Breast-Specific Epigenetic Regulation of DeltaNp73 and Its Role in DNA-Damage-Response of BRCA1-Mutated Human Mammary Epithelial Cells
Abstract
1. Introduction
2. Results
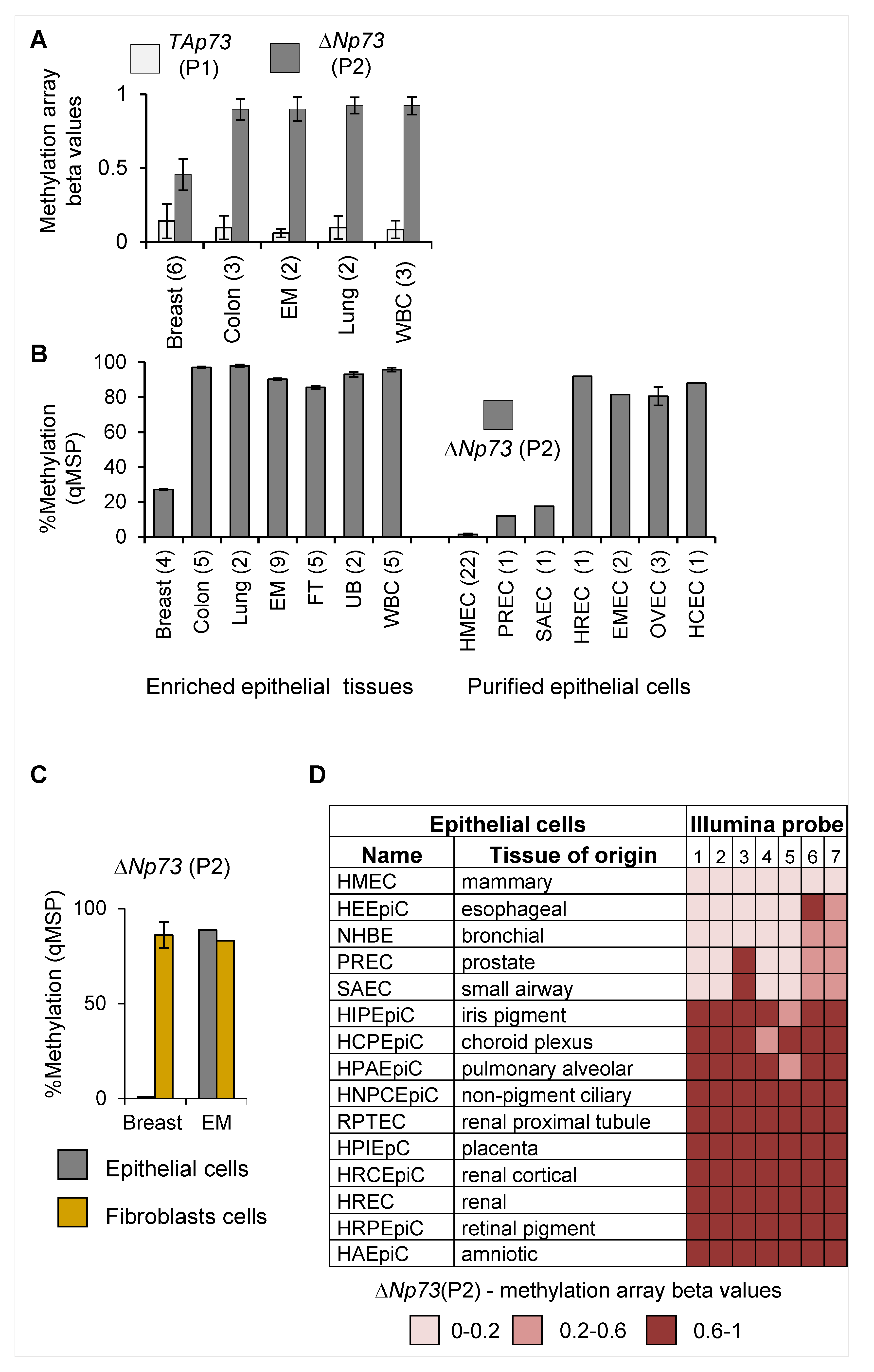
2.1. Tissue-Specific Hypomethylation at the deltaNp73 Promoter in Mammary Epithelial Cells
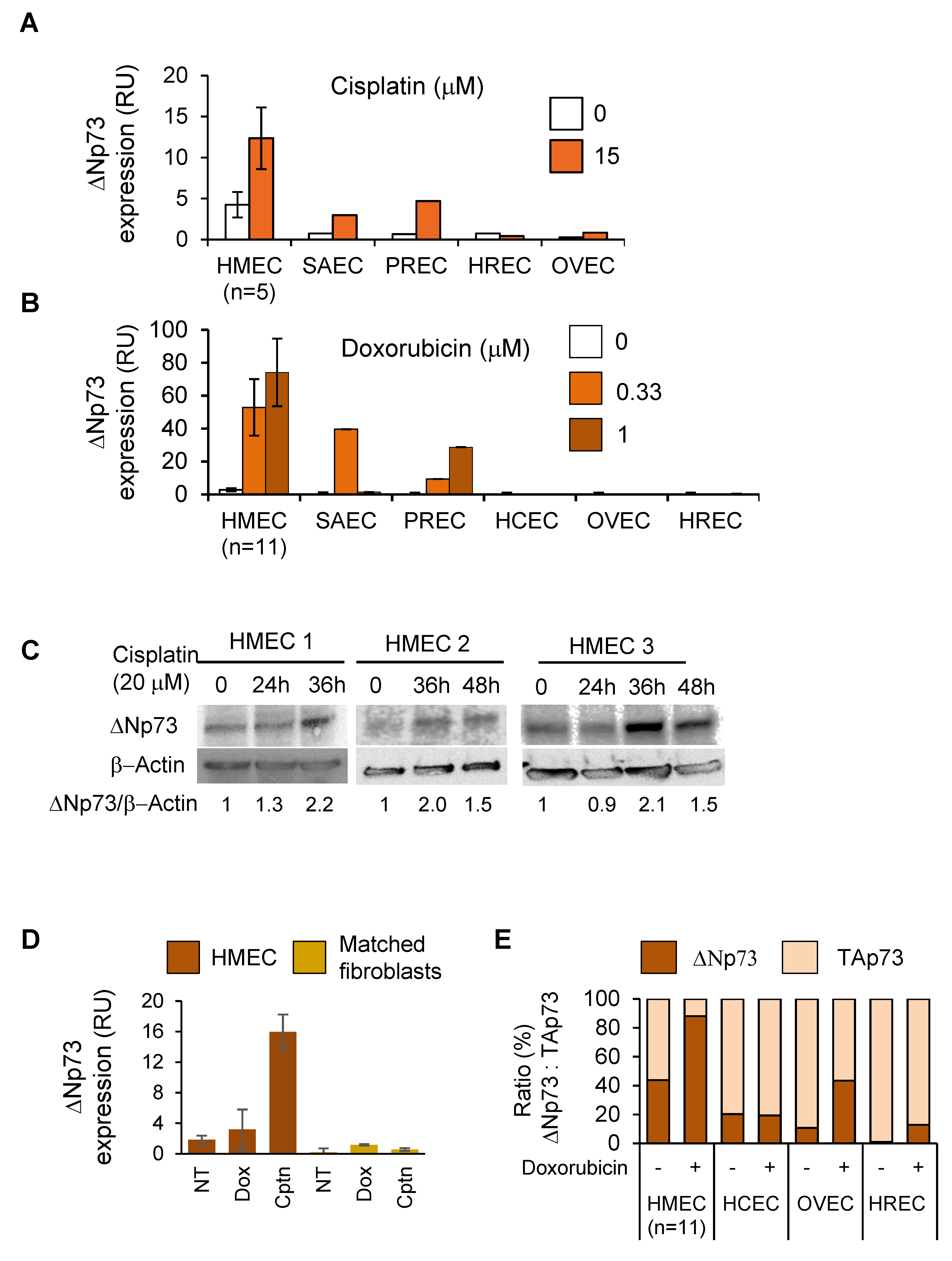
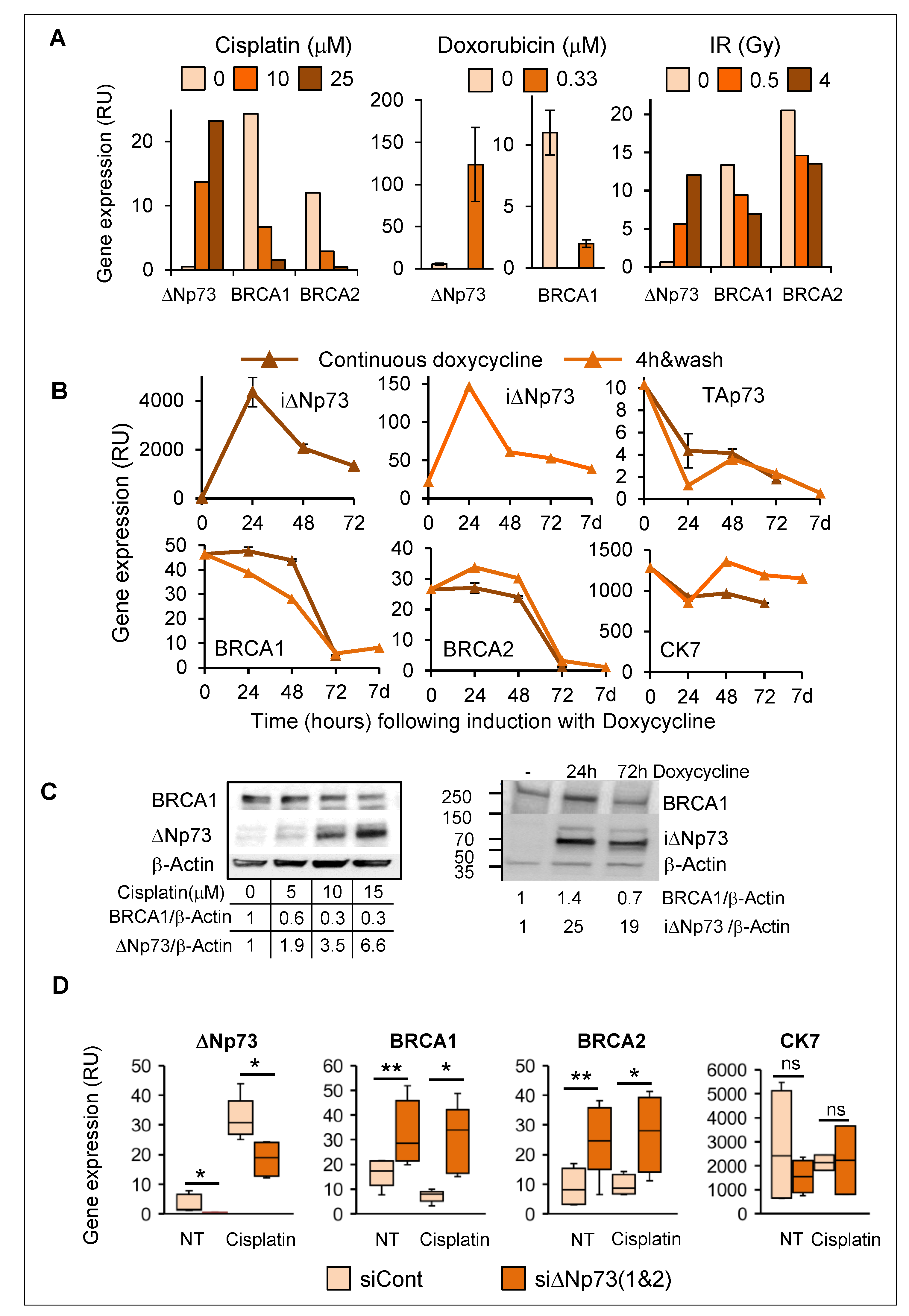
2.2. DeltaNp73 Expression Was Markedly Induced by DNA Damage in Normal HMECs but Not in Other Epithelial Cell Types
2.3. DeltaNp73 Protected HMECs from Cell Death Following Exposure to Cisplatin
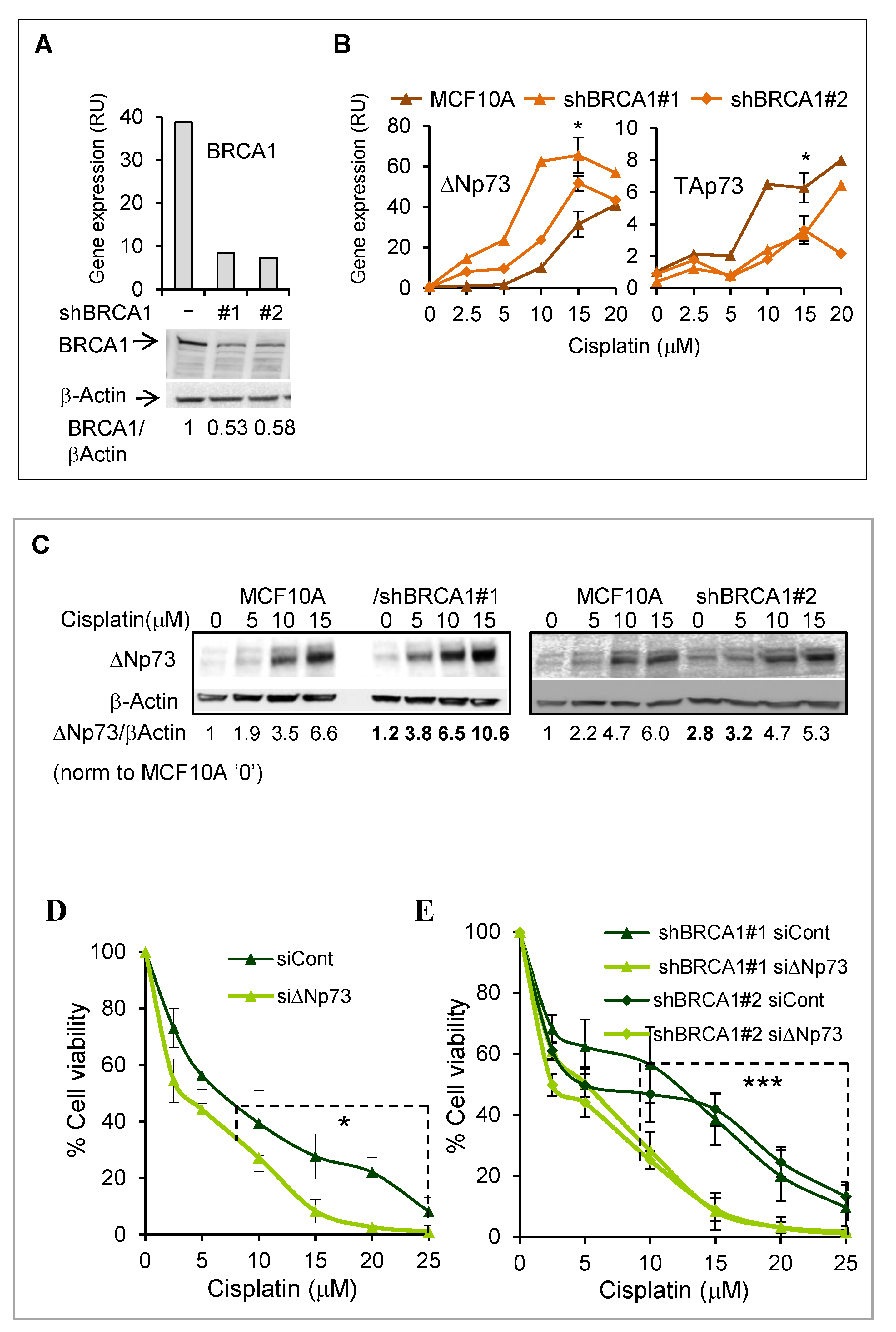
2.4. DeltaNp73 Expression and Antiapoptotic Activity Following DNA Damage Were Augmented in BRCA1 Knocked-Down MCF10A Cells
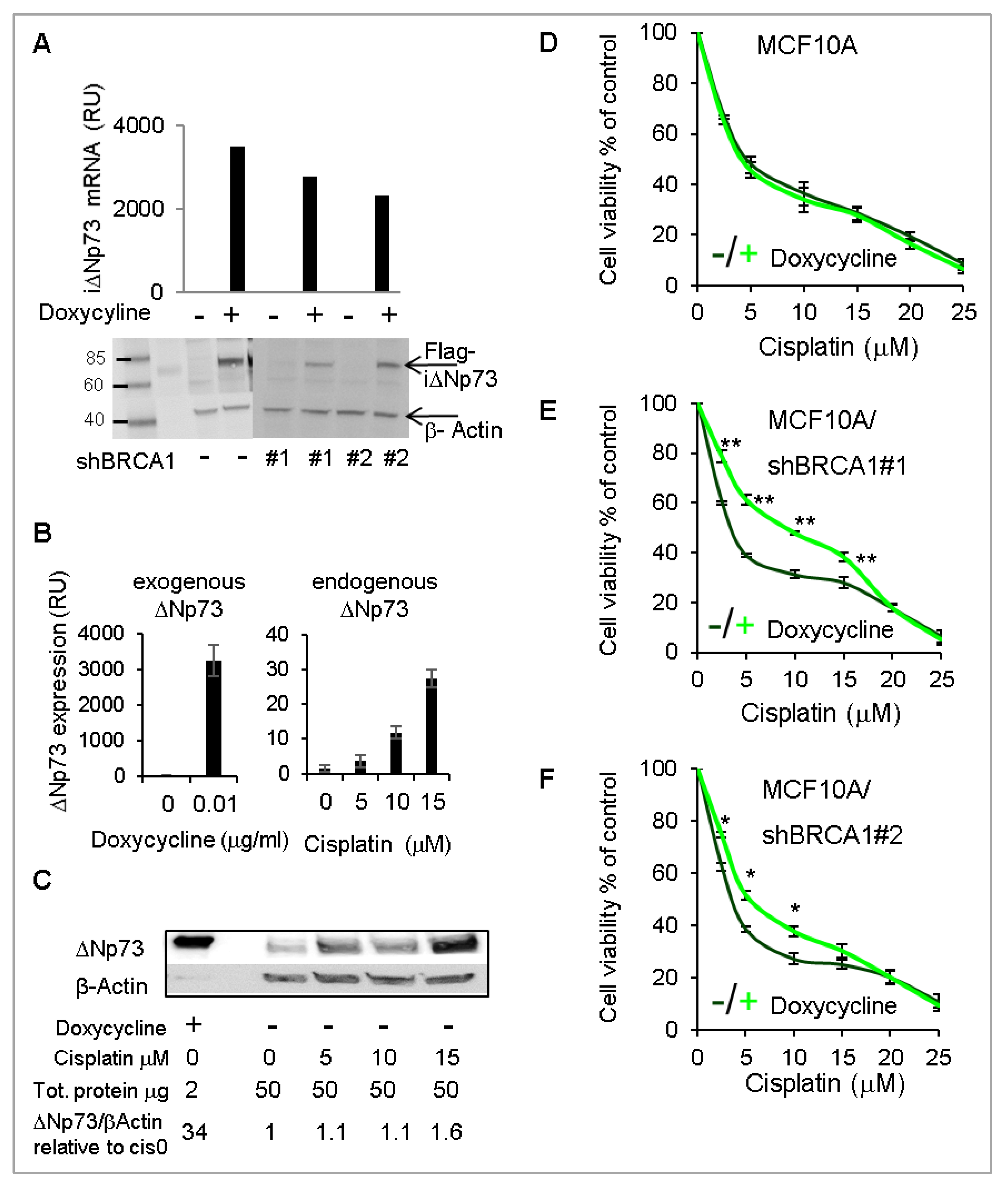
2.5. Overexpression of DeltaNp73 Protected BRCA1-Deficient MCF10A Cells from DNA-Damage-Induced Cell Death
2.6. Induction of DeltaNp73 by DNA Damage Was Associated with Down-Regulation of BRCA1 and BRCA2 in Breast Epithelial Cells
2.7. Hypermethylation of the DeltaNp73 Promoter in Breast Cancers
3. Discussion
4. Materials and Methods
4.1. Primary Tissues, Primary Cells, and Cell Lines
4.2. DNA Methylation
4.3. Quantitative RT-PCR
4.4. siRNA/shRNA Knockdown
4.5. DeltaNp73 Overexpression
4.6. Western Blot and Immunoprecipitation
4.7. Cell Viability
4.8. TCGA Data Analysis
4.9. Statistical Analysis
4.10. Ethical Approval
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levy-Lahad, E.; Friedman, E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br. J. Cancer 2007, 96, 11–16. [Google Scholar] [CrossRef]
- Venkitaraman, A.R. Cancer suppression by the chromosome custodians, BRCA1 and BRCA2. Science 2014, 343, 1470–1475. [Google Scholar] [CrossRef]
- Monteiro, A.N. BRCA1: The enigma of tissue-specific tumor development. Trends. Genet. 2003, 19, 312–317. [Google Scholar] [CrossRef]
- Sedic, M.; Kuperwasser, C. BRCA1-hapoinsufficiency: Unraveling the molecular and cellular basis for tissue-specific cancer. Cell. Cycle 2016, 15, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Venkitaraman, A.R. Linking the cellular functions of BRCA genes to cancer pathogenesis and treatment. Annu. Rev. Pathol. 2009, 4, 4461–4487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, R. BRCA1-Dependent Transcriptional Regulation: Implication in Tissue-Specific Tumor Suppression. Cancers 2018, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Sasanuma, H.; Tsuda, M.; Morimoto, S.; Saha, L.K.; Rahman, M.M.; Kiyooka, Y.; Fujiike, H.; Cherniack, A.D.; Itou, J.; Moreu, E.C.; et al. BRCA1 ensures genome integrity by eliminating estrogen-induced pathological topoisomerase II-DNA complexes. Proc. Natl. Acad. Sci. USA 2018, 115, E10642–E10651. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.I.; Matchett, K.B.; Barros, E.M.; Cooper, K.M.; Irwin, G.W.; Gorski, J.J.; Orr, K.S.; Vohhdina, J.; Kavanagh, J.N.; Madden, A.F.; et al. BRCA1 deficiency exacerbates estrogen-induced DNA damage and genomic instability. Cancer. Res. 2014, 74, 2773–2784. [Google Scholar] [CrossRef]
- Elledge, S.J.; Amon, A. The BRCA1 suppressor hypothesis: An explanation for the tissue-specific tumor development in BRCA1 patients. Cancer. Cell 2002, 1, 129–132. [Google Scholar] [CrossRef]
- Konishi, H.; Mohseni, M.; Tamaki, A.; Garay, J.P.; Croessmann, S.; Karnan, S.; Ota, A.; Wong, H.Y.; Konishi, Y.; Karakas, B.; et al. Mutation of a single allele of the cancer susceptibility gene BRCA1 leads to genomic instability in human breast epithelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17773–17778. [Google Scholar] [CrossRef]
- Pathania, S.; Bade, S.; Le Guillou, M.; Burke, K.; Reed, R.; Bowman-Colin, C.; Su, Y.; Ting, D.T.; Polyak, K.; Richardson, A.L.; et al. BRCA1 haploinsufficiency for replication stress suppression in primary cells. Nat. Commun. 2014, 5, 5496. [Google Scholar] [CrossRef] [PubMed]
- Sedic, M.; Skibinski, A.; Brown, N.; Gallardo, M.; Mulligan, P.; Martinez, P.; Keller, P.J.; Glover, E.; Richardson, A.L.; Cowan, J.; et al. Haploinsufficiency for BRCA1 leads to cell-type-specific genomic instability and premature senescence. Nat. Commun. 2015, 6, 7505. [Google Scholar] [CrossRef] [PubMed]
- Dor, Y.; Cedar, H. Principles of DNA methylation and their implications for biology and medicine. Lancet 2018, 392, 777–786. [Google Scholar] [CrossRef]
- Avraham, A.; Cho, S.S.; Uhlmann, R.; Polak, M.L.; Sandbank, J.; Karni, T.; Pappo, I.; Halperin, R.; Vaknin, Z.; Sella, A.; et al. Tissue specific DNA methylation in normal human breast epithelium and in breast cancer. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Stiewe, T. The p53 family in differentiation and tumorigenesis. Nat. Rev. Cancer 2007, 7, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Stiewe, T.; Zimmermann, S.; Frilling, A.; Esche, H.; Putzer, B.M. Transactivation-deficient DeltaTA-p73 acts as an oncogene. Cancer. Res. 2002, 62, 3598–3602. [Google Scholar]
- Tomasini, R.; Tsuchihara, K.; Wilhelm, M.; Fujitani, M.; Rufini, A.; Cheung, C.C.; Khan, F.; Itie-Youten, A.; Wakeham, A.; Tsao, M.S.; et al. TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes. Dev. 2008, 22, 2677–2691. [Google Scholar] [CrossRef] [PubMed]
- Tomasini, R.; Tsuchihara, K.; Tsuda, C.; Lau, S.K.; Wilhelm, M.; Ruffini, A.; Tsao, M.S.; Iovanna, J.L.; Jurisicova, A.; Melino, G.; et al. TAp73 regulates the spindle assembly checkpoint by modulating BubR1 activity. Proc. Natl. Acad. Sci. USA 2009, 106, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Zaika, A.I.; Slade, N.; Erster, S.H.; Sansome, C.; Joseph, T.W.; Pearl, M.; Chalas, E.; Moll, U.M. DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J. Exp. Med. 2002, 196, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Vernersson-Lindahl, E.; Mills, A.A. DeltaNp73beta puts the brakes on DNA repair. Genes 2010, 24, 517–520. [Google Scholar] [CrossRef]
- Emmrich, S.; Wang, W.; John, K.; Li, W.; Putzer, B.M. Antisense gapmers selectively suppress individual oncogenic p73 splice isoforms and inhibit tumor growth in vivo. Mol. Cancer 2009, 8, 61. [Google Scholar] [CrossRef]
- Wilhelm, M.T.; Rufini, A.; Wetzel, M.K.; Tsuchihara, K.; Inoue, S.; Tomasini, R.; Itie-Youten, A.; Wakeham, A.; Arsenian-Henriksson, M.; Melino, G.; et al. Isoform-specific p73 knockout mice reveal a novel role for delta Np73 in the DNA damage response pathway. Genes 2010, 24, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, D.; Meier, C.; Alla, V.; Putzer, B.M. A balancing act: Orchestrating amino-truncated and full-length p73 variants as decisive factors in cancer progression. Oncogene 2015, 34, 4287–4299. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffery, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Danaei, G.; Vander Hoorn, S.; Lopez, A.D.; Murray, C.J.; Ezzati, M. Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005, 366, 1784–1793. [Google Scholar] [CrossRef]
- Fearon, E.R. Human cancer syndromes: Clues to the origin and nature of cancer. Science 1997, 278, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Vogelstein, B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef]
- Klutstein, M.; Moss, J.; Kaplan, T.; Cedar, H. Contribution of epigenetic mechanisms to variation in cancer risk among tissues. Proc. Natl. Acad. Sci. USA 2017, 114, 2230–2234. [Google Scholar] [CrossRef]
- Chao, E.C.; Lipkin, S.M. Molecular models for the tissue specificity of DNA mismatch repair-deficient carcinogenesis. Nucleic. Acids. Res. 2006, 34, 840–852. [Google Scholar] [CrossRef]
- Petrenko, O.; Zaika, A.; Moll, U.M. deltaNp73 facilitates cell immortalization and cooperates with oncogenic Ras in cellular transformation in vivo. Mol. Cell. Biol. 2003, 23, 5540–5555. [Google Scholar] [CrossRef]
- Cao, L.; Xu, X.; Bunting, S.F.; Liu, J.; Wang, R.H.; Cao, L.L.; Wu, J.J.; Peng, T.N.; Chen, J.; Nussenzweig, A.; et al. A selective requirement for 53BP1 in the biological response to genomic instability induced by BRCA1 deficiency. Mol. Cell 2009, 35, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Bunting, S.F.; Callen, E.; Wong, N.; Chen, H.T.; Polato, F.; Gunn, A.; Bothmer, A.; Feldhahn, N.; Fernandez-Capetillo, O.; Cao, L.; et al. 53BP1 inhibits homologous recombination in BRCA1-deficient cells by blocking resection of DNA breaks. Cell 2010, 141, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ning, S.; Wei, Z.; Xu, R.; Xu, X.; Xing, M.; Guo, R.; Xu, D. 53BP1 and BRCA1 control pathway choice for stalled replication restart. Elife 2017, 6, e30523. [Google Scholar] [CrossRef] [PubMed]
- Grob, T.J.; Novak, U.; Maisse, C.; Barcaroli, D.; Lüthi, A.U.; Pirnia, F.; Hügli, B.; Graber, H.U.; De Laurenzi, V.; Fey, M.F.; et al. Human delta Np73 regulates a dominant negative feedback loop for TAp73 and p53. Cell. Death. Differ. 2001, 8, 1213–1223. [Google Scholar] [CrossRef]
- Arizti, P.; Fang, L.; Park, I.; Yin, Y.; Solomon, E.; Ouchi, T.; Aaronson, S.A.; Lee, S.W. Tumor suppressor p53 is required to modulate BRCA1 expression. Mol. Cell. Biol. 2000, 20, 7450–7459. [Google Scholar] [CrossRef]
- MacLachlan, T.K.; Dash, B.C.; Dicker, D.T.; El-Deiry, W.S. Repression of BRCA1 through a feedback loop involving p53. J. Biol. Chem. 2000, 275, 31869–31875. [Google Scholar] [CrossRef]
- Tan, S.L.W.; Chadha, S.; Liu, Y.; Gabasova, E.; Perera, D.; Ahmed, K.; Constantinou, S.; Renaudin, X.; Lee, M.Y.; Aberesold, R.; et al. A Class of Environmental and Endogenous Toxins Induces BRCA2 Haploinsufficiency and Genome Instability. Cell 2017, 169, 1105–1118. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Ladd-Acosta, C.; Wen, B.; Wu, Z.; Montano, C.; Onyango, P.; Cui, H.; Gabo, K.; Rongione, M.; Webster, M.; et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat. Genet. 2009, 41, 178–186. [Google Scholar] [CrossRef]
- Gomez, L.C.; Sottile, M.L.; Guerrero-Gimenez, M.E.; Zoppino, F.C.M.; Redondo, A.L.; Gago, F.E.; Orozco, J.I.; Tello, O.M.; Roqué, M.; Nadin, B.S.; et al. TP73 DNA methylation and upregulation of DeltaNp73 are associated with an adverse prognosis in breast cancer. J. Clin. Pathol. 2018, 71, 52–58. [Google Scholar] [CrossRef]
- Labarge, M.A.; Garbe, J.C.; Stampfer, M.R. Processing of human reduction mammoplasty and mastectomy tissues for cell culture. J. Vis. Exp. 2013. [Google Scholar] [CrossRef]
- Shepherd, T.G.; Theriault, B.L.; Campbell, E.J.; Nachtigal, M.W. Primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients. Nat. Protoc. 2006, 1, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Kutner, R.H.; Zhang, X.Y.; Reiser, J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 2009, 4, 495–505. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avraham, A.; Feldman, S.; Cho, S.S.; Kol, A.; Heler, L.; Riklin-Nahmias, E.; Sella, A.; Karni, T.; Allweis, T.M.; Sukumar, S.; et al. Breast-Specific Epigenetic Regulation of DeltaNp73 and Its Role in DNA-Damage-Response of BRCA1-Mutated Human Mammary Epithelial Cells. Cancers 2020, 12, 2367. https://doi.org/10.3390/cancers12092367
Avraham A, Feldman S, Cho SS, Kol A, Heler L, Riklin-Nahmias E, Sella A, Karni T, Allweis TM, Sukumar S, et al. Breast-Specific Epigenetic Regulation of DeltaNp73 and Its Role in DNA-Damage-Response of BRCA1-Mutated Human Mammary Epithelial Cells. Cancers. 2020; 12(9):2367. https://doi.org/10.3390/cancers12092367
Chicago/Turabian StyleAvraham, Ayelet, Susanna Feldman, Sean Soonweng Cho, Ayala Kol, Lior Heler, Emmanuela Riklin-Nahmias, Avishay Sella, Tamar Karni, Tanir M. Allweis, Saraswati Sukumar, and et al. 2020. "Breast-Specific Epigenetic Regulation of DeltaNp73 and Its Role in DNA-Damage-Response of BRCA1-Mutated Human Mammary Epithelial Cells" Cancers 12, no. 9: 2367. https://doi.org/10.3390/cancers12092367
APA StyleAvraham, A., Feldman, S., Cho, S. S., Kol, A., Heler, L., Riklin-Nahmias, E., Sella, A., Karni, T., Allweis, T. M., Sukumar, S., & Evron, E. (2020). Breast-Specific Epigenetic Regulation of DeltaNp73 and Its Role in DNA-Damage-Response of BRCA1-Mutated Human Mammary Epithelial Cells. Cancers, 12(9), 2367. https://doi.org/10.3390/cancers12092367





