; Shashikala, A.; Ramji, A.S. Epidermal Growth Factor Receptor Expression in Triple Negative and Nontriple Negative Breast Carcinomas. J. Lab. Phys. 2015, 7, 079–083. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Mei, Y.; Pan, X.; Liu, M.; Wu, S. Expression of HER2/c-erbB-2, EGFR protein in gastric carcinoma and its clinical significance. Open Life Sci. 2019, 14, 119–125. [Google Scholar] [CrossRef]
- Gao, M.; Liang, X.-J.; Zhang, Z.-S.; Ma, W.; Chang, Z.-W.; Zhang, M.-Z. Relationship between expression of EGFR in gastric cancer tissue and clinicopathological features. Asian Pac. J. Trop. Med. 2013, 6, 260–264. [Google Scholar] [CrossRef]
- Galizia, G.; Lieto, E.; Orditura, M.; Castellano, P.; La Mura, A.; Imperatore, V.; Pinto, M.; Zamboli, A.; De Vita, F.; Ferraraccio, F. Epidermal Growth Factor Receptor (EGFR) Expression is Associated with a Worse Prognosis in Gastric Cancer Patients Undergoing Curative Surgery. World J. Surg. 2007, 31, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Chen, Y.-T.; Tsai, H.-L.; Yeh, Y.-S.; Su, W.-C.; Ma, C.-J.; Tsai, T.-N.; Wang, J.-Y. EGFR expression in patients with stage III colorectal cancer after adjuvant chemotherapy and on cancer cell function. Oncotarget 2017, 8, 114663–114676. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Q.; Xu, J.; Wang, J.; Zhang, Y. Detection of EGFR expression in patients with colorectal cancer and the therapeutic effect of cetuximab. J. B.U.ON. Off. J. Balk. Union Oncol. 2016, 21, 95–100. [Google Scholar]
- Theodoropoulos, G.E.; Karafoka, E.; Papailiou, J.G.; Stamopoulos, P.; Zambirinis, C.P.; Bramis, K.; Panoussopoulos, S.-G.; Leandros, E.; Bramis, J. P53 and EGFR expression in colorectal cancer: A reappraisal of ‘old’ tissue markers in patients with long follow-up. Anticancer. Res. 2009, 29, 785–791. [Google Scholar] [PubMed]
- Spano, J.-P.; Lagorce, C.; Atlan, D.; Milano, G.; Domont, J.; Benamouzig, R.; Attar, A.; Benichou, J.; Martin, A.; Morere, J.-F.; et al. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann. Oncol. 2005, 16, 102–108. [Google Scholar] [CrossRef]
- Park, S.J.; Gu, M.J.; Lee, D.S.; Yun, S.S.; Kim, H.J.; Choi, J.H. EGFR expression in pancreatic intraepithelial neoplasia and ductal adenocarcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 8298–8304. [Google Scholar] [PubMed]
- Handra-Luca, A.; Hammel, P.; Sauvanet, A.; Lesty, C.; Ruszniewski, P.; Couvelard, A. EGFR expression in pancreatic adenocarcinoma. Relationship to tumour morphology and cell adhesion proteins. J. Clin. Pathol. 2014, 67, 295–300. [Google Scholar] [CrossRef]
- Bloomston, M.; Bhardwaj, A.; Ellison, E.C.; Frankel, W.L. Epidermal Growth Factor Receptor Expression in Pancreatic Carcinoma Using Tissue Microarray Technique. Dig. Surg. 2006, 23, 74–79. [Google Scholar] [CrossRef]
- Chow, N.H.; Chan, S.H.; Tzai, T.S.; Ho, C.L.; Liu, H.S. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin. Cancer Res. 2001, 7, 1957–1962. [Google Scholar] [PubMed]
- Sheng, Q.; Liu, J. The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. Br. J. Cancer 2011, 104, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Soonthornthum, T.; Arias-Pulido, H.; Joste, N.; Lomo, L.; Muller, C.; Rutledge, T.; Verschraegen, C. Epidermal growth factor receptor as a biomarker for cervical cancer. Ann. Oncol. 2011, 22, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- King, C.R.; Kraus, M.H.; Aaronson, S.A. Amplification of a novel verbB-related gene in a human mammary carcinoma. Science 1985, 229, 974–976. [Google Scholar] [CrossRef]
- Arkhipov, A.; Shan, Y.; Kim, E.T.; Dror, R.O.; Shaw, D.E. Her2 activation mechanism reflects evolutionary preservation of asymmetric ectodomain dimers in the human EGFR family. eLife 2013, 2, e00708. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Johnson, A.M.; Dumbrava, E.E.I.; Raghav, K.; Balaji, K.; Bhatt, M.; Murthy, R.K.; Rodon, J.; Piha-Paul, S.A. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin. Cancer Res. 2019, 25, 2033–2041. [Google Scholar] [CrossRef]
- Krishnamurti, U.; Silverman, J.F. HER2 in Breast Cancer. Adv. Anat. Pathol. 2014, 21, 100–107. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Bang, Y.-J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Gravalos, C.; Jimeno, A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann. Oncol. 2008, 19, 1523–1529. [Google Scholar] [CrossRef]
- Zhan, N.; Dong, W.-G.; Tang, Y.-F.; Wang, Z.-S.; Xiong, C.-L. Analysis of HER2 gene amplification and protein expression in esophageal squamous cell carcinoma. Med. Oncol. 2012, 29, 933–940. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Bang, Y.-J.; Feng-Yi, F.; Xu, J.M.; Lee, K.-W.; Jiao, S.-C.; Chong, J.L.; López-Sanchez, R.I.; Price, T.; Gladkov, O.; et al. HER2 screening data from ToGA: Targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015, 18, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Hartmans, E.; Linssen, M.D.; Sikkens, C.; Levens, A.; Witjes, M.J.; Van Dam, G.M.; Nagengast, W.B. Tyrosine kinase inhibitor induced growth factor receptor upregulation enhances the efficacy of near-infrared targeted photodynamic therapy in esophageal adenocarcinoma cell lines. Oncotarget 2017, 8, 29846–29856. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy in the Treatment of Pleural Disseminated NSCLC: Preclinical Experience. Theranostics 2015, 5, 698–709. [Google Scholar] [CrossRef]
- Sato, K.; Hanaoka, H.; Watanabe, R.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy in the Treatment of Disseminated Peritoneal Ovarian Cancer. Mol. Cancer Ther. 2015, 14, 141–150. [Google Scholar] [CrossRef]
- Ito, K.; Mitsunaga, M.; Arihiro, S.; Saruta, M.; Matsuoka, M.; Kobayashi, H.; Tajiri, H. Molecular targeted photoimmunotherapy for HER2-positive human gastric cancer in combination with chemotherapy results in improved treatment outcomes through different cytotoxic mechanisms. BMC Cancer 2016, 16, 37. [Google Scholar] [CrossRef]
- Ito, K.; Mitsunaga, M.; Nishimura, T.; Kobayashi, H.; Tajiri, H. Combination photoimmunotherapy with monoclonal antibodies recognizing different epitopes of human epidermal growth factor receptor 2: An assessment of phototherapeutic effect based on fluorescence molecular imaging. Oncotarget 2016, 7, 14143–14152. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Lu, J.; Xiong, H.; Shi, X.; Gong, L. Significance of CD44 expression in head and neck cancer: A systemic review and meta-analysis. BMC Cancer 2014, 14, 15. [Google Scholar] [CrossRef]
- Nagaya, T.; Nakamura, Y.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Allen, C.; Kobayashi, H. Syngeneic Mouse Models of Oral Cancer are Effectively Targeted by Anti-CD44-Based NIR-PIT. Mol. Cancer Res. 2017, 15, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Wakiyama, H.; Furusawa, A.; Okada, R.; Inagaki, F.; Kato, T.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Increased Immunogenicity of a Minimally Immunogenic Tumor after Cancer-Targeting Near Infrared Photoimmunotherapy. Cancers 2020, 12, 3747. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, Y.; Furusawa, A.; Okada, R.; Inagaki, F.; Fujimura, D.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Near-Infrared Photoimmunotherapy Combined with CTLA4 Checkpoint Blockade in Syngeneic Mouse Cancer Models. Vaccines 2020, 8, 528. [Google Scholar] [CrossRef]
- Kemper, K.; Sprick, M.R.; De Bree, M.; Scopelliti, A.; Vermeulen, L.; Hoek, M.; Zeilstra, J.; Pals, S.T.; Mehmet, H.; Stassi, G.; et al. The AC133 Epitope, but not the CD133 Protein, Is Lost upon Cancer Stem Cell Differentiation. Cancer Res. 2010, 70, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Weidensteiner, C.; Reichardt, W.; Gaedicke, S.; Zhu, X.; Grosu, A.-L.; Kobayashi, H.; Niedermann, G. Imaging and Selective Elimination of Glioblastoma Stem Cells with Theranostic Near-Infrared-Labeled CD133-Specific Antibodies. Theranostics 2016, 6, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Near-Infrared Photoimmunotherapy Targeting Prostate Cancer with Prostate-Specific Membrane Antigen (PSMA) Antibody. Mol. Cancer Res. 2017, 15, 1153–1162. [Google Scholar] [CrossRef]
- Haberkorn, U.; Eder, M.; Kopka, K.; Babich, J.W.; Eisenhut, M. New Strategies in Prostate Cancer: Prostate-Specific Membrane Antigen (PSMA) Ligands for Diagnosis and Therapy. Clin. Cancer Res. 2016, 22, 9–15. [Google Scholar] [CrossRef]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef]
- Grunnet, M.; Sorensen, J. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012, 76, 138–143. [Google Scholar] [CrossRef]
- Campos-Da-Paz, M.; Dórea, J.G.; Galdino, A.S.; Lacava, Z.G.M.; Santos, M.D.F.M.A. Carcinoembryonic Antigen (CEA) and Hepatic Metastasis in Colorectal Cancer: Update on Biomarker for Clinical and Biotechnological Approaches. Recent Pat. Biotechnol. 2018, 12, 269–279. [Google Scholar] [CrossRef]
- Tiernan, J.P.; Perry, S.L.; Verghese, E.T.; West, N.P.; Yeluri, S.; Jayne, D.G.; Hughes, T.A. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br. J. Cancer 2013, 108, 662–667. [Google Scholar] [CrossRef]
- Maawy, A.A.; Hiroshima, Y.; Zhang, Y.; Heim, R.; Makings, L.; Garcia-Guzman, M.; Luiken, G.A.; Kobayashi, H.; Hoffman, R.M.; Bouvet, M. Near Infra-Red Photoimmunotherapy with Anti-CEA-IR700 Results in Extensive Tumor Lysis and a Significant Decrease in Tumor Burden in Orthotopic Mouse Models of Pancreatic Cancer. PLoS ONE 2015, 10, e0121989. [Google Scholar] [CrossRef] [PubMed]
- Shirasu, N.; Yamada, H.; Shibaguchi, H.; Kuroki, M.; Kuroki, M. Potent and specific antitumor effect of CEA-targeted photoimmunotherapy. Int. J. Cancer 2014, 135, 2697–2710. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Maawy, A.; Zhang, Y.; Guzman, M.G.; Heim, R.; Makings, L.; Luiken, G.A.; Kobayashi, H.; Tanaka, K.; Endo, I.; et al. Photoimmunotherapy Inhibits Tumor Recurrence After Surgical Resection on a Pancreatic Cancer Patient-Derived Orthotopic Xenograft (PDOX) Nude Mouse Model. Ann. Surg. Oncol. 2015, 22, 1469–1474. [Google Scholar] [CrossRef]
- Maawy, A.A.; Hiroshima, Y.; Zhang, Y.; Garcia-Guzman, M.; Luiken, G.A.; Kobayashi, H.; Hoffman, R.M.; Bouvet, M. Photoimmunotherapy lowers recurrence after pancreatic cancer surgery in orthotopic nude mouse models. J. Surg. Res. 2015, 197, 5–11. [Google Scholar] [CrossRef]
- Nishinaga, Y.; Sato, K.; Yasui, H.; Taki, S.; Takahashi, K.; Shimizu, M.; Endo, R.; Koike, C.; Kuramoto, N.; Nakamura, S.; et al. Targeted Phototherapy for Malignant Pleural Mesothelioma: Near-Infrared Photoimmunotherapy Targeting Podoplanin. Cells 2020, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Bibby, A.C.; Tsim, S.; Kanellakis, N.; Ball, H.; Talbot, D.C.; Blyth, K.G.; Maskell, N.A.; Psallidas, I. Malignant pleural mesothelioma: An update on investigation, diagnosis and treatment. Eur. Respir. Rev. 2016, 25, 472–486. [Google Scholar] [CrossRef]
- Schacht, V.; Ramirez, M.I.; Hong, Y.; Hirakawa, S.; Feng, D.; Harvey, N.; Williams, M.; Dvorak, A.M.; Dvorak, H.F.; Oliver, G.; et al. T1 /podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003, 22, 3546–3556. [Google Scholar] [CrossRef]
- Quintanilla, M.; Montero-Montero, L.; Renart, J.; Martín-Villar, E. Podoplanin in Inflammation and Cancer. Int. J. Mol. Sci. 2019, 20, 707. [Google Scholar] [CrossRef]
- Chuang, W.-Y.; Chang, Y.-S.; Yeh, C.-J.; Wu, Y.-C.; Hsueh, C. Role of podoplanin expression in squamous cell carcinoma of upper aerodigestive tract. Histol. Histopathol. 2013, 28, 293–299. [Google Scholar]
- Wicki, A.; Christofori, G. The potential role of podoplanin in tumour invasion. Br. J. Cancer 2006, 96, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Lehembre, F.; Wick, N.; Hantusch, B.; Kerjaschki, D.; Christofori, G. Tumor invasion in the absence of epithelial-mesenchymal transition: Podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell 2006, 9, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, N.G. Application of Mesothelin Immunostaining in Tumor Diagnosis. Am. J. Surg. Pathol. 2003, 27, 1418–1428. [Google Scholar] [CrossRef]
- Hassan, R.; Ho, M. Mesothelin targeted cancer immunotherapy. Eur. J. Cancer 2008, 44, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.; Sato, K.; Zhang, Y.-F.; Ni, M.; Choyke, P.L.; Ho, M.; Kobayashi, H. Near infrared photoimmunotherapy with an anti-mesothelin antibody. Oncotarget 2016, 7, 23361–23369. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, J.; Kojima, H.; Kato, J.; Hamashima, H.; Suzuki, H. Organ-specific expression of the intestinal epithelium-related antigen A33, a cell surface target for antibody-based imaging and treatment in gastrointestinal cancer. Cancer Chemother. Pharmacol. 2000, 46, S27–S32. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.K.; White, S.J.; Johnstone, C.N.; Catimel, B.; Simpson, R.J.; Moritz, R.L.; Tu, G.-F.; Ji, H.; Whitehead, R.H.; Groenen, L.C.; et al. The human A33 antigen is a transmembrane glycoprotein and a novel member of the immunoglobulin superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 469–474. [Google Scholar] [CrossRef]
- Wei, D.; Tao, Z.; Shi, Q.; Wang, L.; Liu, L.; She, T.; Yi, Q.; Wen, X.; Liu, L.; Li, S.; et al. Selective Photokilling of Colorectal Tumors by Near-Infrared Photoimmunotherapy with a GPA33-Targeted Single-Chain Antibody Variable Fragment Conjugate. Mol. Pharm. 2020, 17, 2508–2517. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496–22512. [Google Scholar] [CrossRef]
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer 2014, 6, 84–105. [Google Scholar] [CrossRef]
- Nishimura, T.; Mitsunaga, M.; Sawada, R.; Saruta, M.; Kobayashi, H.; Matsumoto, N.; Kanke, T.; Yanai, H.; Nakamura, K. Photoimmunotherapy targeting biliary-pancreatic cancer with humanized anti-TROP2 antibody. Cancer Med. 2019, 8, 7781–7792. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, R.A.; Barderas, R.; Torres, S.; Fernandez-Aceñero, M.J.; Mendes, M.; García-Foncillas, J.; Lopez-Lucendo, M.; Casal, J.I. Cadherin-17 interacts with α2β1 integrin to regulate cell proliferation and adhesion in colorectal cancer cells causing liver metastasis. Oncogene 2013, 33, 1658–1669. [Google Scholar] [CrossRef]
- Panarelli, N.C.; Yantiss, R.K.; Yeh, M.M.; Liu, Y.; Chen, Y.-T. Tissue-Specific Cadherin CDH17 Is a Useful Marker of Gastrointestinal Adenocarcinomas with Higher Sensitivity Than CDX2. Am. J. Clin. Pathol. 2012, 138, 211–222. [Google Scholar] [CrossRef]
- Lum, Y.-L.; Luk, J.M.; Staunton, D.E.; Ng, D.K.P.; Fong, W.-P. Cadherin-17 Targeted Near-Infrared Photoimmunotherapy for Treatment of Gastrointestinal Cancer. Mol. Pharm. 2020, 17, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.H.; Giffin, M.J.; Bailis, J.M.; Smit, M.-A.D.; Carbone, D.P.; He, K. DLL3: An emerging target in small cell lung cancer. J. Hematol. Oncol. 2019, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.R.; Bankovich, A.J.; Anderson, W.C.; Aujay, M.A.; Bheddah, S.; Black, K.; Desai, R.; Escarpe, P.A.; Hampl, J.; Laysang, A.; et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med. 2015, 7, 302ra136. [Google Scholar] [CrossRef] [PubMed]
- Isobe, Y.; Sato, K.; Nishinaga, Y.; Takahashi, K.; Taki, S.; Yasui, H.; Shimizu, M.; Endo, R.; Koike, C.; Kuramoto, N.; et al. Near infrared photoimmunotherapy targeting DLL3 for small cell lung cancer. EBioMedicine 2020, 52, 102632. [Google Scholar] [CrossRef]
- Rudin, C.M.; Pietanza, M.C.; Bauer, T.M.; Ready, N.; Morgensztern, D.; Glisson, B.S.; Byers, L.A.; Johnson, M.L.; Burris, H.A.; Robert, F.; et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: A first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017, 18, 42–51. [Google Scholar] [CrossRef]
- Baumhoer, D.; Tornillo, L.; Stadlmann, S.; Roncalli, M.; Diamantis, E.K.; Terracciano, L.M. Glypican 3 Expression in Human Nonneoplastic, Preneoplastic, and Neoplastic Tissues. Am. J. Clin. Pathol. 2008, 129, 899–906. [Google Scholar] [CrossRef]
- Silsirivanit, A. Glycosylation markers in cancer. Adv. Clin. Chem. 2019, 89, 189–213. [Google Scholar] [CrossRef]
- Hanaoka, H.; Nagaya, T.; Sato, K.; Nakamura, Y.; Watanabe, R.; Harada, T.; Gao, W.; Feng, M.; Phung, Y.; Kim, I.; et al. Glypican-3 Targeted Human Heavy Chain Antibody as a Drug Carrier for Hepatocellular Carcinoma Therapy. Mol. Pharm. 2015, 12, 2151–2157. [Google Scholar] [CrossRef]
- Hanaoka, H.; Nakajima, T.; Sato, K.; Watanabe, R.; Phung, Y.; Gao, W.; Harada, T.; Kim, I.; Paik, C.H.; Choyke, P.L.; et al. Photoimmunotherapy of hepatocellular carcinoma-targeting Glypican-3 combined with nanosized albumin-bound paclitaxel. Nanomedicine 2015, 10, 1139–1147. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kimmey, M.B. The incidental upper gastrointestinal subepithelial mass. Gastroenterology 2004, 126, 301–307. [Google Scholar] [CrossRef]
- Fujimoto, S.; Muguruma, N.; Okamoto, K.; Kurihara, T.; Sato, Y.; Miyamoto, Y.; Kitamura, S.; Miyamoto, H.; Taguchi, T.; Tsuneyama, K.; et al. A Novel Theranostic Combination of Near-infrared Fluorescence Imaging and Laser Irradiation Targeting c-KIT for Gastrointestinal Stromal Tumors. Theranostics 2018, 8, 2313–2328. [Google Scholar] [CrossRef]
- Nishida, T.; Blay, J.-Y.; Hirota, S.; Kitagawa, Y.; Kang, Y.-K. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016, 19, 3–14. [Google Scholar] [CrossRef]
- Sabattini, E.; Bacci, F.; Sagramoso, C.; Pileri, S.A. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: An overview. Pathologica 2010, 102, 83–87. [Google Scholar] [PubMed]
- Chung, C. Current targeted therapies in lymphomas. Am. J. Health Pharm. 2019, 76, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.; Sato, K.; Harada, T.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy of B-cell lymphoma. Mol. Oncol. 2016, 10, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Heryanto, Y.-D.; Hanaoka, H.; Nakajima, T.; Yamaguchi, A.; Tsushima, Y. Applying near-infrared photoimmunotherapy to B-cell lymphoma: Comparative evaluation with radioimmunotherapy in tumor xenografts. Ann. Nucl. Med. 2017, 31, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Alhothali, G.I. Review of the Treatment of Mycosis Fungoides and Sézary Syndrome: A Stage-Based Approach. Int. J. Health Sci. 2013, 7, 220–239. [Google Scholar] [CrossRef]
- Magro, C.M.; Dyrsen, M.E. Cutaneous lymphocyte antigen expression in benign and neoplastic cutaneous B- and T-cell lymphoid infiltrates. J. Cutan. Pathol. 2008, 35, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Silic-Benussi, M.; Saponeri, A.; Michelotto, A.; Russo, I.; Colombo, A.; Pelizzo, M.G.; Ciminale, V.; Alaibac, M. Near infrared photoimmunotherapy targeting the cutaneous lymphocyte antigen for mycosis fungoides. Expert Opin. Biol. Ther. 2020, 1–5. [Google Scholar] [CrossRef]
- Berkowitz, J.L.; Janik, J.E.; Stewart, D.M.; Jaffe, E.S.; Stetler-Stevenson, M.; Shih, J.H.; Fleisher, T.A.; Turner, M.; Urquhart, N.E.; Wharfe, G.H.; et al. Safety, efficacy, and pharmacokinetics/pharmacodynamics of daclizumab (anti-CD25) in patients with adult T-cell leukemia/lymphoma. Clin. Immunol. 2014, 155, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Dye, D.E.; Emedic, S.; Eziman, M.; Coombe, D.R. Melanoma Biomolecules: Independently Identified but Functionally Intertwined. Front. Oncol. 2013, 3, 252. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jiang, D.; Ehlerding, E.B.; Barnhart, T.E.; Yang, Y.; Engle, J.W.; Luo, Q.-Y.; Huang, P.; Cai, W. CD146-Targeted Multimodal Image-Guided Photoimmunotherapy of Melanoma. Adv. Sci. 2019, 6, 1801237. [Google Scholar] [CrossRef]
- Hama, Y.; Urano, Y.; Koyama, Y.; Kamiya, M.; Bernardo, M.; Paik, R.S.; Krishna, M.C.; Choyke, P.L.; Kobayashi, H. In Vivo Spectral Fluorescence Imaging of Submillimeter Peritoneal Cancer Implants Using a Lectin-Targeted Optical Agent. Neoplasia 2006, 8, 607–612. [Google Scholar] [CrossRef]
- Harada, T.; Nakamura, Y.; Sato, K.; Nagaya, T.; Okuyama, S.; Ogata, F.; Choyke, P.L.; Kobayashi, H. Near-infrared photoimmunotherapy with galactosyl serum albumin in a model of diffuse peritoneal disseminated ovarian cancer. Oncotarget 2016, 7, 79408–79416. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nat. Cell Biol. 2005, 439, 682–687. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.; Sato, K.; Harada, T.; Choyke, P.L.; Hodge, J.W.; Schlom, J.; Kobayashi, H. Near infrared photoimmunotherapy with avelumab, an anti-programmed death-ligand 1 (PD-L1) antibody. Oncotarget 2017, 8, 8807–8817. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Fesnak, A.; June, C.H.; Levine, A.D.F.C.H.J.B.L. Engineered T cells: The promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Alshawa, A.; Suarez-Almazor, M.E. Adverse Events in Cancer Immunotherapy. Adv. Exp. Med. Biol. 2017, 995, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells ex-pressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar]
- Colombo, M.P.; Piconese, S. Regulatory T-cell inhibition versus depletion: The right choice in cancer immunotherapy. Nat. Rev. Cancer 2007, 7, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef]
- Facciabene, A.; Motz, G.T.; Coukos, G. T-Regulatory Cells: Key Players in Tumor Immune Escape and Angiogenesis. Cancer Res. 2012, 72, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Nishikawa, H. Roles of regulatory T cells in cancer immunity. Int. Immunol. 2016, 28, 401–409. [Google Scholar] [CrossRef]
- Spolski, R.; Li, P.; Leonard, W.J. Biology and regulation of IL-2: From molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018, 18, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Maruoka, Y.; Furusawa, A.; Inagaki, F.; Nagaya, T.; Fujimura, D.; Choyke, P.L.; Kobayashi, H. The Effect of Antibody Fragments on CD25 Targeted Regulatory T Cell Near-Infrared Photoimmunotherapy. Bioconjugate Chem. 2019, 30, 2624–2633. [Google Scholar] [CrossRef]
- Yu, G.-T.; Bu, L.-L.; Zhao, Y.-Y.; Mao, L.; Deng, W.-W.; Wu, T.-F.; Zhang, W.-F.; Sun, Z.-J. CTLA4 blockade reduces immature myeloid cells in head and neck squamous cell carcinoma. OncoImmunology 2016, 5, e1151594. [Google Scholar] [CrossRef]
- Wang, X.B.; Fan, Z.Z.; Anton, D.; Vollenhoven, A.V.; Ni, Z.H.; Chen, X.F.; Lefvert, A.K. CTLA4 is expressed on mature dendritic cells derived from human monocytes and influences their maturation and antigen presentation. BMC Immunol. 2011, 12, 21–28. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Kato, T.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Choyke, P.L.; Kobayashi, H. Local Depletion of Immune Checkpoint Ligand CTLA4 Expressing Cells in Tumor Beds Enhances Antitumor Host Immunity. Adv. Ther. 2021, 4, 2000269. [Google Scholar] [CrossRef]
- Dubbs, S.B. The Latest Cancer Agents and Their Complications. Emerg. Med. Clin. North Am. 2018, 36, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, L.; Cai, Y.; Liu, H.; Gao, D.; Lai, J.; Jia, B.; Wang, F.; Liu, Z. Inhibition of tumor growth and metastasis by photoimmunotherapy targeting tumor-associated macrophage in a sorafenib-resistant tumor model. Biomaterials 2016, 84, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Noma, K.; Smalley, K.S.; Lioni, M.; Naomoto, Y.; Tanaka, N.; El–Deiry, W.; King, A.J.; Nakagawa, H.; Herlyn, M. The Essential Role of Fibroblasts in Esophageal Squamous Cell Carcinoma-Induced Angiogenesis. Gastroenterology 2008, 134, 1981–1993. [Google Scholar] [CrossRef]
- Kashima, H.; Noma, K.; Ohara, T.; Kato, T.; Katsura, Y.; Komoto, S.; Sato, H.; Katsube, R.; Ninomiya, T.; Tazawa, H.; et al. Cancer-associated fibroblasts (CAFs) promote the lymph node metastasis of esophageal squamous cell carcinoma. Int. J. Cancer 2019, 144, 828–840. [Google Scholar] [CrossRef]
- Katsube, R.; Noma, K.; Ohara, T.; Nishiwaki, N.; Kobayashi, T.; Komoto, S.; Sato, H.; Kashima, H.; Kato, T.; Kikuchi, S.; et al. Fibroblast activation protein targeted near infrared photoimmunotherapy (NIR PIT) overcomes therapeutic resistance in human esophageal cancer. Sci. Rep. 2021, 11, 1693. [Google Scholar] [CrossRef]
- Kato, T.; Noma, K.; Ohara, T.; Kashima, H.; Katsura, Y.; Sato, H.; Komoto, S.; Katsube, R.; Ninomiya, T.; Tazawa, H.; et al. Cancer-Associated Fibroblasts Affect Intratumoral CD8+ and FoxP3+ T Cells Via IL6 in the Tumor Microenvironment. Clin. Cancer Res. 2018, 24, 4820–4833. [Google Scholar] [CrossRef]
- Roberts, E.W.; Deonarine, A.; Jones, J.O.; Denton, A.E.; Feig, C.; Lyons, S.; Espeli, M.; Kraman, M.; McKenna, B.; Wells, R.J.; et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 2013, 210, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Noma, K.; Ohara, T.; Kashima, H.; Sato, H.; Kato, T.; Urano, S.; Katsube, R.; Hashimoto, Y.; Tazawa, H.; et al. Photoimmunotherapy for cancer-associated fibroblasts targeting fibroblast activation protein in human esophageal squamous cell carcinoma. Cancer Biol. Ther. 2019, 20, 1234–1248. [Google Scholar] [CrossRef]
- Chao, M.P.; Jaiswal, S.; Weissman-Tsukamoto, R.; Alizadeh, A.A.; Gentles, A.J.; Volkmer, J.; Weiskopf, K.; Willingham, S.B.; Raveh, T.; Park, C.Y.; et al. Calreticulin Is the Dominant Pro-Phagocytic Signal on Multiple Human Cancers and Is Counterbalanced by CD47. Sci. Transl. Med. 2010, 2, 63ra94. [Google Scholar] [CrossRef]
- Kiss, B.; Berg, N.S.V.D.; Ertsey, R.; McKenna, K.; Mach, K.E.; Zhang, C.A.; Volkmer, J.-P.; Weissman, I.L.; Rosenthal, E.L.; Liao, J.C. CD47-Targeted Near-Infrared Photoimmunotherapy for Human Bladder Cancer. Clin. Cancer Res. 2019, 25, 3561–3571. [Google Scholar] [CrossRef]
- Aguayo, A.; Kantarjian, H.; Manshouri, T.; Gidel, C.; Estey, E.; Thomas, D.; Koller, C.; Estrov, Z.; O’Brien, S.; Keating, M.; et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood 2000, 96, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nat. Cell Biol. 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-esophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.-C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.-Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Krupitskaya, Y.; Wakelee, H.A. Ramucirumab, a fully human mAb to the transmembrane signaling tyrosine kinase VEGFR-2 for the potential treatment of cancer. Curr. Opin. Investig. Drugs 2009, 10, 597–605. [Google Scholar] [PubMed]
- Nishimura, T.; Mitsunaga, M.; Ito, K.; Kobayashi, H.; Saruta, M. Cancer neovasculature-targeted near-infrared photoimmunotherapy (NIR-PIT) for gastric cancer: Different mechanisms of phototoxicity compared to cell membrane-targeted NIR-PIT. Gastric Cancer 2019, 23, 82–94. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Ishida, M.; Sekine, S.; Taniguchi, H.; Fukagawa, T.; Katai, H.; Kushima, R. Consistent absence of HER2 expression, regardless ofHER2amplification status, in neuroendocrine carcinomas of the stomach. Histopathology 2014, 64, 1027–1031. [Google Scholar] [CrossRef]
- Yoshida, R.; Tazawa, H.; Hashimoto, Y.; Yano, S.; Onishi, T.; Sasaki, T.; Shirakawa, Y.; Kishimoto, H.; Uno, F.; Nishizaki, M.; et al. Mechanism of resistance to trastuzumab and molecular sensitization via ADCC activation by exogenous expression of HER2-extracellular domain in human cancer cells. Cancer Immunol. Immunother. 2012, 61, 1905–1916. [Google Scholar] [CrossRef]
- Shimoyama, K.; Kagawa, S.; Ishida, M.; Watanabe, S.; Noma, K.; Takehara, K.; Tazawa, H.; Hashimoto, Y.; Tanabe, S.; Matsuoka, J.; et al. Viral transduction of the HER2-extracellular domain expands trastuzumab-based photoimmunotherapy for HER2-negative breast cancer cells. Breast Cancer Res. Treat. 2015, 149, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Kagawa, S.; Shimoyama, K.; Takehara, K.; Noma, K.; Tanabe, S.; Shirakawa, Y.; Tazawa, H.; Kobayashi, H.; Fujiwara, T. Trastuzumab-Based Photoimmunotherapy Integrated with Viral HER2 Transduction Inhibits Peritoneally Disseminated HER2-Negative Cancer. Mol. Cancer Ther. 2016, 15, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, Y.; Furusawa, A.; Okada, R.; Inagaki, F.; Fujimura, D.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Combined CD44- and CD25-Targeted Near-Infrared Photoimmunotherapy Selectively Kills Cancer and Regulatory T Cells in Syngeneic Mouse Cancer Models. Cancer Immunol. Res. 2020, 8, 345–355. [Google Scholar] [CrossRef] [PubMed]

| Malignant Neoplasma | Target Moleclue | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EGFR | HER2 | CD44 | CEA | PDPN | MSLN | GPA33 | TROP2 | CDH-17 | PD-L1 | Cancer Specific Target | |
| Glioblastoma multiforme | + | + | + | ||||||||
| Head and neck Ca. | ++ | + | ++ | + | ++ | ||||||
| Lung Ca. | ++ | ± | + | + | + | + | ++ | DLL3 (SCLC) | |||
| MPM | + | + | |||||||||
| Breast Ca. | ++ | + | + | + | + | ||||||
| Gastrointestinal Ca. | |||||||||||
| Esophageal Ca. | ++ | + | ± | + | + | ||||||
| Gastric Ca. | + | + | ++ | + | + | + | + | + | |||
| Colorectal Ca. | + | ± | + | ++ | + | + | + | + | |||
| Hepatic cell Ca. | + | + | GPC-3 | ||||||||
| cholangiocarcinoma | + | + | + | + | |||||||
| Pancreatic Ca. | + | + | + | + | + | + | + | + | + | ||
| GIST | c-KIT | ||||||||||
| Bladder Ca. | ++ | + | + | + | |||||||
| Prostate Ca. | ± | + | + | PSMA | |||||||
| Cervical Ca. | ++ | + | + | ± | + | + | |||||
| Ovarian Ca. | + | + | + | + | + | + | + | ||||
| Malignant melanoma | ++ | CD146 | |||||||||
| Lymphoma | ++ | CD20, CD25, CLA | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, T.; Wakiyama, H.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy; A Review of Targets for Cancer Therapy. Cancers 2021, 13, 2535. https://doi.org/10.3390/cancers13112535
Kato T, Wakiyama H, Furusawa A, Choyke PL, Kobayashi H. Near Infrared Photoimmunotherapy; A Review of Targets for Cancer Therapy. Cancers. 2021; 13(11):2535. https://doi.org/10.3390/cancers13112535
Chicago/Turabian StyleKato, Takuya, Hiroaki Wakiyama, Aki Furusawa, Peter L. Choyke, and Hisataka Kobayashi. 2021. "Near Infrared Photoimmunotherapy; A Review of Targets for Cancer Therapy" Cancers 13, no. 11: 2535. https://doi.org/10.3390/cancers13112535
APA StyleKato, T., Wakiyama, H., Furusawa, A., Choyke, P. L., & Kobayashi, H. (2021). Near Infrared Photoimmunotherapy; A Review of Targets for Cancer Therapy. Cancers, 13(11), 2535. https://doi.org/10.3390/cancers13112535






