Are Volatile Organic Compounds Accurate Markers in the Assessment of Colorectal Cancer and Inflammatory Bowel Diseases? A Review
Abstract
Simple Summary
Abstract
1. Introduction
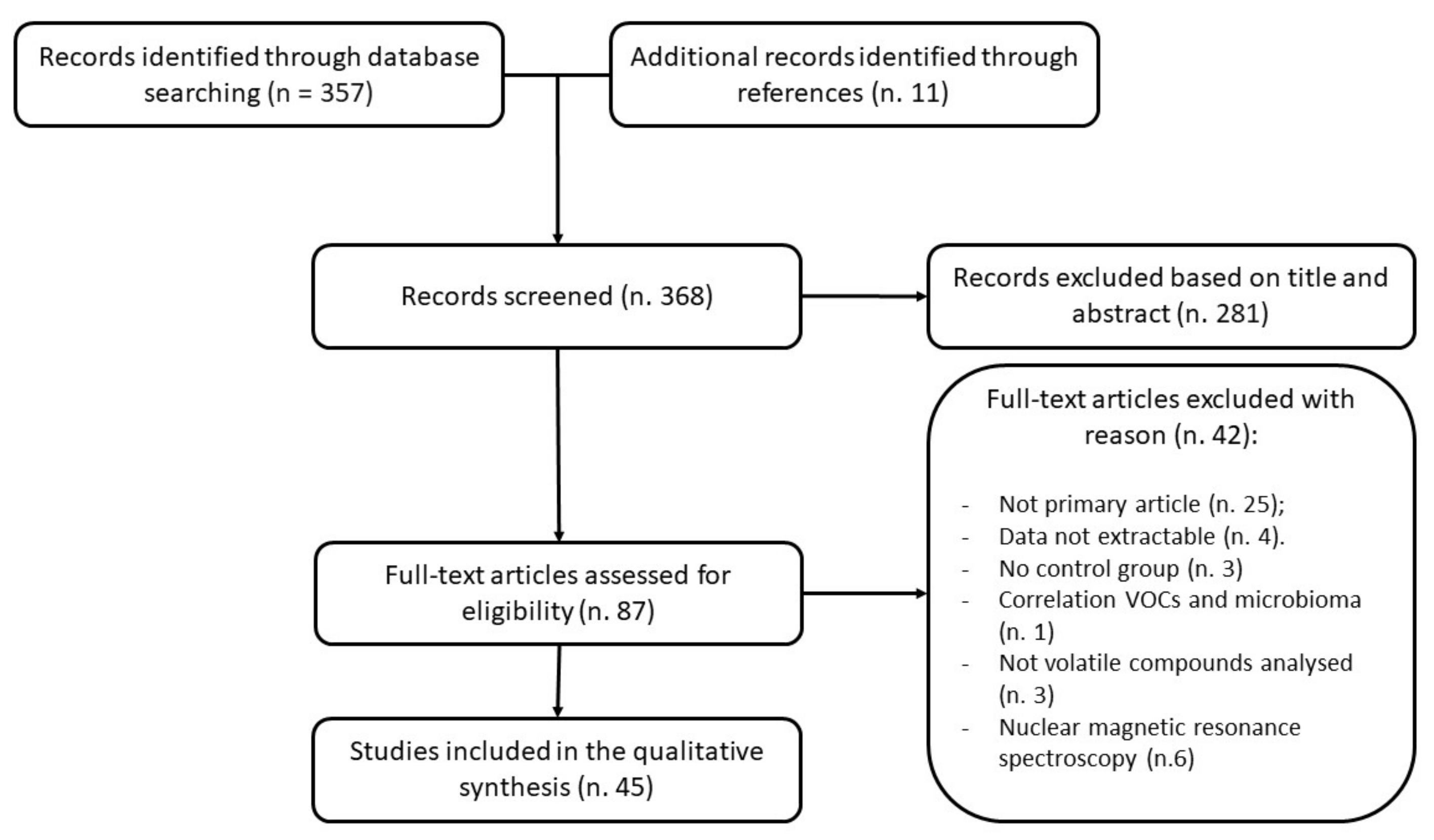
2. Materials and Methods
(“volatile organic compounds” [MeSH Terms] OR “volatile organic metabolites” [All Fields] OR “VOC” [All Fields] OR “VOM” [All Fields]) AND ((“colorectal neoplasms” [MeSH Terms] OR “colorectal neoplasia” [All Fields] OR “colorectal cancer” [All Fields] OR “digestive system neoplasms” [MeSH Terms] OR “colonic neoplasms” [MeSH Terms]) OR (“inflammatory bowel diseases” [MeSH Terms] OR “colitis, ulcerative” [MeSH Terms] OR “Crohn disease” [MeSH Terms] OR “IBD” [All Fields] OR (“UC” [All Fields] OR “CD” [All Fields]).
3. Results
3.1. Colorectal Cancer
3.1.1. Breath Exhaled VOCs
3.1.2. Fecal VOCs
| Reference | Aim | Population | Analysis Method | Different VOCs between Groups | Accuracy | ||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention Group CRC/Adenoma | CRC Stage (I, II, III, IV) | Control Group | Sens | %Spec | %AUC | ||||
| Bosch 2020 [50] | Disease detection | 14 CRC | n.a. | 227 | GC-IMS (FlavourSpec®) | VOCs Panel | 1 | 1 | 0.96 |
| Disease detection | 64 AA | n.a. | 227 | 0.96 | 0.93 | 0.96 | |||
| Disease detection | 69 LA | n.a. | 227 | 0.98 | 0.91 | 0.96 | |||
| Disease detection | 127 SA | n.a. | 227 | 0.96 | 0.93 | 0.96 | |||
| Disease detection | 14 CRC | n.a. | 260 Adenomas † | n.s. | n.s. | n.s. | |||
| Bond 2019 [54] | Disease detection | 21 CRC | n.a. | 60 ‡ | GC-IMS | A, B, C, D, E, F, G, H | 0.88 | 0.85 | 0.82 |
| Ishibe 2018 [49] | Disease detection | 30 CRC | I = 9, II = 12 II = 6, IV = 3 | 26 HC | GC | I, L | 0.90 | 0.57 | 0.78 |
| Batty 2015 [47] | Disease detection | 31 CRC | n.a. | 31 ‡ | SIFT-MS | VOCs Panel | 0.78 | 0.72 | n.a. |
| Zonta 2017 [48] | Disease detection | 28 CRC and adenomas | n.a. | 58 | eNose (SCENT A1) | VOCs Panel | 0.95 | 0.95 | n.a. |
| De Meij 2013 [46] | Disease detection | 40 CRC | n.a. | 57 | eNose (Cyranose 320®) | VOCs Panel | 0.85 | 0.87 | 0.92 |
| Disease detection | 60 AA | n.a. | 57 | 0.62 | 0.86 | 0.79 | |||
| Disease detection | 40 CRC | n.a. | 60 AA | 0.75 | 0.73 | 0.82 | |||
3.1.3. Urinary VOCs
| Reference | Aim | Population | Analysis Method | Different VOCs between Groups | Accuracy | ||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention Group CRC/Adenoma | CRC Stage (I, II, III, IV) | Control Group | Sens | %Spec | %AUC | ||||
| McFarlane 2019 [58] | Disease detection | 56 CRC | n.a. | 82 HC (relatives + spouses) | FAIMS-MS | 0.69 | 0.69 | 0.71 | |
| Widlak 2018 [57] | Disease detection | 35 CRC | n.a. | n.a. | FAIMS | VOCs Panel | 0.63 | 0.63 | 0.67 |
| Disease detection | 27 AA | n.a. | n.a. | FAIMS | 0.93 | 0.16 | 0.56 | ||
| Disease detection | 94 A | n.a. | n.a. | FAIMS | 0.91 | 0.15 | 0.55 | ||
| Arasaradnam 2014 [55] | Disease detection | 83 CRC | 65 non-metastatic, 9 metastatic, 9 not-fully staged | 50 HC | FAIMS | A, B, C, D, E, F, G, H, I, J, K, L, M, N, O, P, Q, R, S, T, U, V, W | 0.88 | 0.60 | n.a. |
| Mozdiak 2019 [60] | Disease detection | 12 CRC | n.a. | 12 HC ‡ | FAIMS | VOCs Panel | 1 | 0.92 | 0.98 |
| Disease detection | 12 CRC + 93 A | n.a. | 37 HC ‡ | FAIMS | 0.48 | 0.89 | 0.64 | ||
| Disease detection | 12 CRC + 18 AA | n.a. | 37 HC ‡ | FAIMS | 0.57 | 0.68 | 0.62 | ||
| Disease detection | 12 CRC | n.a. | 7 AA ‡ | FAIMS | 0.83 | 1 | 0.92 | ||
| Disease detection | 10 CRC | n.a. | 24 HC ‡ | GC-IMS | 0.80 | 0.83 | 0.82 | ||
| Disease detection | 10 CRC + 55 adenomas | n.a. | 42 HC ‡ | GC-IMS | 0.71 | 0.55 | 0.61 | ||
| Disease detection | 10 CRC + 13 AA | n.a. | 24 HC ‡ | GC-IMS | 0.48 | 0.67 | 0.53 | ||
| Disease detection | 10 CRC | n.a. | 13 AA ‡ | GC-IMS | n.a. | n.a. | n.a. | ||
| Silva 2011 [59] | Disease detection | 12 CRC | n.a. | 21 HC | GC-MS | a, b, c, d, e, f, g, h, i, j | n.a. | n.a. | n.a. |
| Westenbrink 2015 [56] | Disease detection | 39 CRC | n.a. | 35 IBS | e-Nose (Warwick OLFaction ®) | VOCs Panel | 0.78 | 0.79 | n.a. |
3.2. Inflammatory Bowel Diseases
3.2.1. Breath Exhaled VOCs
| Reference | Aim | Population | Analysis Method | Different VOCs between Groups | Accuracy | ||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention Group (Samples Collected) [CD; UC] | Control Group (Samples Collected) | Sample Collection Method | Sens% | Spec% | AUC | ||||
| Arasaradnam 2016 [55] | Disease diagnosis | 54 [25 CD; 29 UC] | 22 HC | FAIMS | Tedlar® bags | n.a. | 0.74 | 0.75 | 0.82 |
| Differential diagnosis | 25 CD | 29 UC | n.a. | 0.67 | 0.67 | 0.70 | |||
| Tiele 2019 [15] | Disease diagnosis | 30 IBD [14 CD; 16 UC] | 9 HC | GC-IMS | Direct measurement | A, B | 0.87 | 0.89 | 0.93 |
| eNose (Warwick OLFaction) | Bio-VOC sampling device | . | 0.67 | 0.89 | 0.81 | ||||
| Differential diagnosis | 14 CD | 16 UC | GC-IMS | . | 0.86 | 0.62 | 0.71 | ||
| eNose (Warwick OLFaction) | . | 0.71 | 0.88 | 0.88 | |||||
| Smolinska 2017 [35] | Disease activity | UC remission (70) | UC active (62) | GC-tof-MS | Tedlar bags | C, D, E, F, G | 0.92 | 0.77 | 0.94 |
| Bodelier 2015 [40] | Disease diagnosis | 140 active CD (725 †) | 110 HC (110) | GC-tof-MS (and PCA) | Tedlar bags | VOCs Panel ¶ | 0.96 | 0.99 | 0.99 |
| Disease diagnosis | 135 inactive CD (725 †) | 110 HC (110) | VOCs Panel ¶ | 0.96 | 0.97 | 0.98 | |||
| Disease activity | 140 active CD | 135 remission CD | VOCs Panel ¶ | 0.81 | 0.80 | 0.88 | |||
| Pelli 1999 [42] | Disease diagnosis | 10 CD | 10 HC | GC | Tedlar bags | H | n.a. | n.a. | n.a. |
| Disease diagnosis | 10 UC | 10 HC | H | n.a. | n.a. | n.a. | |||
| Sedghi 1994 [43] | Disease diagnosis | 17 UC (56) | 14 HC | GC | Plastic syringes | Z | n.a. | n.a. | n.a. |
| Dryahina 2017 [36] | Disease diagnosis | 187 IBD [136 CD; 51 UC] | 14 HC | SIFT-MS | Nalophan bags | F, H, I, L, M, N, O, P, Q | n.a. | n.a. | n.a. |
| Rieder 2016 [37] | Disease diagnosis | 36 IBD [24 CD; 11 UC] | 53 HC | SIFT-MS | Mylar bag | I, L, M, R, S, T, U | n.a. | n.a. | n.a. |
| Disease diagnosis | 36 IBD [24 CD; 11 UC] | 6 OGDs | I, M, V | n.a. | n.a. | n.a. | |||
| Disease activity | n.a. | n.a. | VOCs Panel ¶ ** | n.a. | n.a. | n.a. | |||
| Differential diagnosis | 24 CD | 11 UC | VOCs Panel ¶ ** | n.a. | n.a. | n.a. | |||
| Hicks 2015 [39] | Disease diagnosis | 18 CD | 18 HC | SIFT-MS (and PCA and OSC-PLS-DA) | Nalophan bags | N, T, W, X | 0.94 | 0.94 | 0.86 |
| Disease diagnosis | 20 UC | 18 HC | V | 0.90 | 0.94 | 0.74 | |||
| Differential diagnosis | 18 CD | 20 UC | N, W, Y | 0.89 | 0.90 | 0.82 | |||
| Dryahina 2013 [41] | Disease diagnosis | 20 CD | 140 HC | SIFT-MS | Direct measurement | H | n.a. | n.a. | n.a. |
| Disease diagnosis | 28 UC | 140 HC | H | n.a. | n.a. | n.a. | |||
| Monasta 2017 [44] (pediatric patients) | Disease diagnosis | 67 IBD (124) [34 CD; 33 UC] | 167 (334) [102 HC; 65 GIC] | IMR-MS | Bio-VOC sam-pling device | VOCs Panel ¶ | 0.95 | 0.69 | 0.92 |
| Differential diagnosis | 34 CD | 33 UC | VOCs Panel ¶ | 0.94 | 0.71 | 0.88 | |||
| Patel 2014 [45] (pediatric patients) | Disease diagnosis | 62 IBD [51 CD; 11 UC] | 55 HC | SIFT-MS | Mylar bags | Aa, Ab, Ac | n.a. | n.a. | 0.96 |
| Differential diagnosis | 51 CD | 11 UC | VOCs Panel ¶ ** | n.a. | n.a. | n.a. | |||
3.2.2. Fecal VOCs
| Reference | Aim | Population | Analysis Method | Different VOCs between Groups | Accuracy | |||
|---|---|---|---|---|---|---|---|---|
| Intervention Group (Samples Collected) [CD; UC] | Control Group (Samples Collected) | Sens% | Spec% | AUC | ||||
| Bosch 2020 [50] | Disease diagnosis | 276 IBD (495) † | 227 HC (227) | GC-IMS (FlavourSpec®) | VOCs Panel | 0.97 | 0.92 | 0.96 |
| Disease diagnosis | 164 CD (292) † | 227 HC (227) | 0.96 | 0.97 | 0.96 | |||
| Disease diagnosis | 112 UC (197) † | 227 HC (227) | 0.91 | 0.88 | 0.95 | |||
| Differential diagnosis | 164 CD (187) † | 112 UC (147) | 0.17 | 0.96 | 0.55 | |||
| Disease activity | active CD (107) | inactive CD (84) | 0.76 | 0.43 | 0.52 | |||
| Disease activity | active UC (80) | inactive UC (63) | 0.67 | 0.57 | 0.63 | |||
| Shepherd 2014 [70] | Disease diagnosis | 101 IBD | 46 HC | GC | VOCs Panel | 0.78 | 0.80 | n.a. |
| Ahmed 2013 [67] | Disease diagnosis | 110 IBD | 30 IBS | GC-MS | A, B, C, D, E, F, G, H, I, J, K | 0.96 | 0.80 | n.a. |
| Disease diagnosis | 62 CD | 30 IBS | 1 | 0.80 | n.a. | |||
| Disease diagnosis | 48 UC | 30 IBS | 0.94 | 0.87 | n.a. | |||
| El Manouni El Hassani 2019 [68] (Pediatric patients) | Differential diagnosis | 17 IBD [15 CD; 2 UC] | 25 HC | FAIMS | VOCs Panel | 0.94 | 0.96 | 0.99 |
| Bosch 2018 [65] (paediatric patients) | Disease diagnosis | 30 IBD [15 CD; 15 UC] | 15 IBS-FAP/NOS | FAIMS | VOCs Panel | 1 | 0.87 | 0.94 |
| Disease diagnosis | 30 IBD [15 CD; 15 UC] | 30 HC | 0.93 | 0.97 | 0.96 | |||
| Disease diagnosis | 15 CD | 30 HC | 0.93 | 0.93 | 0.95 | |||
| Disease diagnosis | 15 UC | 30 HC | 0.93 | 0.97 | 0.98 | |||
| Differential diagnosis | 15 CD | 15 UC | 0.60 | 0.80 | 0.67 | |||
| Van Gaal 2017 [66] (Pediatric patients) | Disease diagnosis | 36 IBD [23 CD; 13 UC] | 24 HC | FAIMS | VOCs Panel | 0.79 | 0.78 | 0.76 |
| Disease diagnosis | 23 CD | 24 HC | 0.83 | 0.83 | 0.90 | |||
| Disease diagnosis | 13 UC | 24 HC | 0.77 | 0.75 | 0.74 | |||
| Differential diagnosis | 23 CD | 13 UC | n.s. | n.s. | n.s. | |||
| Bosch 2018 [64] (paediatric patients) | Disease diagnosis | 10 IBD (10) [5 CD; 5 UC] | 10 OGDs (10) | GC-IMS (FlavourSpec®) | VOCs Panel | 0.70 | 0.90 | 0.73 |
| De Meij 2014 [69] (Paediatric patients) | Disease diagnosis | active UC (26) | 28 HC | eNose (Cyranose 320®) | VOCs Panel | 1 | 1 | 1 |
| Disease diagnosis | Inactive UC (17) | 28 HC | 0.94 | 0.94 | 0.94 | |||
| Disease diagnosis | Active CD (6) | 28 HC | 0.87 | 0.67 | 0.85 | |||
| Disease diagnosis | Inactive CD (20) | 28 HC | 0.94 | 0.94 | 0.94 | |||
| Differential diagnosis | Active CD (6) | Active UC (12) | 0.97 | 0.92 | 0.96 | |||
| Differential diagnosis | Inactive CD (20) | Inactive UC (17) | 0.86 | 0.72 | 0.81 | |||
3.2.3. Urinary VOCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Ewing, M.; Naredi, P.; Zhang, C.; Månsson, J. Identification of patients with nonmetastatic colorectal cancer in primary care: A case-control study. Br. J. Gen. Pract. 2016, 66, e880–e886. [Google Scholar] [CrossRef] [PubMed]
- Săftoiu, A.; Hassan, C.; Areia, M.; Bhutani, M.; Bisschops, R.; Bories, E.; Cazacu, I.; Dekker, E.; Deprez, P.; Pereira, S.; et al. Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2020, 52, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Bertels, L.; Lucassen, P.; van Asselt, K.; Dekker, E.; van Weert, H.; Knottnerus, B. Motives for non-adherence to colonoscopy advice after a positive colorectal cancer screening test result: A qualitative study. Scand. J. Prim. Health Care 2020, 38, 487–498. [Google Scholar] [CrossRef]
- Di Ruscio, M.; Vernia, F.; Ciccone, A.; Frieri, G.; Latella, G. Surrogate Fecal Biomarkers in Inflammatory Bowel Disease: Rivals or Complementary Tools of Fecal Calprotectin? Inflamm. Bowel Dis. 2017, 24, 78–92. [Google Scholar] [CrossRef]
- Vernia, F.; Di Ruscio, M.; Stefanelli, G.; Viscido, A.; Frieri, G.; Latella, G. Is fecal calprotectin an accurate marker in the management of Crohn’s disease? J. Gastroenterol. Hepatol. 2020, 35, 390–400. [Google Scholar] [CrossRef]
- Jess, T.; Rungoe, C.; Peyrin-Biroulet, L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of pop-ulation-based cohort studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of Dietary Protein and Peptides by intestinal Microbes and their Impacts on Gut. Curr. Protein. Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef]
- Davis, V.; Bathe, O.; Schiller, D.; Slupsky, C.M.; Sawyer, M.B. Metabolomics and surgical oncology: Potential role for small molecule biomarkers. J. Surg. Oncol. 2011, 103, 451–459. [Google Scholar] [CrossRef]
- Rıos-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-Gavilan, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA: Acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef]
- Smolinska, A.; Tedjo, D.I.; Blanchet, L.; Bodelier, A.; Pierik, M.; Masclee, A.; Dallinga, J.; Savelkoul, P.; Jonkers, D.; Penders, J.; et al. Volatile metabolites in breath strongly correlate with gut microbiome in CD patients. Anal. Chim. Acta 2018, 1025, 1–11. [Google Scholar] [CrossRef]
- Gagnière, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef]
- De Boer, N.K.; de Meij, T.G.J.; Oort, F.A.; Larbi, I.B.; Mulder, C.J.J.; van Bodegraven, A.A.; van der Schee, M.P. The scent of colorectal cancer: Detection by volatile organic compound analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 1085–1089. [Google Scholar] [CrossRef]
- Tiele, A.; Wicaksono, A.; Kansara, J.; Arasaradnam, R.P.; Covington, J.A. Breath Analysis Using eNose and Ion Mobility Technology to Diagnose Inflammatory Bowel Disease-A Pilot Study. Biosensors 2019, 9, 55. [Google Scholar] [CrossRef]
- Keshteli, A.H.; Madsen, K.L.; Mandal, R.; Boeckxstaens, G.E.; Bercik, P.; De Palma, G.; Reed, D.E.; Wishart, D.; Vanner, S.; Dieleman, L.A. Comparison of the metabolomic profiles of irritable bowel syndrome patients with ulcerative colitis patients and healthy controls: New insights into pathophysiology and potential biomarkers. Aliment. Pharmacol. Ther. 2019, 49, 723–732. [Google Scholar] [CrossRef]
- Baranska, A.; Tigchelaar, E.; Smolinska, A.; Dallinga, J.W.; Moonen, E.J.C.; Dekens, J.A.M.; Wijmenga, C.; Zhernakova, A.; van Schooten, F.J. Profile of volatile organic compounds in exhaled breath changes as a result of gluten-free diet. J. Breath Res. 2013, 7, 037104. [Google Scholar] [CrossRef]
- Ruzsanyi, V.; Kalapos, M.P.; Schmidl, C.; Karall, D.; Scholl-Burgi, S.; Baumann, M. Breath profiles of children on ketogenic therapy. J. Breath Res. 2018, 12, 036021. [Google Scholar] [CrossRef]
- Rossi, M.; Aggio, R.; Staudacher, H.M.; Lomer, M.C.; Lindsay, J.O.; Irving, P.; Probert, C.; Whelan, K. Volatile organic compounds in feces associate with response to dietary intervention in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2018, 16, 385–391. [Google Scholar] [CrossRef]
- Couch, R.D.; Dailey, A.; Zaidi, F.; Navarro, K.; Forsyth, C.B.; Mutlu, E.; Engen, P.A.; Keshavarzian, A. Alcohol induced alterations to the human fecal VOC metabolome. PLoS ONE 2015, 10, e0119362. [Google Scholar] [CrossRef]
- Amann, A.; Spanel, P.; Smith, D. Breath analysis: The approach towards clinical application. Mini Rev. Med. Chem. 2007, 7, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Di Lena, M.; Porcelli, F.; Altomare, D.F. Volatile organic compounds as new biomarkers for colorectal cancer: A review. Colorectal. Dis. 2016, 18, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Van Keulen, K.E.; Jansen, M.E.; Schrauwen, R.W.M.; Kolkman, J.J.; Siersema, P.D. Volatile organic compounds in breath can serve as a non-invasive diagnostic biomarker for the detection of advanced adenomas and colorectal cancer. Aliment. Pharmacol. Ther. 2020, 51, 334–346. [Google Scholar] [CrossRef]
- Van de Goor, R.; van Hooren, M.; Dingemans, A.M.; Kremer, B.; Kross, K. Training and validating a portable electronic nose for lung cancer screening. J. Thorac. Oncol. 2018, 13, 676–681. [Google Scholar] [CrossRef]
- Lewis, N.S. Comparisons between mammalian and artificial olfaction based on arrays of carbon black-polymer composite vapor detectors. Acc. Chem. Res. 2004, 37, 663–672. [Google Scholar] [CrossRef]
- Altomare, D.F.; Picciariello, A.; Rotelli, M.T.; De Fazio, M.; Aresta, A.; Zambonin, C.G.; Vincenti, L.; Trerotoli, P.; De Vietro, N. Chemical signature of colorectal cancer: Case-control study for profiling the breath print. BJS Open 2020. [Google Scholar] [CrossRef]
- Steenhuis, E.G.M.; Schoenaker, I.J.H.; de Groot, J.W.B.; Fiebrich, H.B.; de Graaf, J.C.; Brohet, R.M.; van Dijk, J.D.; van Westreenen, H.L.; Siersema, P.D.; de Vos Tot Nederveen Cappel, W.H. Feasibility of volatile organic compound in breath analysis in the follow-up of colorectal cancer: A pilot study. Eur. J. Surg. Oncol. 2020, 46, 2068–2073. [Google Scholar] [CrossRef]
- Markar, S.R.; Chin, S.T.; Romano, A.; Wiggins, T.; Antonowicz, S.; Paraskeva, P.; Ziprin, P.; Darzi, A.; Hanna, G.B. Breath volatile organic compound profiling of colorectal cancer using selected ion flow-tube mass spectrometry. Ann. Surg. 2019, 269, 903–910. [Google Scholar] [CrossRef]
- Altomare, D.F.; Porcelli, F.; Picciariello, A.; Pinto, M.; Di Lena, M.; Caputi Iambrenghi, O.; Ugenti, I.; Guglielmi, A.; Vincenti, L.; de Gennaro, G. The use of the PEN3 e-nose in the screening of colorectal cancer and polyps. Tech. Coloproctol. 2016, 20, 405–409. [Google Scholar] [CrossRef]
- Amal, H.; Leja, M.; Funka, K.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Kikuste, I.; Vanags, A.; Tolmanis, I.; et al. Breath testing as potential colorectal cancer screening tool. Int. J. Cancer 2016, 138, 229–236. [Google Scholar] [CrossRef]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Travaglio, E.; Longobardi, F.; Tutino, M.; Depalma, N.; Tedesco, G.; Sardaro, A.; Memeo, R.; et al. Effects of curative colorectal cancer surgery on exhaled volatile organic compounds and potential implications in clinical follow-up. Ann. Surg. 2015, 262, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ke, C.; Wang, X.; Chi, C.; Guo, L.; Luo, S.; Guo, Z.; Xu, G.; Zhang, F.; Li, E. Noninvasive detection of colorectal cancer by analysis of exhaled breath. Anal. Bioanal. Chem. 2014, 406, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; de Gennaro, G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br. J. Surg. 2013, 100, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef]
- Smolinska, A.; Bodelier, A.G.; Dallinga, J.W.; Masclee, A.A.; Jonkers, D.M.; van Schooten, F.J.; Pierik, M.J. The potential of volatile organic compounds for the detection of active disease in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 45, 1244–1254. [Google Scholar] [CrossRef]
- Dryahina, K.; Smith, D.; Bortlík, M.; Machková, N.; Lukáš, M.; Španěl, P. Pentane and other volatile organic compounds, including carboxylic acids, in the exhaled breath of patients with Crohn’s disease and ulcerative colitis. J. Breath Res. 2017, 12, 016002. [Google Scholar] [CrossRef]
- Rieder, F.; Kurada, S.; Grove, D.; Cikach, F.; Lopez, R.; Patel, N.; Singh, A.; Alkhouri, N.; Shen, B.; Brzezinski, A.; et al. A Distinct Colon-Derived Breath Metabolome is Associated with Inflammatory Bowel Disease, but not its Complications. Clin. Transl. Gastroenterol. 2016, 7, e201. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; McFarlane, M.; Daulton, E.; Skinner, J.; Connell, N.; Wuriea, S.; Chambers, S.; Nwokolo, C.; Bardhan, K.; Savage, R.; et al. Non-invasive exhaled volatile organic biomarker analysis to detect inflammatory bowel disease (IBD). Dig. Liver Dis. 2016, 48, 148–153. [Google Scholar] [CrossRef]
- Hicks, L.C.; Huang, J.; Kumar, S.; Powles, S.T.; Orchard, T.R.; Hanna, G.B.; Williams, H.R.T. Analysis of Exhaled Breath Volatile Organic Compounds in Inflammatory Bowel Disease: A Pilot Study. J. Crohns Colitis 2015, 9, 731–737. [Google Scholar] [CrossRef]
- Bodelier, A.G.L.; Smolinska, A.; Baranska, A.; Dallinga, J.; Mujagic, Z.; Vanhees, K.; Heuvel, T.; Masclee, A.; Jonkers, D.; Pierik, M.; et al. Volatile Organic Compounds in Exhaled Air as Novel Marker for Disease Activity in Crohn’s Disease: A Metabolomic Approach. Inflamm. Bowel Dis. 2015, 21, 1776–1785. [Google Scholar] [CrossRef]
- Dryahina, K.; Španěl, P.; Pospíšilová, V.; Sovová, K.; Hrdlička, L.; Machková, N.; Lukáš, M.; Smith, D. Quantification of pentane in exhaled breath, a potential biomarker of bowel disease, using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 1983–1992. [Google Scholar] [CrossRef]
- Pelli, M.A.; Trovarelli, G.; Capodicasa, E.; De Medio, G.E.; Bassotti, G. Breath alkanes determination in ulcerative colitis and Crohn’s disease. Dis. Colon Rectum 1999, 42, 71–76. [Google Scholar] [CrossRef]
- Sedghi, S.; Keshavarzian, A.; Klamut, M.; Eiznhamer, D.; Zarling, E.J. Elevated breath ethane levels in active ulcerative colitis: Evidence for excessive lipid peroxidation. Am. J. Gastroenterol. 1994, 89, 2217–2221. [Google Scholar]
- Monasta, L.; Pierobon, C.; Princivalle, A.; Martelossi, S.; Marcuzzi, A.; Pasini, F.; Perbellini, L. Inflammatory bowel disease and patterns of volatile organic compounds in the exhaled breath of children: A case-control study using Ion Molecule Reaction-Mass Spectrometry. PLoS ONE 2017, 12, e0184118. [Google Scholar] [CrossRef]
- Patel, N.; Alkhouri, N.; Eng, K.; Cikach, F.; Mahajan, L.; Yan, C.; Grove, D.; Rome, E.S.; Lopez, R.; Dweik, R.A. Metabolomic analysis of breath volatile organic compounds reveals unique breathprints in children with inflammatory bowel disease: A pilot study. Aliment. Pharmacol. Ther. 2014, 40, 498–507. [Google Scholar] [CrossRef]
- De Meij, T.G.; Larbi, I.B.; van der Schee, M.P.; Lentferink, Y.E.; Paff, T.; Terhaar Sive Droste, J.S.; Mulder, C.J.; van Bodegraven, A.A.; de Boer, N.K. Electronic nose can discriminate colorectal carcinoma and advanced adenomas by fecal volatile biomarker analysis: Proof of principle study. Int. J. Cancer 2014, 134, 1132–1138. [Google Scholar] [CrossRef]
- Batty, C.A.; Cauchi, M.; Lourenço, C.; Hunter, J.O.; Turner, C. Use of the Analysis of the Volatile Faecal Metabolome in Screening for Colorectal Cancer. PLoS ONE 2015, 10, e0130301. [Google Scholar] [CrossRef]
- Zonta, G.; Anania, G.; de Togni, A.; Gaiardo, A.; Gherardi, S.; Giberti, A.; Guidi, V.; Landini, N.; Palmonari, C.; Ricci, L.; et al. Use of gas sensors and FOBT for the early detection of colorectal cancer. Proc. Eurosensors 2017, 1, 398. [Google Scholar] [CrossRef]
- Ishibe, A.; Ota, M.; Takeshita, A.; Tsuboi, H.; Kizuka, S.; Oka, H.; Suwa, Y.; Suzuki, S.; Nakagawa, K.; Suwa, H.; et al. Detection of gas components as a novel diagnostic method for colorectal cancer. Ann. Gastroenterol. Surg. 2018, 2, 147–153. [Google Scholar] [CrossRef]
- Bosch, S.; Bot, R.; Wicaksono, A.; Savelkoul, E.; Hulst, R.; Kuijvenhoven, J.; Stokkers, P.; Daulton, E.; Covington, J.A.; Meij, T.G. Early detection and follow-up of colorectal neoplasia based on faecal volatile organic compounds. Colorectal Dis. 2020, 22, 1119–1129. [Google Scholar] [CrossRef]
- Bezabeh, T.; Somorjai, R.; Dolenko, B.; Bryskina, N.; Levin, B.; Bernstein, C.N.; Jeyarajah, E.; Steinhart, A.H.; Rubin, D.T.; Smith, I.C.P. Detecting colorectal cancer by 1H magnetic resonance spectroscopy of fecal extracts. NMR Biomed. 2009, 22, 593–600. [Google Scholar] [CrossRef]
- Monleón, D.; Morales, J.M.; Barrasa, A.; López, J.A.; Vázquez, C.; Celda, B. Metabolite profiling of fecal water extracts from human colorectal cancer. NMR Biomed. 2009, 22, 342–348. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, C.; Liu, C.; Wang, Z.; Yang, J.; Liu, X.; Shen, Z.; Wu, R. NMR-based fecal metabolomics fingerprinting as predictors of earlier diagnosis in patients with colorectal cancer. Oncotarget 2016, 7, 29454–29464. [Google Scholar] [CrossRef]
- Bond, A.; Greenwood, R.; Lewis, S.; Corfe, B.; Sarkar, S.; O’Toole, P.; Rooney, P.; Burkitt, M.; Hold, G.; Probert, C. Volatile organic compounds emitted from faeces as a biomarker for colorectal cancer. Aliment. Pharmacol. Ther. 2019, 49, 1005–1012. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; McFarlane, M.J.; Ryan-Fisher, C.; Westenbrink, E.; Hodges, P.; Thomas, M.; Chambers, S.; O’Connell, N.; Bailey, C.; Harmston, C.; et al. Detection of colorectal cancer (CRC) by urinary volatile organic compound analysis. PLoS ONE 2014, 9, e108750. [Google Scholar] [CrossRef]
- Westenbrink, E.; Arasaradnam, R.P.; O’Connell, N.; Bailey, C.; Nwokolo, C.; Bardhan, K.D.; Covington, J.A. Development and application of a new electronic nose instrument for the detection of colorectal cancer. Biosens Bioelectron. 2015, 67, 733–738. [Google Scholar] [CrossRef]
- Widlak, M.M.; Neal, M.; Daulton, E.; Thomas, C.L.; Tomkins, C.; Singh, B.; Harmston, C.; Wicaksono, A.; Evans, C. Risk stratification of symptomatic patients suspected of colorectal cancer using faecal and urinary markers. Colorectal Dis. 2018, 20, O335–O342. [Google Scholar] [CrossRef]
- McFarlane, M.; Millard, A.; Hall, H.; Savage, R.; Constantinidou, C.; Arasaradnam, R.; Nwokolo, C. Urinary volatile organic compounds and faecal microbiome profiles in colorectal cancer. Colorectal Dis. 2019, 21, 1259–1269. [Google Scholar] [CrossRef]
- Silva, C.L.; Passos, M.; Câmara, J.S. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br. J. Cancer 2011, 105, 1894–1904. [Google Scholar] [CrossRef]
- Mozdiak, E.; Wicaksono, A.N.; Covington, J.A.; Arasaradnam, R.P. Colorectal cancer and adenoma screening using urinary volatile organic compound (VOC) detection: Early results from a single-centre bowel screening population (UK BCSP). Tech. Coloproctol. 2019, 23, 343–351. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Y.; Liang, J.; Huang, Y.; Ma, C.; Liu, X.; Yang, J. NMR-based metabolomic techniques identify potential urinary biomarkers for early colorectal cancer detection. Oncotarget 2017, 8, 105819–105831. [Google Scholar] [CrossRef] [PubMed]
- Latella, G.; Papi, C. Crucial steps in the natural history of inflammatory bowel disease. World J. Gastroenterol. 2012, 18, 3790–3799. [Google Scholar] [CrossRef] [PubMed]
- Wheat, C.L.; Clark-Snustad, K.; Devine, B.; Grembowski, D.; Thornton, T.A.; Ko, C.W. Worldwide Incidence of Colorectal Cancer, Leukemia, and Lymphoma in Inflammatory Bowel Disease: An Updated Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 1632439. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.; van Gaal, N.; Zuurbier, R.P.; Covington, J.A.; Wicaksono, A.N.; Biezeveld, M.H.; Benninga, M.A.; Mulder, C.J.; de Boer, N.K.H.; de Meij, T.G.J. Differentiation Between Pediatric Irritable Bowel Syndrome and Inflammatory Bowel Disease Based on Fecal Scent: Proof of Principle Study. Inflamm. Bowel Dis. 2018, 24, 2468–2475. [Google Scholar] [CrossRef]
- Bosch, S.; El Manouni El Hassani, S.; Covington, J.A.; Wicaksono, A.N.; Bomers, M.K.; Benninga, M.A.; Mulder, C.J.J.; de Boer, N.K.H.; de Meij, T.G.J. Optimized Sampling Conditions for Fecal Volatile Organic Compound Analysis by Means of Field Asymmetric Ion Mobility Spectrometry. Anal. Chem. 2018, 90, 7972–7981. [Google Scholar] [CrossRef]
- Van Gaal, N.; Lakenman, R.; Covington, J.; Savage, R.; de Groot, E.; Bomers, M.; Benninga, M.; Mulder, C.; de Boer, N.; de Meij, T. Faecal volatile organic compounds analysis using field asymmetric ion mobility spectrometry: Non-invasive diagnostics in paediatric inflammatory bowel disease. J. Breath Res. 2017, 12, 016006. [Google Scholar] [CrossRef]
- Ahmed, I.; Greenwood, R.; Costello, B.; Ratcliffe, N.M.; Probert, C.S. An investigation of fecal volatile organic metabolites in irritable bowel syndrome. PLoS ONE 2013, 8, e58204. [Google Scholar] [CrossRef]
- El Manouni El Hassani, S.; Bosch, S.; Lemmen, J.P.M.; Brizzio Brentar, M.; Ayada, I.; Wicaksono, A.N.; Covington, J.A.; Benninga, M.A.; de Boer, N.K.H.; de Meij, T.G.J. Simultaneous Assessment of Urinary and Fecal Volatile Organic Compound Analysis in De Novo Pediatric IBD. Sensors 2019, 19, 4496. [Google Scholar] [CrossRef]
- De Meij, T.G.; de Boer, N.K.; Benninga, M.A.; Lentferink, Y.E.; de Groot, E.F.; van de Velde, M.E.; van Bodegraven, A.A.; van der Schee, M.P. Faecal gas analysis by electronic nose as novel, non-invasive method for assessment of active and quiescent paediatric inflammatory bowel disease: Proof of principle study. J. Crohns Colitis 2014. [Google Scholar] [CrossRef]
- Shepherd, S.F.; McGuire, N.D.; de Lacy Costello, B.P.; Ewen, R.J.; Jayasena, D.H.; Vaughan, K.; Ahmed, I.; Probert, C.S.; Ratcliffe, N.M. The use of a gas chromatograph coupled to a metal oxide sensor for rapid assessment of stool samples from irritable bowel syndrome and inflammatory bowel disease patients. J. Breath Res. 2014, 8, 026001. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; Pharaoh, M.W.; Williams, G.J.; Nwokolo, C.U.; Bardhan, K.D.; Kumar, S. Colonic fermentation--more than meets the nose. Med. Hypotheses 2009, 73, 753–756. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; Ouaret, N.; Thomas, M.G.; Quraishi, N.; Heatherington, E.; Nwokolo, C.U.; Bardhan, K.D.; Covington, J.A. A novel tool for noninvasive diagnosis and tracking of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013, 19, 999–1003. [Google Scholar] [CrossRef]
- Dawiskiba, T.; Deja, S.; Mulak, A.; Ząbek, A.; Jawień, E.; Pawełka, D.; Banasik, M.; Mastalerz-Migas, A.; Balcerzak, W.; Kaliszewski, K.; et al. Serum and urine metabolomic fingerprinting in diagnostics of inflammatory bowel diseases. World J. Gastroenterol. 2014, 20, 163–174. [Google Scholar] [CrossRef]
- Williams, H.R.T.; Cox, I.J.; Walker, D.G.; North, B.; Patel, V.; Marshall, S.; Jewell, D.; Ghosh, S.; Thomas, H.; Teare, J.; et al. Characterization of inflammatory bowel disease with urinary metabolic profiling. Am. J. Gastroenterol. 2009, 104, 1435–1444. [Google Scholar] [CrossRef]
- Bannaga, A.S.; Farrugia, A.; Arasaradnam, R.P. Diagnosing Inflammatory bowel disease using noninvasive applications of volatile organic compounds: A systematic review. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1113–1122. [Google Scholar] [CrossRef]
- Sola Martínez, R.A.; Pastor Hernández, J.M.; Yanes Torrado, Ó.; Cánovas Díaz, M.; de Diego Puente, T.; Vinaixa Crevillent, M. Exhaled volatile organic compounds analysis in clinical pediatrics: A systematic review. Pediatr. Res. 2020. [Google Scholar] [CrossRef]
- Lee, J.H.; Zhu, J. Analyses of short-chain fatty acids and exhaled breath volatiles in dietary intervention trials for metabolic diseases. Exp. Biol. Med. 2020. [Google Scholar] [CrossRef]
- Zou, S.; Fang, L.; Lee, M.H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol. Rep. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Bosch, S.; Lemmen, J.P.; Menezes, R.; Van der Hulst, R.; Kuijvenhoven, J.; Stokkers, P.C.; De Meij, T.G.; DeBoer, N.K. The influence of lifestyle factors on fecal volatile organic compound composition as measured by an electronic nose. J. Breath Res. 2019, 13, 046001. [Google Scholar] [CrossRef]
- Caprilli, R.; Frieri, G.; Latella, G.; Vernia, P.; Santoro, M.L. Fecal excretion of bicarbonate in ulcerative colitis. Digestion 1986, 35, 136–142. [Google Scholar] [CrossRef]
- Caprilli, R.; Vernia, P.; Latella, G.; Frieri, G. Consequence of colonic involvement on electrolyte and acid base homeostasis in Crohn’s disease. Am. J. Gastroenterol. 1985, 80, 509–512. [Google Scholar]
- Vernia, P.; Latella, G.; Magliocca, F.M.; Caprilli, R. Fecal organic anions in diarrhoeal diseases. Scand J. Gastroenterol. 1987, 22 (Suppl. 129), 105–109. [Google Scholar] [CrossRef]
- Leja, M.; Amal, H.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Tolmanis, I.; Vanags, A.; Kupcinskas, J.; Ramonaite, R.; et al. Analysis of the effects of microbiome-related confounding factors on the reproducibility of the volatolomic test. J. Breath Res. 2016, 10, 037101. [Google Scholar] [CrossRef]
- Krilaviciute, A.; Heiss, J.A.; Leja, M.; Kupcinskas, J.; Haick, H.; Brenner, H. Detection of cancer through exhaled breath: A systematic review. Oncotarget 2015, 6, 38643–38657. [Google Scholar] [CrossRef]
- Esfahani, S.; Sagar, N.M.; Kyrou, I.; Mozdiak, E.; O’Connell, N.; Nwokolo, C.; Bardhan, K.D.; Arasaradnam, R.P.; Covington, J.A. Variation in gas and volatile compound emissions from human urine as it ages, measured by an electronic nose. Biosensors 2016, 6, 4. [Google Scholar] [CrossRef]
- Berkhout, D.J.; Benninga, M.A.; van Stein, R.M.; Brinkman, P.; Niemarkt, H.J.; de Boer, N.K.H.; de Meij, T.G.J. Effects of sampling conditions and environmental factors on fecal volatile organic compound analysis by an electronic nose device. Sensors 2016, 16, 1967. [Google Scholar] [CrossRef]
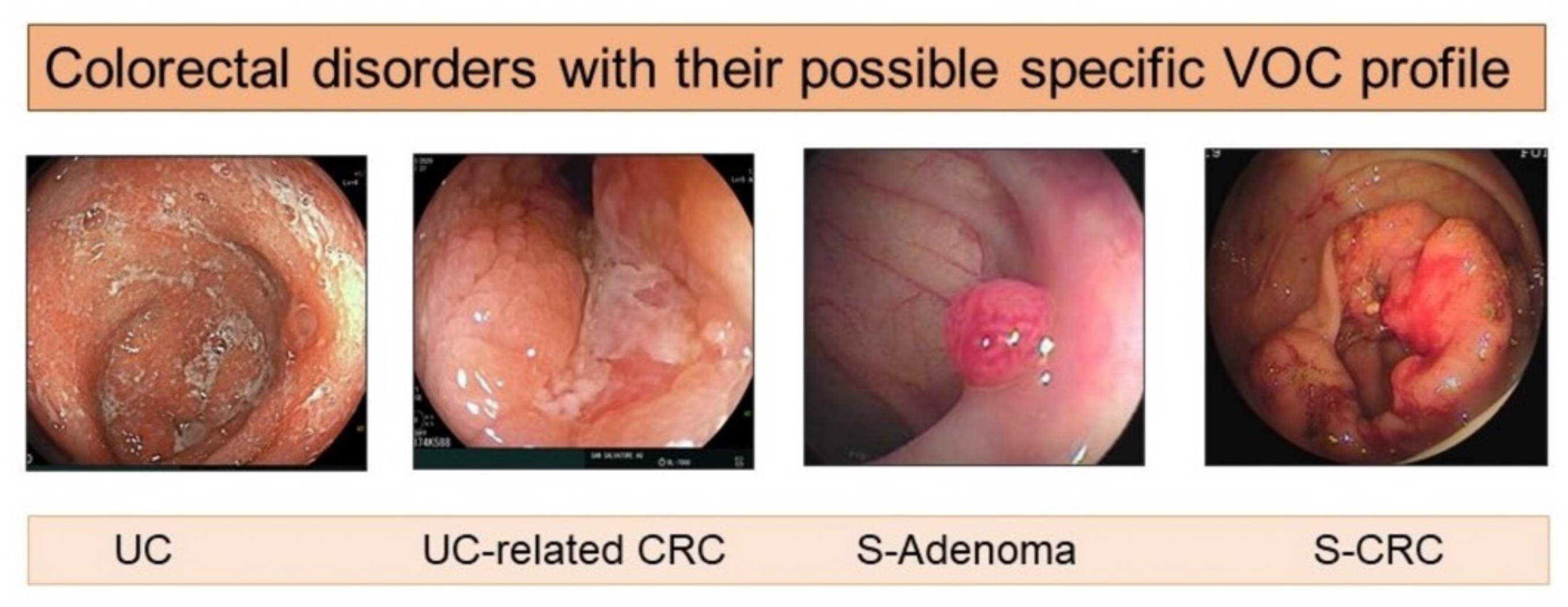
| Colorectal Carcinoma (CRC) | |
|---|---|
| References | Identified VOCs |
| Altomare 2020 [26] | Tetradecane; ethylbenzene; methylbenzene; 5,9-undecadien-2-one + 6,10-dimethyl (E); tridecane; benzaldehyde; dodecane; benzoic acid; 1,3-bis(1-methylethenyl) benzene; decanal; 2-ethyl-hexanol; ethenone + 1[4-(1-methylethenyl) phenyl]; acetic acid; butyl hydroxy toluene; unknown VOC. |
| Steenhuis 2020 [27] | n.a. |
| Van Keulen 2020 [23] | n.a. |
| Markar 2019 [28] | Propanal; ammonia; ethanol; acrolein; propanol; butanol; carbon disulfide. |
| Altomare 2016 [29] | Methane. |
| Amal 2016 [30] | Acetone; ethyl acetate; 4-methyl-octane; ethanol. |
| Altomare 2015 [31] | 1,2-pentadiene; 2-methylbutane; 3-ethylpentane; methylcyclo-pentane; cyclohexane; nonanal; methylcyclohexane; 4-methyl-2-pentanone; 1,4-dimethylbenzene; 1,3-dimethylbenzene; decanal. |
| Wang 2014 [32] | Cyclohexanone; 2,2-dimethyldecane; 4-ethyl-1-octyn-3-ol; ethylaniline; cyclooctylmethanol; trans-2-dodecen-1-ol; 3-hydroxy-2,4,4-trimethylpentyl 2-methylpropanoate; dodecane. |
| Altomare 2013 [33] | 4-methyl-octane; 1,2-pentadiene; 2-methylbutane; cyclohexane; nonanal; methylcyclohexane; 4-methyl-2-pentanone; 1,4-dimethylbenzene; 1,3-dimethylbenzene; 2-methylpentane; 3-methylpentane; 4-methylundecane; trimethyldecane; decanal. |
| Peng 2010 [34] | 1,3-dimethylbenzene; 1,10-(1-butenylidiene) bis-benzene; 1-iodonane; [(1,1-dimethylethyl) thio] acetic acid; 4-(4-propylciclohexyl)-4′-cyano [1,10-biphenyl]-4-yl ester benzoic acid; 2-amino-5-isopropyl-8-methyl-1-azulenecarbonitrile. |
| Inflammatory Bowel Disease (IBD) | |
| Identified VOCs | |
| Tiele 2019 [15] | 2-methyl-, propyl ester; 3-methyl-1-butyl ester |
| Smolinska 2017 [35] | Unknown VOCs; cumene; 2,4-dimethylpentane; methylcyclopentene; C14H30 branched; C15H30 (pentadecene); 3-methyl-1-butanlo, octane, acetic acid, α-pinene; m-cymene. |
| Dryahina 2017 [36] | Pentane; isoprene; ethanol; propanol; hydrogen sulfide; acetone; acetic acid; propanoic acid; butanoic acid. |
| Rieder 2016 [37] | 2-propanol; acetaldehyde; acetone; acetonitrile; acrylonitrile; benzene; carbon disulfide; dimethyl sulfide; ethanol; isoprene; pentane; 1-decene; 1-heptene; 1-nonene; 1-octene; 3-methylhexane; (e)-2-nonene; ammonia; ethane; hydrogen sulfide; triethyl amine; trimethyl amine. |
| Arasaradnam 2016 [38] | n.a. |
| Hicks 2015 [39] | Acetic acid; pentanoic acid; hexanoic acid; propanal; butanal; pentanal; hexanal; heptanal; octanal; nonanal; decanal; methanonal; propanol; butanol; pentanol; phenol; methyl phenol; Ethyl phenol; acetone; dimethyl sulfide; dimethyl disulfide; hydrogen sulfide; carbon disulfide; ammonia; hydrogen cyanide; isoprene |
| Bodelier 2015 [40] | Isoprene; acetone; 2,2,4-trimethylpentane; heptadecane; saturated C12; 1-butoxy-2-propanol; unknown VOC (healthy control vs. active Crohn’s disease)—isoprene; acetone; heptadecane; undecanal; two unknown VOCs (healthy control vs. remission Crohn’s disease)—decanal; 1-hydroxy-2-propanone; hexadecanal; 2,2,4-trimethylhexane; 2,2,4,4-tetramethyloctane; acetic acid methyl ester; four unknown VOCs (active vs. remission Crohn’s disease) |
| Dryahina 2013 [41] | Pentane |
| Pelli 1999 [42] | Ethane; Propane; Butane; Pentane; Isoprene |
| Sedghi 1994 [43] | Ethane; Pentane |
| Monasta 2017 [44] (pediatric patients) | Methane *; ammonia; propene *; acetonitrile ł; nitrous oxide ł; nitrous acid *; acetic acid *; methyl ethyl ketone; methanimineł; cyclopentane ł; carbon disulfide *; methyl nitrate ł; pyridine; methylpyrrole *; ethyl cyanoformate *; dimethyylpyridine; trimethylpentane ł; ammonia; ethylene; acetaldehyde; acetone; isoprene; toluene; n-heptane. |
| Patel 2014 [45] (Pediatric patients) | 2-propanol; acetaldhyde; acetone; acrylonitrile; benzene; carbon disulfide; dimethyl sulfide; ethanol; isoprene; pentane; 1-decene; 1-heptene; 1-nonene; 1-octene; 3-methylhexane; (E)-2-nonene; ammonia; ethane; hydrogen sulfide; triethyl amine; trimethyl amine. |
| Reference | Aim | Population | Analysis Method | Sample Collection Method | Different VOCs between Groups | Accuracy | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention Group CRC/Adenoma | CRC Stage (I, II, III, IV) | Control Group | Sens | %Spec | %AUC | |||||
| Altomare 2020 [26] | Disease detection | 83 | I–II = 38 III–IV = 42 n.a. = 3 | 90 | GC-MS | ReCIVA® sorbent tubes | VOCs Panel ¶ | 0.90 | 0.93 | 0.98 |
| 38 (early cancer) | I–II = 38 | 90 | VOCs Panel ¶ | 0.86 | 0.94 | 0.98 | ||||
| Amal 2016 [30] | Disease detection | 65 CRC | CIS = 1 I = 21 II = 22 III = 18 IV = 2 n.a. = 1 | 122 | GC-MS | Tedlar bags | A, B, C, D (lower in CRC). | 0.85 | 0.94 | n.a. |
| Altomare 2015 [31] | POR detection | 32 in FU | I–II = 20 III–IV = 12 | 55 | GC-MS | Tedlar bags | VOCs Panel ¶ | 1 | 0.96 | 0.96 |
| 32 CRC in FUAS | VOCs Panel ¶ | 1 | 0.95 | 1 | ||||||
| Wang 2014 [32] | Disease detection | 20 CRC | I–II = 12 III = 8 | 20 | GC-MS | Gas-tight syringe and glass vials | VOCs Panel ¶ E (lower in CRC). | n.a. | n.a. | n.a. |
| Altomare 2013 [33] | Disease detection | 37 CRC | I–II = 19 III–IV = 18 | 41 | GC-MS | Tedlar bags | VOCs Panel ¶ | 0.86 | 0.83 | 0.85 |
| Peng 2010 [34] | Disease detection | 26 CRC | PM = 2 I–II = 10 III–IV = 14 | 22 | GC-MS | Mylar polyvinyl fluoride bags | VOCs Panel ¶ | n.a. | n.a. | n.a. |
| Markar 2019 [28] | Disease detection | 50 CRC | I = 9, II = 15 III = 18, IV = 1 | 50 * | SIFT-MS | Direct measurement | VOCs Panel ¶ | 0.90 | 0.66 | 0.83 |
| 50 HC | 0.96 | 0.76 | 0.90 | |||||||
| 25 CRC | n.a. | 54 | F | 0.83 | 0.84 | 0.79 | ||||
| POR detection | 21 POR | 19 CRC (no POR) | F | 0.71 | 0.90 | 0.81 | ||||
| Steenhuis 2020 [27] | Disease detection | 62 in FU | I = 13, II = 12, III = 19, IV = 18 | n.a. | eNose Aeonose® | Direct measurement | n.a. | 0.88 | 0.75 | 0.86 |
| Van Keulen 2020 [23] | Disease detection | 62 CRC | I = 22, II = 22 III = 23, IV = 3 | 104 | eNose Aeonose® | Direct measurement | n.a. | 0.95 | 0.64 | 0.84 |
| 174 (CRC + AA) | 104 | 0.83 | 0.54 | 0.72 | ||||||
| Altomare 2016 [29] | Disease detection | 15 CRC, 15 polyps | I = 1, II = 12 III = 2 | 15 | eNose PEN3 | Tedlar Bags | G | 0.93 | 0.10 | n.a. |
| Reference | Aim | Population | Analysis Method | Different VOCs between Groups | Accuracy | |||
|---|---|---|---|---|---|---|---|---|
| Intervention Group [CD; UC] | Control Group | Sens% | Spec% | AUC | ||||
| Keshteli 2019 [16] * | Disease diagnosis | 53 IBD [0;53] | 39 IBS | GC-MS | A, B, C, D, E, F, G, H, I, J, K, L, M, N | 0.99 | 0.99 | 0.99 |
| El Manouni El Hassani 2019 [68] (Paediatric patients) | Differential diagnosis | 10 IBD [5 CD; 5 UC] | 10 HC | GC-IMS | VOCs Panel | 0.80 | 0.70 | 0.78 |
| Arasaradnam 2013 [72] | Disease diagnosis | 48 IBD [24 CD; 24 UC] | 14 HC | FAIMS | VOCs Panel | n.a. | n.a. | n.a. |
| Disease diagnosis | 48 IBD [24 CD; 24 UC] | 14 HC | e-nose (Fox 4000®) | VOCs Panel | n.a | n.a. | n.a. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernia, F.; Valvano, M.; Fabiani, S.; Stefanelli, G.; Longo, S.; Viscido, A.; Latella, G. Are Volatile Organic Compounds Accurate Markers in the Assessment of Colorectal Cancer and Inflammatory Bowel Diseases? A Review. Cancers 2021, 13, 2361. https://doi.org/10.3390/cancers13102361
Vernia F, Valvano M, Fabiani S, Stefanelli G, Longo S, Viscido A, Latella G. Are Volatile Organic Compounds Accurate Markers in the Assessment of Colorectal Cancer and Inflammatory Bowel Diseases? A Review. Cancers. 2021; 13(10):2361. https://doi.org/10.3390/cancers13102361
Chicago/Turabian StyleVernia, Filippo, Marco Valvano, Stefano Fabiani, Gianpiero Stefanelli, Salvatore Longo, Angelo Viscido, and Giovanni Latella. 2021. "Are Volatile Organic Compounds Accurate Markers in the Assessment of Colorectal Cancer and Inflammatory Bowel Diseases? A Review" Cancers 13, no. 10: 2361. https://doi.org/10.3390/cancers13102361
APA StyleVernia, F., Valvano, M., Fabiani, S., Stefanelli, G., Longo, S., Viscido, A., & Latella, G. (2021). Are Volatile Organic Compounds Accurate Markers in the Assessment of Colorectal Cancer and Inflammatory Bowel Diseases? A Review. Cancers, 13(10), 2361. https://doi.org/10.3390/cancers13102361






