Nitric Oxide (NO) and NO Synthases (NOS)-Based Targeted Therapy for Colon Cancer
Abstract
1. Introduction
2. Paradox Criteria of NO in Cancers
3. Biphasic Role of NO in Cancers
4. Role of NOS in Colon Carcinogenesis
4.1. nNOS/NOS1
4.2. iNOS/NOS2
4.3. eNOS/NOS3
5. NOS Inhibitors: Targeting NOS in Colon Cancer
5.1. NOS Inhibitors from Natural Extracts
5.1.1. Celastrol (Tripterine)
5.1.2. Maqui Berry (MB) Extracts
5.1.3. Cannabidiol (CBD)
5.1.4. Phaleria Macrocarpa
5.1.5. All-Trans Retinoic Acid (AtRA)
5.1.6. Dietary Polyphenol Ellagic Acid
5.2. Synthesized NOS Inhibitors
5.2.1. 1400 W and L-NIO
5.2.2. Se,Se’-1,4-Phenylenebis(1,2-Ethanediyl)Bis-Isoselenourea (PBISe)
5.2.3. S,S9-1,4-Phenylene-Bis(1,2-Ethanediyl)Bis-Isothiourea (PBIT)/L-N6-(1-Iminoethyl)Lysinetetrazole-Amide (SC-51)
5.3. NOS Inhibitors from Nutraceuticals
5.3.1. Nobiletin and Its Colonic Metabolites (NOB-Met)
5.3.2. Omega-3 Fatty Acid Docosahexaenoic Acid (DHA)
6. Traditional and Innovative NO Donor-Based Therapy
6.1. Nitric-Oxide-Donating Nonsteroidal Anti-Inflammatory Drugs (NO-NSAIDs) in Cancers
6.2. NO-NSAIDs in CRC
6.3. NO- and H2S-Releasing NSAIDs in CRC
6.4. Nitric Oxide Donor Doxorubicin (NO-DOXOs)
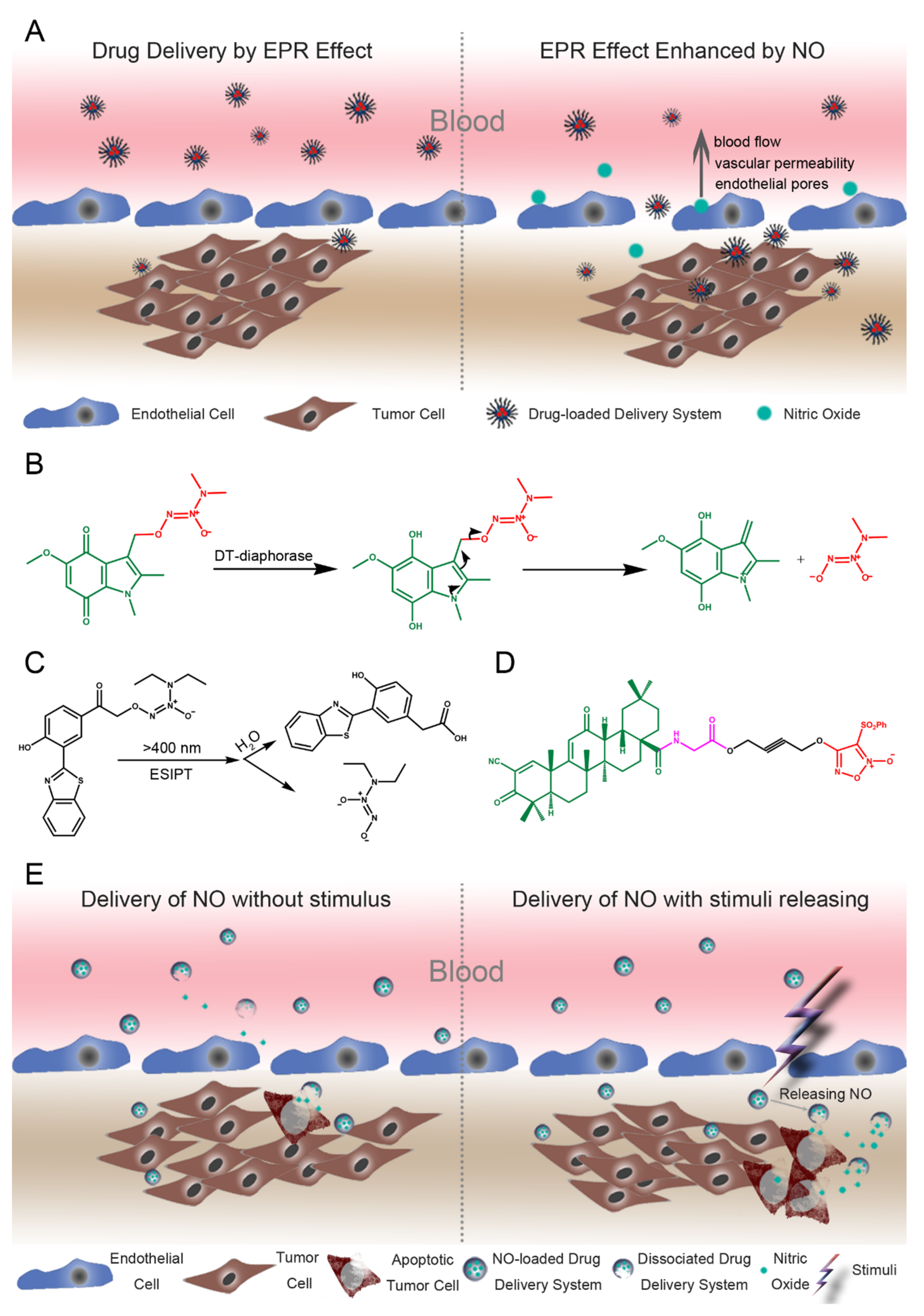
7. Application and Delivery of Nitric Oxide for Colon Cancer
7.1. Augmented EPR Effects by the NO Drug Delivery System
7.2. Delivery of NO for Targeted Cancer Therapy
8. Clinical Manifestation of NO/NOS in Colon Cancer
9. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Cho, K.B.; Li, Y.; Tao, G.; Xie, Z.; Guo, B. Long Noncoding RNA (lncRNA)-Mediated competing endogenous rna networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int. J. Mol. Sci. 2019, 20, 5758. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. Ca Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, M.; Ohta, Y.; Nishikori, S.; Matano, M.; Takano, A.; Fujii, M.; Date, S.; Sugimoto, S.; Kanai, T.; Sato, T. Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature 2017, 545, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Fang, J.; Liao, L.; Maeda, H.; Su, Q. Upregulation of heme oxygenase-1 in colorectal cancer patients with increased circulation carbon monoxide levels, potentially affects chemotherapeutic sensitivity. Bmc Cancer 2014, 14, 436. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.N.; Kopetz, E.S. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: Clinical characteristics, clinical behavior, and response to targeted therapies. J. Gastrointest Oncol. 2015, 6, 660–667. [Google Scholar]
- Szabo, C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016, 15, 185–203. [Google Scholar] [CrossRef]
- Mandal, P. Insight of nitric oxide signaling: A potential biomarker with multifaceted complex mechanism in colorectal carcinogenesis. Biochem. Biophys. Res. Commun. 2018, 495, 1766–1768. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric oxide donor-based cancer therapy: Advances and prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- De Oliveira, G.A.; Cheng, R.Y.S.; Ridnour, L.A.; Basudhar, D.; Somasundaram, V.; McVicar, D.W.; Monteiro, H.P.; Wink, D.A. Inducible Nitric Oxide Synthase in the carcinogenesis of gastrointestinal cancers. Antioxid. Redox Signal. 2017, 26, 1059–1077. [Google Scholar] [CrossRef]
- Rabender, C.S.; Alam, A.; Sundaresan, G.; Cardnell, R.J.; Yakovlev, V.A.; Mukhopadhyay, N.D.; Graves, P.; Zweit, J.; Mikkelsen, R.B. The Role of nitric oxide synthase uncoupling in tumor progression. Mol. Cancer. Res. 2015, 13, 1034–1043. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, L.; Mollica, M.; Re, A.T.; Wu, S.; Zuo, L. Nitric oxide in cancer metastasis. Cancer Lett. 2014, 353, 1–7. [Google Scholar] [CrossRef]
- Salimian Rizi, B.; Achreja, A.; Nagrath, D. Nitric oxide: The forgotten child of tumor metabolism. Trends Cancer 2017, 3, 659–672. [Google Scholar] [CrossRef]
- Pervin, S.; Singh, R.; Sen, S.; Chaudhuri, G. Dual Role of nitric oxide in cancer biology. Nitric Oxide (No) Cancer 2010, 8, 39–57. [Google Scholar]
- Vahora, H.; Khan, M.A.; Alalami, U.; Hussain, A. The potential role of nitric oxide in halting cancer progression through chemoprevention. J. Cancer Prev. 2016, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Ridnour, L.A.; Isenberg, J.S.; Flores-Santana, W.; Switzer, C.H.; Donzelli, S.; Hussain, P.; Vecoli, C.; Paolocci, N.; Ambs, S.; et al. The chemical biology of nitric oxide: Implications in cellular signaling. Free Radic. Biol. Med. 2008, 45, 18–31. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.K. Nitric oxide: Role in tumour biology and iNOS/NO-based anticancer therapies. Cancer Chemother Pharm. 2011, 67, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Prueitt, R.L.; Boersma, B.J.; Howe, T.M.; Goodman, J.E.; Thomas, D.D.; Ying, L.; Pfiester, C.M.; Yfantis, H.G.; Cottrell, J.R.; Lee, D.H.; et al. Inflammation and IGF-I activate the Akt pathway in breast cancer. Int. J. Cancer 2007, 120, 796–805. [Google Scholar] [CrossRef]
- Pervin, S.; Singh, R.; Hernandez, E.; Wu, G.; Chaudhuri, G. Nitric oxide in physiologic concentrations targets the translational machinery to increase the proliferation of human breast cancer cells: Involvement of mammalian target of rapamycin/eIF4E pathway. Cancer Res. 2007, 67, 289–299. [Google Scholar] [CrossRef]
- Thomas, D.D.; Ridnour, L.A.; Espey, M.G.; Donzelli, S.; Ambs, S.; Hussain, S.P.; Harris, C.C.; DeGraff, W.; Roberts, D.D.; Mitchell, J.B.; et al. Superoxide fluxes limit nitric oxide-induced signaling. J. Biol. Chem. 2006, 281, 25984–25993. [Google Scholar] [CrossRef]
- Thomas, D.D.; Espey, M.G.; Ridnour, L.A.; Hofseth, L.J.; Mancardi, D.; Harris, C.C.; Wink, D.A. Hypoxic inducible factor 1α, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc. Natl. Acad. Sci. USA 2004, 101, 8894–8899. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Ridnour, L.A.; Perruccio, E.M.; Espey, M.G.; Wink, D.A.; Roberts, D.D. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc. Natl. Acad. Sci. USA 2005, 102, 13141–13146. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Smith, R.S., Jr.; Hsieh, C.M.; Sun, J.; Chao, J.; Liao, J.K. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol. Cell. Biol. 2003, 23, 5726–5737. [Google Scholar] [CrossRef] [PubMed]
- Olson, N.; Van Der Vliet, A. Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease. Nitric Oxide 2011, 25, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Amstad, P.; Raja, K.; Ambs, S.; Nagashima, M.; Bennett, W.P.; Shields, P.G.; Ham, A.-J.; Swenberg, J.A.; Marrogi, A.J. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: A cancer-prone chronic inflammatory disease. Cancer Res. 2000, 60, 3333–3337. [Google Scholar]
- Rao, C.V.; Kawamori, T.; Hamid, R.; Reddy, B.S. Chemoprevention of colonic aberrant crypt foci by an inducible nitric oxide synthase-selective inhibitor. Carcinogenesis 1999, 20, 641–644. [Google Scholar] [CrossRef]
- Ambs, S.; Merriam, W.G.; Bennett, W.P.; Felley-Bosco, E.; Ogunfusika, M.O.; Oser, S.M.; Klein, S.; Shields, P.G.; Billiar, T.R.; Harris, C.C. Frequent nitric oxide synthase-2 expression in human colon adenomas: Implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998, 58, 334–341. [Google Scholar]
- Mandal, P. Molecular signature of nitric oxide on major cancer hallmarks of colorectal carcinoma. Inflammopharmacology 2018, 26, 331–336. [Google Scholar] [CrossRef]
- Arias-Salvatierra, D.; Silbergeld, E.K.; Acosta-Saavedra, L.C.; Calderon-Aranda, E.S. Role of nitric oxide produced by iNOS through NF-kappaB pathway in migration of cerebellar granule neurons induced by Lipopolysaccharide. Cell Signal. 2011, 23, 425–435. [Google Scholar] [CrossRef]
- Hickok, J.R.; Thomas, D.D. Nitric oxide and cancer therapy: The emperor has NO clothes. Curr. Pharm. Des. 2010, 16, 381–391. [Google Scholar] [CrossRef]
- Rao, C.V. Nitric oxide signaling in colon cancer chemoprevention. Mutat. Res. 2004, 555, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Liaudet, L.; Soriano, F.G.; Szabó, C. Biology of nitric oxide signaling. Crit. Care Med. 2000, 28, N37–N52. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Xie, Q.-W. Nitric oxide synthases: Roles, tolls, and controls. Cell 1994, 78, 915–918. [Google Scholar] [CrossRef]
- Babykutty, S.; Suboj, P.; Srinivas, P.; Nair, A.S.; Chandramohan, K.; Gopala, S. Insidious role of nitric oxide in migration/invasion of colon cancer cells by upregulating MMP-2/9 via activation of cGMP-PKG-ERK signaling pathways. Clin. Exp. Metastasis 2012, 29, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Módis, K.; Coletta, C.; Chao, C.; Papapetropoulos, A.; Hellmich, M.; Szabo, C. P35 Cystathionine-β-synthase (CBS)-derived hydrogen sulfide (H2S) supports cellular bioenergetics in colon cancer cells in vitro. Nitric Oxide 2013, 31, S50. [Google Scholar] [CrossRef]
- Alimoradi, H.; Greish, K.; Gamble, A.B.; Giles, G.I. Controlled delivery of nitric oxide for cancer therapy. Pharm. Nanotechnol. 2019, 7, 279–303. [Google Scholar] [CrossRef]
- Seabra, A.B.; Duran, N. Nitric oxide donors for prostate and bladder cancers: Current state and challenges. Eur. J. Pharm. 2018, 826, 158–168. [Google Scholar] [CrossRef]
- Burke, A.J.; Sullivan, F.J.; Giles, F.J.; Glynn, S.A. The yin and yang of nitric oxide in cancer progression. Carcinogenesis 2013, 34, 503–512. [Google Scholar] [CrossRef]
- Sonveaux, P.; Jordan, B.F.; Gallez, B.; Feron, O. Nitric oxide delivery to cancer: Why and how? Eur. J. Cancer 2009, 45, 1352–1369. [Google Scholar] [CrossRef]
- Coulter, J.A.; McCarthy, H.O.; Xiang, J.; Roedl, W.; Wagner, E.; Robson, T.; Hirst, D.G. Nitric oxide--a novel therapeutic for cancer. Nitric Oxide 2008, 19, 192–198. [Google Scholar] [CrossRef]
- Bonavida, B.; Khineche, S.; Huerta-Yepez, S.; Garbán, H. Therapeutic potential of nitric oxide in cancer. Drug Resist. Updates 2006, 9, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, L.; Zhang, Q.; Yang, X.; Zou, Z.; Hao, B.; Marincola, F.M.; Liu, Z.; Zhong, Z.; Wang, M.; et al. NOS1 S-nitrosylates PTEN and inhibits autophagy in nasopharyngeal carcinoma cells. Cell Death Discov. 2017, 3, 17011. [Google Scholar] [CrossRef] [PubMed]
- Augsten, M.; Sjoberg, E.; Frings, O.; Vorrink, S.U.; Frijhoff, J.; Olsson, E.; Borg, A.; Ostman, A. Cancer-associated fibroblasts expressing CXCL14 rely upon NOS1-derived nitric oxide signaling for their tumor-supporting properties. Cancer Res. 2014, 74, 2999–3010. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, V.; Basudhar, D.; Bharadwaj, G.; No, J.H.; Ridnour, L.A.; Cheng, R.Y.S.; Fujita, M.; Thomas, D.D.; Anderson, S.K.; McVicar, D.W.; et al. Molecular mechanisms of nitric oxide in cancer progression, signal transduction, and metabolism. Antioxid. Redox Signal. 2019, 30, 1124–1143. [Google Scholar] [CrossRef]
- Vecchini, F.; Pringault, E.; Billiar, T.R.; Geller, D.A.; Hausel, P.; Felley-Bosco, E. Decreased activity of inducible nitric oxide synthase type 2 and modulation of the expression of glutathione S-transferase alpha, bcl-2, and metallothioneins during the differentiation of CaCo-2 cells. Cell Growth Differ. 1997, 8, 261–268. [Google Scholar]
- Penarando, J.; Lopez-Sanchez, L.M.; Mena, R.; Guil-Luna, S.; Conde, F.; Hernandez, V.; Toledano, M.; Gudino, V.; Raponi, M.; Billard, C.; et al. A role for endothelial nitric oxide synthase in intestinal stem cell proliferation and mesenchymal colorectal cancer. Bmc Biol. 2018, 16, 3. [Google Scholar] [CrossRef]
- Jess, T.; Rungoe, C.; Peyrin-Biroulet, L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef]
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric oxide in inflammatory bowel disease: A universal messenger in an unsolved puzzle. Immunology 2004, 113, 427–437. [Google Scholar] [CrossRef]
- Erdman, S.; Rao, V.; Poutahidis, T.; Rogers, A.; Taylor, C.; Jackson, E.; Ge, Z.; Lee, C.; Schauer, D.; Wogan, G. Nitric oxide and TNF-α trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc. Natl. Acad. Sci. USA 2009, 106, 1027–1032. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, A.; Urbanski, S.J.; McCafferty, D.-M. Induction of inducible nitric oxide synthase: A protective mechanism in colitis-induced adenocarcinoma. Carcinogenesis 2007, 28, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.P.; Pretlow, T.G.; Rao, J.S.; Pretlow, T.P. Inducible nitric oxide synthase (iNOS) is expressed similarly in multiple aberrant crypt foci and colorectal tumors from the same patients. Cancer Res. 2001, 61, 419–422. [Google Scholar]
- McCafferty, D.-M.; Sihota, E.; Muscara, M.; Wallace, J.L.; Sharkey, K.A.; Kubes, P. Spontaneously developing chronic colitis in IL-10/iNOS double-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G90–G99. [Google Scholar] [CrossRef] [PubMed]
- Gochman, E.; Mahajna, J.; Shenzer, P.; Dahan, A.; Blatt, A.; Elyakim, R.; Reznick, A.Z. The expression of iNOS and nitrotyrosine in colitis and colon cancer in humans. Acta Histochem. 2012, 114, 827–835. [Google Scholar] [CrossRef]
- Sun, M.-H.; Han, X.-C.; Jia, M.-K.; Jiang, W.-D.; Wang, M.; Zhang, H.; Han, G.; Jiang, Y. Expressions of inducible nitric oxide synthase and matrix metalloproteinase-9 and their effects on angiogenesis and progression of hepatocellular carcinoma. World J. Gastroenterol. Wjg 2005, 11, 5931. [Google Scholar] [CrossRef]
- Cianchi, F.; Cortesini, C.; Fantappiè, O.; Messerini, L.; Sardi, I.; Lasagna, N.; Perna, F.; Fabbroni, V.; Di Felice, A.; Perigli, G. Cyclooxygenase-2 activation mediates the proangiogenic effect of nitric oxide in colorectal cancer. Clin. Cancer Res. 2004, 10, 2694–2704. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Ancrile, B.B.; Kashatus, D.F.; Counter, C.M. Tumour maintenance is mediated by eNOS. Nature 2008, 452, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- Jadeski, L.C.; Hum, K.O.; Chakraborty, C.; Lala, P.K. Nitric oxide promotes murine mammary tumour growth and metastasis by stimulating tumour cell migration, invasiveness and angiogenesis. Int. J. Cancer 2000, 86, 30–39. [Google Scholar] [CrossRef]
- Takahashi, M.; Fukuda, K.; Ohata, T.; Sugimura, T.; Wakabayashi, K. Increased expression of inducible and endothelial constitutive nitric oxide synthases in rat colon tumors induced by azoxymethane. Cancer Res. 1997, 57, 1233–1237. [Google Scholar]
- Yu, S.; Jia, L.; Zhang, Y.; Wu, D.; Xu, Z.; Ng, C.F.; To, K.K.; Huang, Y.; Chan, F.L. Increased expression of activated endothelial nitric oxide synthase contributes to antiandrogen resistance in prostate cancer cells by suppressing androgen receptor transactivation. Cancer Lett. 2013, 328, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Hofseth, L.J. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007, 67, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, G.G.; Yao, J.C.; Gong, W.; Wei, D.; Wu, T.T.; Ajani, J.A.; Huang, S.; Xie, K. Expression of endothelial nitric oxide synthase correlates with the angiogenic phenotype of and predicts poor prognosis in human gastric cancer. Gastric Cancer 2005, 8, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, S.; Xu, Y.; Sheng, S.; Qian, S.Y.; Huo, X. Nitric oxide synthase inhibitors 1400W and L-NIO inhibit angiogenesis pathway of colorectal cancer. Nitric Oxide 2019, 83, 33–39. [Google Scholar] [CrossRef]
- Thomsen, L.L.; Scott, J.M.; Topley, P.; Knowles, R.G.; Keerie, A.-J.; Frend, A.J. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: Studies with 1400W, a novel inhibitor. Cancer Res. 1997, 57, 3300–3304. [Google Scholar] [PubMed]
- Gao, Y.; Zhou, S.; Pang, L.; Yang, J.; Li, H.J.; Huo, X.; Qian, S.Y. Celastrol suppresses nitric oxide synthases and the angiogenesis pathway in colorectal cancer. Free Radic. Res. 2019, 53, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Céspedes-Acuña, C.L.; Xiao, J.; Wei, Z.-J.; Chen, L.; Bastias, J.M.; Avila, J.G.; Alarcon-Enos, J.; Werner-Navarrete, E.; Kubo, I. Antioxidant and anti-inflammatory effects of extracts from Maqui berry Aristotelia chilensis in human colon cancer cells. J. Berry Res. 2018, 8, 275–296. [Google Scholar] [CrossRef]
- Deepa, M.; Sureshkumar, T.; Satheeshkumar, P.K.; Priya, S. Antioxidant rich Morus alba leaf extract induces apoptosis in human colon and breast cancer cells by the downregulation of nitric oxide produced by inducible nitric oxide synthase. Nutr. Cancer 2013, 65, 305–310. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Gao, Z.; Sun, Y.; Wang, M.; Li, F.; Zheng, J.; Xiao, H. Nobiletin and its colonic metabolites suppress colitis-associated colon carcinogenesis by down-regulating iNOS, inducing antioxidative enzymes and arresting cell cycle progression. J. Nutr. Biochem. 2017, 42, 17–25. [Google Scholar] [CrossRef]
- Rafa, H.; Benkhelifa, S.; AitYounes, S.; Saoula, H.; Belhadef, S.; Belkhelfa, M.; Boukercha, A.; Toumi, R.; Soufli, I.; Morales, O.; et al. All-Trans retinoic acid modulates TLR4/NF-kappaB signaling pathway targeting TNF-alpha and nitric oxide synthase 2 expression in colonic mucosa during ulcerative colitis and colitis associated cancer. Mediat. Inflamm. 2017, 2017, 7353252. [Google Scholar] [CrossRef]
- Kong, Z.-L.; Kao, N.-J.; Hu, J.-Y.; Wu, C.-S. Fucoxanthin-rich brown algae extract decreases inflammation and attenuates colitis-associated colon cancer in mice. J. Food Nutr. Res 2016, 4, 137–147. [Google Scholar]
- Heo, S.-J.; Yoon, W.-J.; Kim, K.-N.; Oh, C.; Choi, Y.-U.; Yoon, K.-T.; Kang, D.-H.; Qian, Z.-J.; Choi, I.-W.; Jung, W.-K. Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem. Toxicol. 2012, 50, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, B.A.; Narayanan, N.K.; Desai, D.; Pittman, B.; Reddy, B.S. Effects of a combination of docosahexaenoic acid and 1, 4-phenylene bis (methylene) selenocyanate on cyclooxygenase 2, inducible nitric oxide synthase and β-catenin pathways in colon cancer cells. Carcinogenesis 2004, 25, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, B.A.; Narayanan, N.K.; Simi, B.; Reddy, B.S. Modulation of inducible nitric oxide synthase and related proinflammatory genes by the omega-3 fatty acid docosahexaenoic acid in human colon cancer cells. Cancer Res. 2003, 63, 972–979. [Google Scholar]
- Umesalma, S.; Sudhandiran, G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-κB, iNOS, COX-2, TNF-α, and IL-6 in 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2010, 107, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Harmen, F.; Hanifah, R.S.; Novitarani, N.A.; Tedjo, A.; Azizah, N.N.; Putrianingsih, R.; Fachri, W.; Kusmardi, K. The Inhibition of Ethanol Extract of Phaleria macrocarpa Stem Bark on iNOS Expression of HCT116 Colorectal Cancer Cell Line. J. Pharm. Sci. Res. 2019, 11, 892–895. [Google Scholar]
- Jeong, S.; Kim, B.G.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A. Cannabidiol overcomes oxaliplatin resistance by enhancing NOS3-and SOD2-induced autophagy in human colorectal cancer cells. Cancers 2019, 11, 781. [Google Scholar] [CrossRef]
- Benkhelifa, S.; Rafa, H.; Belhadef, S.; Ait-Kaci, H.; Medjeber, O.; Belkhelfa, M.; Hetit, S.; Ait-Younes, S.; Moralès, O.; Mahfouf, H. Aberrant up-regulation of iNOS/NO system is correlated with an increased abundance of Foxp3+ cells and reduced effector/memory cell markers expression during colorectal cancer: Immunomodulatory effects of cetuximab combined with chemotherapy. Inflammopharmacology 2019, 27, 685–700. [Google Scholar] [CrossRef]
- Ding, Q.-G.; Zang, J.; Gao, S.; Gao, Q.; Duan, W.; Li, X.; Xu, W.; Zhang, Y. Nitric oxide donor hybrid compounds as promising anticancer agents. Drug Discov. 2016, 10, 276–284. [Google Scholar] [CrossRef][Green Version]
- Stevens, E.V.; Carpenter, A.W.; Shin, J.H.; Liu, J.; Der, C.J.; Schoenfisch, M.H. Nitric oxide-releasing silica nanoparticle inhibition of ovarian cancer cell growth. Mol. Pharm. 2010, 7, 775–785. [Google Scholar] [CrossRef][Green Version]
- Fang, L.; Lehmann, J. NO donor hybrid compounds as multifunctional therapeutic agents. Expert Opin. Ther. Pat. 2008, 18, 1111–1125. [Google Scholar] [CrossRef]
- Dunlap, T.; Abdul-Hay, S.O.; Chandrasena, R.E.P.; Hagos, G.K.; Sinha, V.; Wang, Z.; Wang, H.; Thatcher, G.R. Nitrates and NO-NSAIDs in cancer chemoprevention and therapy: In vitro evidence querying the NO donor functionality. Nitric Oxide 2008, 19, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Scatena, R.; Bottoni, P.; Martorana, G.E.; Giardina, B. Nitric oxide donor drugs: An update on pathophysiology and therapeutic potential. Expert Opin. Investig. Drugs 2005, 14, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Tashjian, A.H., Jr.; Voelkel, E.F.; Goldhaber, P.; Levine, L. Successful treatment of hypercalcemia by indomethacin in mice bearing a prostaglandin-producing fibrosarcoma. Prostaglandins 1973, 3, 515–524. [Google Scholar] [CrossRef]
- Din, F.V.; Theodoratou, E.; Farrington, S.M.; Tenesa, A.; Barnetson, R.A.; Cetnarskyj, R.; Stark, L.; Porteous, M.E.; Campbell, H.; Dunlop, M.G. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut 2010, 59, 1670–1679. [Google Scholar] [CrossRef]
- Slattery, M.L.; Samowitz, W.; Hoffman, M.; Ma, K.N.; Levin, T.R.; Neuhausen, S. Aspirin, NSAIDs, and colorectal cancer: Possible involvement in an insulin-related pathway. Cancer Epidemiol. Prev. Biomark. 2004, 13, 538–545. [Google Scholar]
- Rayyan, Y.; Williams, J.; Rigas, B. The role of NSAIDs in the prevention of colon cancer. Cancer Investig. 2002, 20, 1002–1011. [Google Scholar] [CrossRef]
- Gupta, R.A.; DuBois, R.N. Aspirin, NSAIDS, and Colon Cancer Prevention: Mechanisms? Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Ahnen, D.J. Colon cancer prevention by NSAIDs: What is the mechanism of action? Eur. J. Surg. 1998, 164, 111–114. [Google Scholar] [CrossRef]
- Thun, M.J. NSAID use and decreased risk of gastrointestinal cancers. Gastroenterol. Clin. North Am. 1996, 25, 333–348. [Google Scholar] [CrossRef]
- Waddell, W.R.; Gerner, R.E. Indomethacin and ascorbate inhibit desmoid tumors. J. Surg. Oncol. 1980, 15, 85–90. [Google Scholar] [CrossRef]
- Lynch, P.M.; Burke, C.A.; Phillips, R.; Morris, J.S.; Slack, R.; Wang, X.; Liu, J.; Patterson, S.; Sinicrope, F.A.; Rodriguez-Bigas, M.A. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut 2016, 65, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Food Drug Administration. Pfizer, Inc.; withdrawal of approval of familial adenomatous polyposis indication for CELEBREX. Federal Register. 2012; Volume 77. Available online: https://www.govinfo.gov/content/pkg/FR-2012-06-08/html/2012-13900.htm (accessed on 20 May 2020).
- Rigas, B.; Kashfi, K. Nitric-oxide-donating NSAIDs as agents for cancer prevention. Trends Mol. Med. 2004, 10, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Borgo, S.; Hasan, I.; Castillo, E.; Traganos, F.; Rigas, B. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NSAIDs) alter the kinetics of human colon cancer cell lines more effectively than traditional NSAIDs: Implications for colon cancer chemoprevention. Cancer Res. 2001, 61, 3285–3289. [Google Scholar] [PubMed]
- Fiorucci, S.; Santucci, L.; Gresele, P.; Faccino, R.M.; Del Soldato, P.; Morelli, A. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: A proof of concept endoscopic study. Gastroenterology 2003, 124, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.W.; McKnight, W.; Li, P.; Del Soldato, P.; Calignano, A.; Cirino, G.; Wallace, J.L. Cyclooxygenase-independent chemoprevention with an aspirin derivative in a rat model of colonic adenocarcinoma. Life Sci. 1998, 62, 367–373. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; Kodela, R.; Olson, K.R.; Kashfi, K. NOSH–aspirin (NBS-1120), a novel nitric oxide-and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem. Biophys. Res. Commun. 2012, 419, 523–528. [Google Scholar] [CrossRef]
- Kashfi, K.; Chattopadhyay, M.; Kodela, R. NOSH-sulindac (AVT-18A) is a novel nitric oxide-and hydrogen sulfide-releasing hybrid that is gastrointestinal safe and has potent anti-inflammatory, analgesic, antipyretic, anti-platelet, and anti-cancer properties. Redox Biol. 2015, 6, 287–296. [Google Scholar] [CrossRef]
- Chegaev, K.; Riganti, C.; Lazzarato, L.; Rolando, B.; Guglielmo, S.; Campia, I.; Fruttero, R.; Bosia, A.; Gasco, A. Nitric oxide donor doxorubicins accumulate into doxorubicin-resistant human colon cancer cells inducing cytotoxicity. Acs Med. Chem. Lett. 2011, 2, 494–497. [Google Scholar] [CrossRef]
- Ai, Y.; Kang, F.; Huang, Z.; Xue, X.; Lai, Y.; Peng, S.; Tian, J.; Zhang, Y. Synthesis of CDDO–amino acid–nitric oxide donor trihybrids as potential antitumor agents against both drug-sensitive and drug-resistant colon cancer. J. Med. Chem. 2015, 58, 2452–2464. [Google Scholar] [CrossRef]
- Millet, A.; Bettaieb, A.; Renaud, F.; Prevotat, L.; Hammann, A.; Solary, E.; Mignotte, B.; Jeannin, J.F. Influence of the nitric oxide donor glyceryl trinitrate on apoptotic pathways in human colon cancer cells. Gastroenterology 2002, 123, 235–246. [Google Scholar] [CrossRef]
- Pathi, S.S.; Jutooru, I.; Chadalapaka, G.; Sreevalsan, S.; Anand, S.; Thatcher, G.R.; Safe, S. GT-094, a NO-NSAID, inhibits colon cancer cell growth by activation of a reactive oxygen species-microRNA-27a: ZBTB10-specificity protein pathway. Mol. Cancer Res. 2011, 9, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Ji, P.; Ouyang, N.; Kopelovich, L.; Rigas, B. Protein nitration and nitrosylation by NO-donating aspirin in colon cancer cells: Relevance to its mechanism of action. Exp. Cell Res. 2011, 317, 1359–1367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hua, A.; Mackenzie, G.G.; Rigas, B. The differential cell signaling effects of two positional isomers of the anticancer NO-donating aspirin. Int. J. Oncol. 2009, 35, 837–844. [Google Scholar] [PubMed]
- Rigas, B. Novel agents for cancer prevention based on nitric oxide. Biochem. Soc. Trans. 2007, 35, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, K.; Rigas, B. The mechanism of action of nitric oxide-donating aspirin. Biochem. Biophys. Res. Commun. 2007, 358, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, N.; Williams, J.L.; Tsioulias, G.J.; Gao, J.; Iatropoulos, M.J.; Kopelovich, L.; Kashfi, K.; Rigas, B. Nitric oxide–donating aspirin prevents pancreatic cancer in a hamster tumor model. Cancer Res. 2006, 66, 4503–4511. [Google Scholar] [CrossRef]
- Kashfi, K.; Rigas, B. Molecular targets of nitric-oxide-donating aspirin in cancer. Biochem. Soc. Trans. 2005, 33, 701–704. [Google Scholar] [CrossRef]
- Karateev, A.; Nasonov, E.; Yakhno, N.; Ivashkin, V.; Chichasova, N.; Alekseeva, L.; Karpov, Y.A.; Evseev, M.; Kukushkin, M.; Danilov, A. Clinical guidelines «Rational use of nonsteroidal anti-inflammatory drugs (NSAIDs) in clinical practice». Mod. Rheumatol. J. 2015, 9, 4–23. [Google Scholar] [CrossRef][Green Version]
- Day, R.O.; Graham, G.G. Non-steroidal anti-inflammatory drugs (NSAIDs). Bmj 2013, 346, f3195. [Google Scholar]
- Mallen, S.R.; Essex, M.N.; Zhang, R. Gastrointestinal tolerability of NSAIDs in elderly patients: A pooled analysis of 21 randomized clinical trials with celecoxib and nonselective NSAIDs. Curr. Med Res. Opin. 2011, 27, 1359–1366. [Google Scholar] [CrossRef]
- Patrono, C.; Baigent, C. Low-dose aspirin, coxibs, and other NSAIDS: A clinical mosaic emerges. Mol. Interv. 2009, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Derry, S.; Phillips, C.J.; McQuay, H.J. Nonsteroidal anti-inflammatory drugs (NSAIDs), cyxlooxygenase-2 selective inhibitors (coxibs) and gastrointestinal harm: Review of clinical trials and clinical practice. Bmc Musculoskelet. Disord. 2006, 7, 79. [Google Scholar] [CrossRef]
- Fiorucci, S.; Antonelli, E. NO-NSAIDs: From inflammatory mediators to clinical readouts. Inflamm. Allergy-Drug Targets (Former. Curr. Drug Targets-Inflamm. Allergy) 2006, 5, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Bergh, M.S.; Budsberg, S.C. The coxib NSAIDs: Potential clinical and pharmacologic importance in veterinary medicine. J. Vet. Intern. Med. 2005, 19, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Tarnawski, A.S.; Jones, M.K. Inhibition of angiogenesis by NSAIDs: Molecular mechanisms and clinical implications. J. Mol. Med. 2003, 81, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Hale, V.; Borga, O.; Stein, R. Predictability of the clinical potency of NSAIDs from the preclinical pharmacodynamics in rats. Inflamm. Res. 1996, 45, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Brater, D.C. Clinical pharmacology of NSAIDs. J. Clin. Pharmacol. 1988, 28, 518–523. [Google Scholar] [CrossRef]
- Roujeau, J.-C. Clinical aspects of skin reactions to NSAIDs. Scand. J. Rheumatol. 1987, 16, 131–134. [Google Scholar] [CrossRef]
- Verbeeck, R.K.; Blackburn, J.L.; Loewen, G.R. Clinical pharmacokinetics of non-steroidal anti-inflammatory drugs. Clin. Pharmacokinet. 1983, 8, 297–331. [Google Scholar] [CrossRef]
- Yeh, R.K.; Chen, J.; Williams, J.L.; Baluch, M.; Hundley, T.R.; Rosenbaum, R.E.; Kalala, S.; Traganos, F.; Benardini, F.; Del Soldato, P. NO-donating nonsteroidal antiinflammatory drugs (NSAIDs) inhibit colon cancer cell growth more potently than traditional NSAIDs: A general pharmacological property? Biochem. Pharmacol. 2004, 67, 2197–2205. [Google Scholar] [CrossRef]
- Gao, J.; Liu, X.; Rigas, B. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc. Natl. Acad. Sci. USA 2005, 102, 17207–17212. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.; Kashfi, K.; Chen, J.; Rigas, B. Nitric oxide-donating aspirin inhibits β-catenin/T cell factor (TCF) signaling in SW480 colon cancer cells by disrupting the nuclear β-catenin–TCF association. Proc. Natl. Acad. Sci. USA 2003, 100, 12584–12589. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Ji, P.; Ouyang, N.; Liu, X.; Rigas, B. NO-donating aspirin inhibits the activation of NF-κB in human cancer cell lines and Min mice. Carcinogenesis 2008, 29, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Nath, N.; Chen, J.; Hundley, T.R.; Gao, J.; Kopelovich, L.; Kashfi, K.; Rigas, B. Growth inhibition of human colon cancer cells by nitric oxide (NO)-donating aspirin is associated with cyclooxygenase-2 induction and β-catenin/T-cell factor signaling, nuclear factor-κB, and NO synthase 2 inhibition: Implications for chemoprevention. Cancer Res. 2003, 63, 7613–7618. [Google Scholar] [PubMed]
- Rao, C.V.; Reddy, B.S.; Steele, V.E.; Wang, C.; Liu, X.; Ouyang, N.; Patlolla, J.M.; Simi, B.; Kopelovich, L.; Rigas, B. Nitric oxide–releasing aspirin and indomethacin are potent inhibitors against colon cancer in azoxymethane-treated rats: Effects on molecular targets. Mol. Cancer Ther. 2006, 5, 1530–1538. [Google Scholar] [CrossRef]
- Fonseca, M.D.; Cunha, F.Q.; Kashfi, K.; Cunha, T.M. NOSH-aspirin (NBS-1120), a dual nitric oxide and hydrogen sulfide-releasing hybrid, reduces inflammatory pain. Pharmacol. Res. Perspect. 2015, 3. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986, 46, 6387–6392. [Google Scholar]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Noguchi, Y.; Sato, K.; Akaike, T. Enhanced vascular permeability in solid tumor is mediated by nitric oxide and inhibited by both new nitric oxide scavenger and nitric oxide synthase inhibitor. Jpn. J. Cancer Res. 1994, 85, 331–334. [Google Scholar] [CrossRef]
- Wu, J.; Akaike, T.; Maeda, H. Modulation of enhanced vascular permeability in tumors by a bradykinin antagonist, a cyclooxygenase inhibitor, and a nitric oxide scavenger. Cancer Res. 1998, 58, 159–165. [Google Scholar] [PubMed]
- Tanaka, S.; Akaike, T.; Wu, J.; Fang, J.; Sawa, T.; Ogawa, M.; Beppu, T.; Maeda, H. Modulation of tumor-selective vascular blood flow and extravasation by the stable prostaglandin I2 analogue beraprost sodium. J. Drug Target. 2003, 11, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Shen, Q.; Xie, C.; Lu, W.; Peng, C.; Wei, X.; Li, X.; Su, B.; Gao, C.; Liu, M. Retro-inverso bradykinin opens the door of blood–brain tumor barrier for nanocarriers in glioma treatment. Cancer Lett. 2015, 369, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wang, R.; Xie, Z.; Ruan, H.; Li, J.; Xie, C.; Lu, W.; Wang, J.; Wang, D.; Liu, M. Effect of Retro-Inverso Isomer of Bradykinin on Size-Dependent Penetration of Blood–Brain Tumor Barrier. Small 2018, 14, 1702331. [Google Scholar] [CrossRef]
- Sessa, W. Molecular control of blood flow and angiogenesis: Role of nitric oxide. J. Thromb. Haemost. 2009, 7, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Islam, W.; Fang, J.; Imamura, T.; Etrych, T.; Subr, V.; Ulbrich, K.; Maeda, H. Augmentation of the Enhanced Permeability and Retention Effect with Nitric Oxide-Generating Agents Improves the Therapeutic Effects of Nanomedicines. Mol. Cancer 2018, 17, 2643–2653. [Google Scholar] [CrossRef]
- Fang, J.; Liao, L.; Yin, H.; Nakamura, H.; Shin, T.; Maeda, H. Enhanced bacterial tumor delivery by modulating the EPR effect and therapeutic potential of Lactobacillus casei. J. Pharm. Sci. 2014, 103, 3235–3243. [Google Scholar] [CrossRef]
- Scatena, R.; Bottoni, P.; Pontoglio, A.; Giardina, B. Pharmacological modulation of nitric oxide release: New pharmacological perspectives, potential benefits and risks. Curr. Med. Chem. 2010, 17, 61–73. [Google Scholar] [CrossRef]
- Belinsky, M.; Jaiswal, A.K. NAD (P) H: Quinone oxidoreductase 1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev. 1993, 12, 103–117. [Google Scholar] [CrossRef]
- Luo, X.; Wu, J.; Lv, T.; Lai, Y.; Zhang, H.; Lu, J.-J.; Zhang, Y.; Huang, Z. Synthesis and evaluation of novel O2-derived diazeniumdiolates as photochemical and real-time monitoring nitric oxide delivery agents. Org. Chem. Front. 2017, 4, 2445–2449. [Google Scholar] [CrossRef]
- Hasegawa, U.; Wang, T.; Chen, J.J.; Uyama, H.; Van Der Vlies, A.J. Furoxan-Bearing Micelles for Nitric Oxide Delivery. Macromol Biosci 2016, 16, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Jaraki, O.; Osborne, J.; Simon, D.I.; Keaney, J.; Vita, J.; Singel, D.; Valeri, C.R.; Loscalzo, J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc. Natl. Acad. Sci. USA 1992, 89, 7674–7677. [Google Scholar] [CrossRef]
- Katayama, N.; Nakajou, K.; Komori, H.; Uchida, K.; Yokoe, J.-i.; Yasui, N.; Yamamoto, H.; Kai, T.; Sato, M.; Nakagawa, T. Design and evaluation of S-nitrosylated human serum albumin as a novel anticancer drug. J. Pharmacol. Exp. Ther. 2008, 325, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ishima, Y.; Fang, J.; Kragh-Hansen, U.; Yin, H.; Liao, L.; Katayama, N.; Watanabe, H.; Kai, T.; Suenaga, A.; Maeda, H.; et al. Tuning of poly-S-nitrosated human serum albumin as superior antitumor nanomedicine. J. Pharm. Sci. 2014, 103, 2184–2188. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, R.; Ishima, Y.; Ikeda, M.; Kragh-Hansen, U.; Fang, J.; Nakamura, H.; Chuang, V.T.; Tanaka, R.; Maeda, H.; Kodama, A.; et al. S-Nitrosated human serum albumin dimer as novel nano-EPR enhancer applied to macromolecular anti-tumor drugs such as micelles and liposomes. J. Control Release 2015, 217, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, R.; Ishima, Y.; Chuang, V.T.G.; Nakamura, H.; Fang, J.; Watanabe, H.; Shimizu, T.; Okuhira, K.; Ishida, T.; Maeda, H.; et al. Improved anticancer effects of albumin-bound paclitaxel nanoparticle via augmentation of EPR effect and albumin-protein interactions using S-nitrosated human serum albumin dimer. Biomaterials 2017, 140, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Gao, H. The application of nitric oxide delivery in nanoparticle-based tumor targeting drug delivery and treatment. Asian J Pharm Sci 2019, 14, 380–390. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; He, Q. Strategies for engineering advanced nanomedicines for gas therapy of cancer. Natl. Sci. Rev. 2020. [Google Scholar] [CrossRef]
- Seabra, A. Nitric Oxide Donors: Novel Biomedical Applications and Perspectives; Academic Press: London, UK, 2017. [Google Scholar]
- Sukhatme, V.; Bouche, G.; Meheus, L.; Sukhatme, V.P.; Pantziarka, P. Repurposing Drugs in Oncology (ReDO)—Nitroglycerin as an anti-cancer agent. Ecancermedicalscience 2015, 9. [Google Scholar] [CrossRef]
- Yasuda, H.; Yamaya, M.; Nakayama, K.; Sasaki, T.; Ebihara, S.; Kanda, A.; Asada, M.; Inoue, D.; Suzuki, T.; Okazaki, T. Randomized phase II trial comparing nitroglycerin plus vinorelbine and cisplatin with vinorelbine and cisplatin alone in previously untreated stage IIIB/IV non–small-cell lung cancer. J. Clin. Oncol. 2006, 24, 688–694. [Google Scholar] [CrossRef]
- Burn, J.; Sheth, H.; Elliott, F.; Reed, L.; Macrae, F.; Mecklin, J.-P.; Möslein, G.; McRonald, F.E.; Bertario, L.; Evans, D.G. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: A double-blind, randomised, placebo-controlled trial. Lancet 2020, 395, 1855–1863. [Google Scholar] [CrossRef]
- Tikk, K.; Czock, D.; Haefeli, W.E.; Kopp-Schneider, A.; Brenner, H. Clinical trial protocol of the ASTER trial: A double-blind, randomized, placebo-controlled phase III trial evaluating the use of acetylsalicylic acid (ASA) for enhanced early detection of colorectal neoplasms. Bmc Cancer 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ján, P.; Roman, H.; Ondřej, B.; Štěpán, T.; Jiří, N.; Eva, M.; Bořivoj, V. Acetylsalicylic Acid and its Potential for Chemoprevention of Colorectal Carcinoma. Klin. Onkol. 2018, 31, 77–81. [Google Scholar]
- Félétou, M. Discovery of Nitric Oxide and Translation to Clinical Application. Physiol. (BethesdaMd.) 2016, 31, 76–77. [Google Scholar] [CrossRef]
- Hays, E.; Bonavida, B. Nitric oxide-mediated enhancement and reversal of resistance of anticancer therapies. Antioxidants 2019, 8, 407. [Google Scholar] [CrossRef]
- Rigas, B.; Williams, J.L. NO-releasing NSAIDs and colon cancer chemoprevention: A promising novel approach. Int. J. Oncol. 2002, 20, 885–890. [Google Scholar] [CrossRef]
- Ohta, T.; Takahashi, M.; Ochiai, A. Increased protein expression of both inducible nitric oxide synthase and cyclooxygenase-2 in human colon cancers. Cancer Lett. 2006, 239, 246–253. [Google Scholar] [CrossRef]
- Holotiuk, V.; Kryzhanivska, A.; Churpiy, I.; Tataryn, B.; Ivasiutyn, D.Y. Role of nitric oxide in pathogenesis of tumor growth and its possible application in cancer treatment. Exp. Oncol. 2019, 41, 210–215. [Google Scholar] [CrossRef]
- Rahat, M.A.; Hemmerlein, B. Macrophage-tumor cell interactions regulate the function of nitric oxide. Front. Physiol. 2013, 4, 144. [Google Scholar] [CrossRef]
| Compound | Target | Mechanism | Inhibited Proteins | Related CRD Hallmark | Inhibited Cells | In Vivo Model | Combined Therapy | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1400 W | iNOS | Angiogenesis pathway | PROK2; MMP2 | Suppress CRC cell proliferation and migration | HT29/ HCT116 | Human CRC xenografts mice model | 5-FU | [64,65] |
| L-NIO | eNOS nNOS | Angiogenesis pathway | Serpin B5; uPA | Suppress CRC cell proliferation and migration | HCT116 | 5-FU | [64] | |
| Celastrol | iNOS/ eNOS | Angiogenesis pathway | IL1b, MMP-9, PDGF, TIMP-4 | Suppress CRC cell proliferation and migration | HT29/HCT116 | 5-FU/ Salinomycin; 1400W, L-NIO | [66] | |
| Maqui berry extracts | iNOS | Antioxidant activity; anti-inflammation | NF-κB; COX-2 | Suppress CRC cell growth | HT29/ Caco-2 | [67] | ||
| M. alba extract | iNOS | Caspase 3 activity | Induce apoptosis in human CRC cells | HCT-15 | [68] | |||
| PBISe | iNOS/Akt | MAP and PI3 kinase signaling | pAkt; Akt2 | Induce apoptosis in human CRC cells | Caco-2 | [68] | ||
| Nobiletin (NOB) | iNOS | Nrf2 signaling pathway; antioxidative activity | cyclin D, CDK6, CDK4, CDK2 | Suppress colitis-associated colon carcinogenesis; caused cell cycle arrest in human CRC cells | RAW 264.7/ HCT116 | AOM/DSS-treated mice | [69] | |
| All-Trans Retinoic Acid (AtRA) | iNOS/ TNF-α | TLR4/NF-κ;B signaling pathway | Improve the clinical prevention of CAC | colonic mucosa of patients with CAC and UC | [70] | |||
| Fucoxanthin-Rich Brown Algae Extract (FX-BAE) | iNOS | Pro-inflammatory cytokines; TNF-α and IL-6 | Reduced intestinal injury caused by chronic inflammation; attenuated colitis-associated colon cancer | DSS-induced colitis and CACC in mice | [71,72] | |||
| DHA | iNOS | Cyclin-dependent kinase | Induce apoptosis in human CRC cells | Caco-2 | [73,74] | |||
| Polyphenol Ellagic Acid | iNOS/COX-2/TNF-α/IL-6 | NF-κB pathway | COX-2; NF-κB; iNOS | Attenuated colonic inflammation | Inflammatory rat model | [75] | ||
| Phaleria macrocarpa Stem Bark | iNOS | Inhibit iNOS expression | HCT116 | [76] | ||||
| Cannabidiol (CBD) | eNOS | Autophagy; antioxidant activity | phospho-eNOS; SOD2 | Overcome oxaliplatin resistance | DLD-1/colo205 | human CRC xenografts mice model | Oxaliplatin and other chemotherapeutics | [77] |
| Cetuximab | iNOS | Immunosuppressive activation | IL-10; TGFβ | Potentiate chemotherapeutic efficacy | Patients with CRC or metastatic CRC | Chemotherapeutics | [78] |
| NO Donor | Properties | Mechanism | Chemo-Preventative Effects | Inhibited Cells | In Vivo Model | Ref. |
|---|---|---|---|---|---|---|
| NO-NSAIDs | Suppress CRC cell proliferation; block cell cycle transition | HT-29 | [95] | |||
| NO-aspirin (NO-ASA) | NO-NSAID | Induce COX-2; Inhibit the β-catenin/TCF4 signaling pathway | Inhibit CRC cell growth | HT-29/DLD-1 | [95] | |
| NCX-4016 | NO aspirin derivate | Independent of any inhibitory activity on COX-1 or COX-2 | Reduce aberrant crypt foci (ACF) in colon | Azoxymethane (AOM)-induced mice | [97] | |
| NOSH–aspirin (NBS-1120) | NO- and H2S-releasing NSAID | Inhibit cyclo-oxygenase enzyme activity | Suppress CRC cell proliferation; induce apoptosis; block cell cycle | HT-29 | Human xenograft mouse model | [98] |
| NOSH-sulindac (AVT-18A) | NO- and H2S-releasing NSAID | Inhibit COX-1 and COX-2 | Anti-colon cancer activity and anti-inflammation | HT-29/SW-480/HCT-15 | Rat | [99] |
| NO donor Doxorubicin (NO-DOXOs) | Inhibit cellular drug efflux; nitration of tyrosine residues of MRP3 protein | Induce cytotoxicity in colon cancer cells; | doxorubicin-resistant HT-29 | [100] | ||
| CDDO-Amino Acid-Nitric Oxide Donor Trihybrids | Inhibit HIF-1α, ERK, Stat 3, and AKT signaling | Anti-tumor effect against chemo-sensitive and chemo-resistant CRC | HCT-8; HCT-8/5-FU | [101] | ||
| Glyceryl trinitrate (GTN) | Activate caspase-1 and caspase-10 | Induce apoptosis in colon cancer cells | HCT116 | In clinics | [102] | |
| GT-094 | NO-NASID | Activate the ROS-miR-27a:ZBTB10-Sp transcription factor pathway | Inhibit CRC cell growth | RKO/SW480 | [103] |
| Compound | In Vivo Model | Mechanism | Treatment (Dose/Duration) | Therapeutic Efficacy | Ref. |
|---|---|---|---|---|---|
| 1400W | Genetically engineered xenograft mice model with constitutive iNOS expression (colon adenocarcinoma DLD-1) | Angiogenesis pathway | 6 mg/kg-1/h-1 1400W/13 days | Inhibited tumor growth | [65] |
| Nobiletin (NOB) | AOM/DSS colon cancer model on CD-1 mice | iNOS ↓ antioxidative enzymes ↑ cell cycle ↓ | AIN93G diet containing NOB (0.05 wt % in diet)/ 1 week after the AOM injection until the end of study | Suppressed colitis-associated colon carcinogenesis | [69] |
| All-Trans Retinoic Acid (AtRA) | Ex vivo; colonic mucosa of patients with CAC and UC | LPS/TLR4/NF-κB signaling pathway NOS2 ↓ TNF-α ↓ | 10–7 M AtRA/6 h; stimulated with 10 μg/mL lipopolysaccharide (LPS) | Clinically prevented the CAC development and progression | [70] |
| Fucoxanthin-Rich Brown Algae Extract (FX-BAE) | DSS-induced colitis and CACC model in BALB/c mice | Oxidative stress↓ | Colitis: fed with FX-BAE 1, 2, or 5 g/kg/day from day 8 to day 14; CACC: fed with FX-BAE at 0.5, 1, or 2.5 g/kg every 2 days | Decreased the incidence of colonic neoplasm; increased superoxide dismutase (SOD) production, lymphocyte proliferation; prolonged survival rate in CACC mice | [71,72] |
| Polyphenol Ellagic Acid | 1,2-dimethylhydrazine-induced colon cancer model on Wistar albino rats | Anti-inflammatory NF-κB pathway ↓ iNOS ↓ COX-2 ↓ TNF-α ↓ IL-6 ↓ | 60 mg/kg ellagic acid/p.o./every day for 15 weeks | Chemo-prevention on colon carcinogenesis | [75] |
| Cannabidiol (CBD) | Colo205 xenograft model on BALB/c nude mice | CBD overcomes NOS-induced oxaliplatin resistance by inducing autophagy | CBD + oxaliplatin (i.p.) | Overcame the resistance to oxaliplatin | [77] |
| Cetuximab | Ex vivo; CRC tissue explant culture | iNOS ↓ immunosuppressive cytokines ↑ | Cetuximab + chemotherapy (CTX + Chemo) | Potentiated the chemo-therapeutic efficacy | [78] |
| NCX-4016 | TNBS-AMO colon cancer model on rats | Independent of any inhibitory activity on COX-1 or COX-2 | 10 mg/kg NCX-4016 after four- weeks administration of AOM | Reduce aberrant crypt foci (ACF) in colon | [97] |
| NOSH–aspirin (NBS-1120) | Human colon cancer xenograft model | cell proliferation ↓ apoptosis ↑ the blockage of G(0)/G(1) cell cycle | Reduced the tumor volume by 85% | [98] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, L.; Xie, Z.; Zhou, S.; Li, Y.; Zhou, Y.; Sun, M. Nitric Oxide (NO) and NO Synthases (NOS)-Based Targeted Therapy for Colon Cancer. Cancers 2020, 12, 1881. https://doi.org/10.3390/cancers12071881
Wang H, Wang L, Xie Z, Zhou S, Li Y, Zhou Y, Sun M. Nitric Oxide (NO) and NO Synthases (NOS)-Based Targeted Therapy for Colon Cancer. Cancers. 2020; 12(7):1881. https://doi.org/10.3390/cancers12071881
Chicago/Turabian StyleWang, Hao, Liye Wang, Zuoxu Xie, Shuang Zhou, Yan Li, Yue Zhou, and Meiyan Sun. 2020. "Nitric Oxide (NO) and NO Synthases (NOS)-Based Targeted Therapy for Colon Cancer" Cancers 12, no. 7: 1881. https://doi.org/10.3390/cancers12071881
APA StyleWang, H., Wang, L., Xie, Z., Zhou, S., Li, Y., Zhou, Y., & Sun, M. (2020). Nitric Oxide (NO) and NO Synthases (NOS)-Based Targeted Therapy for Colon Cancer. Cancers, 12(7), 1881. https://doi.org/10.3390/cancers12071881





