Leucine-Rich Diet Modulates the Metabolomic and Proteomic Profile of Skeletal Muscle during Cancer Cachexia
Abstract
1. Introduction
2. Results
2.1. Tumour-Induced Morphometric Damages Time-Course Dependent Were Modulated by Leucine-Rich Diet
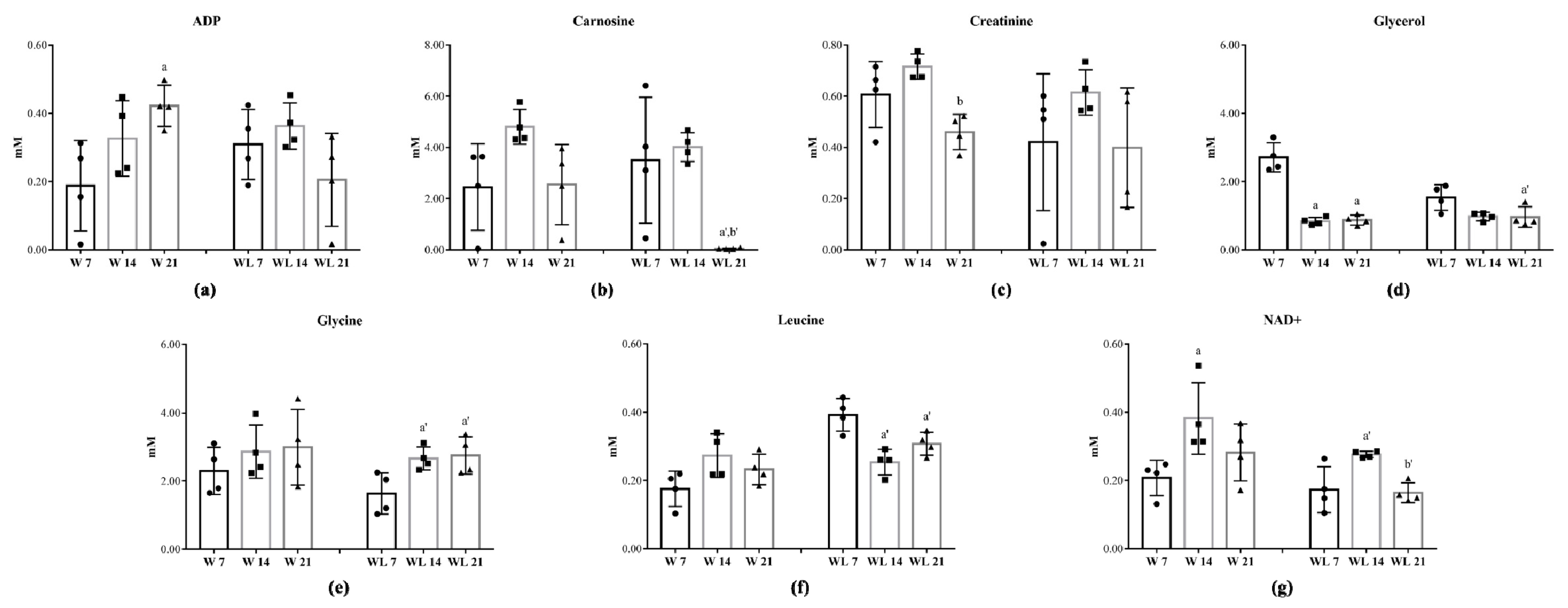
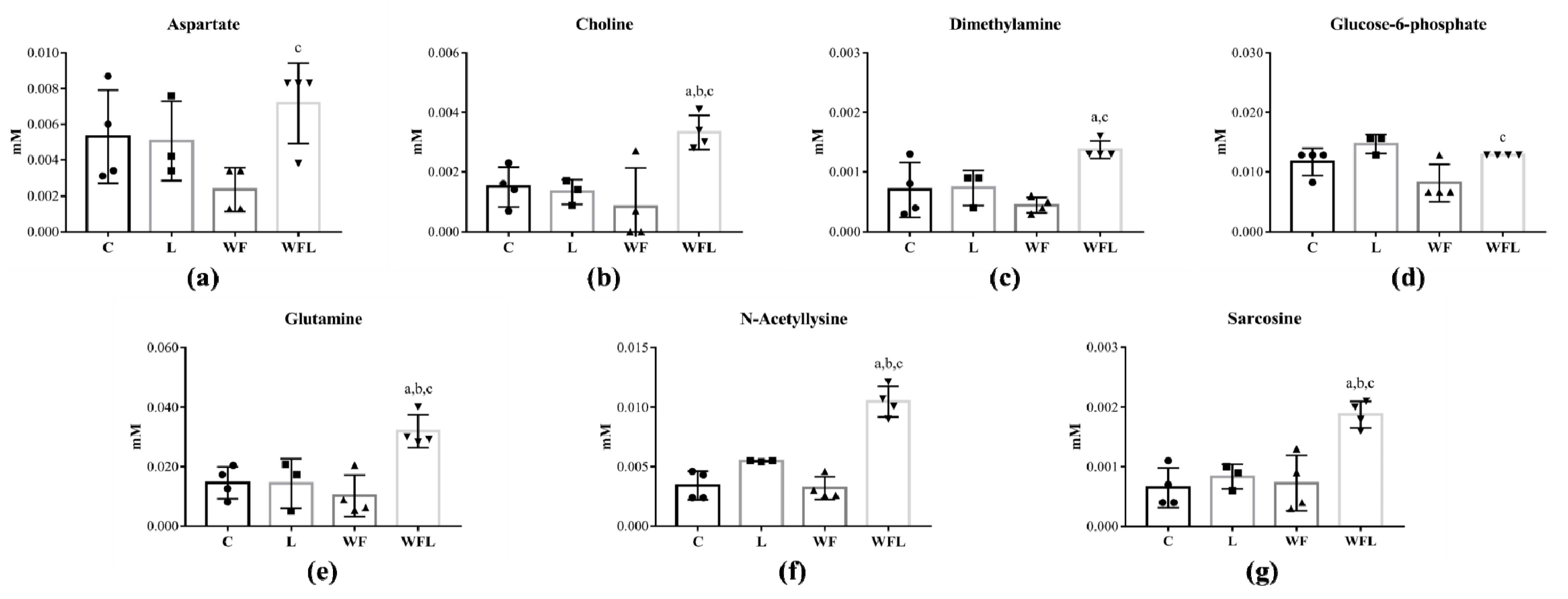
2.2. Tumour Growth Induced Metabolomic Profile Changes in Skeletal Muscle
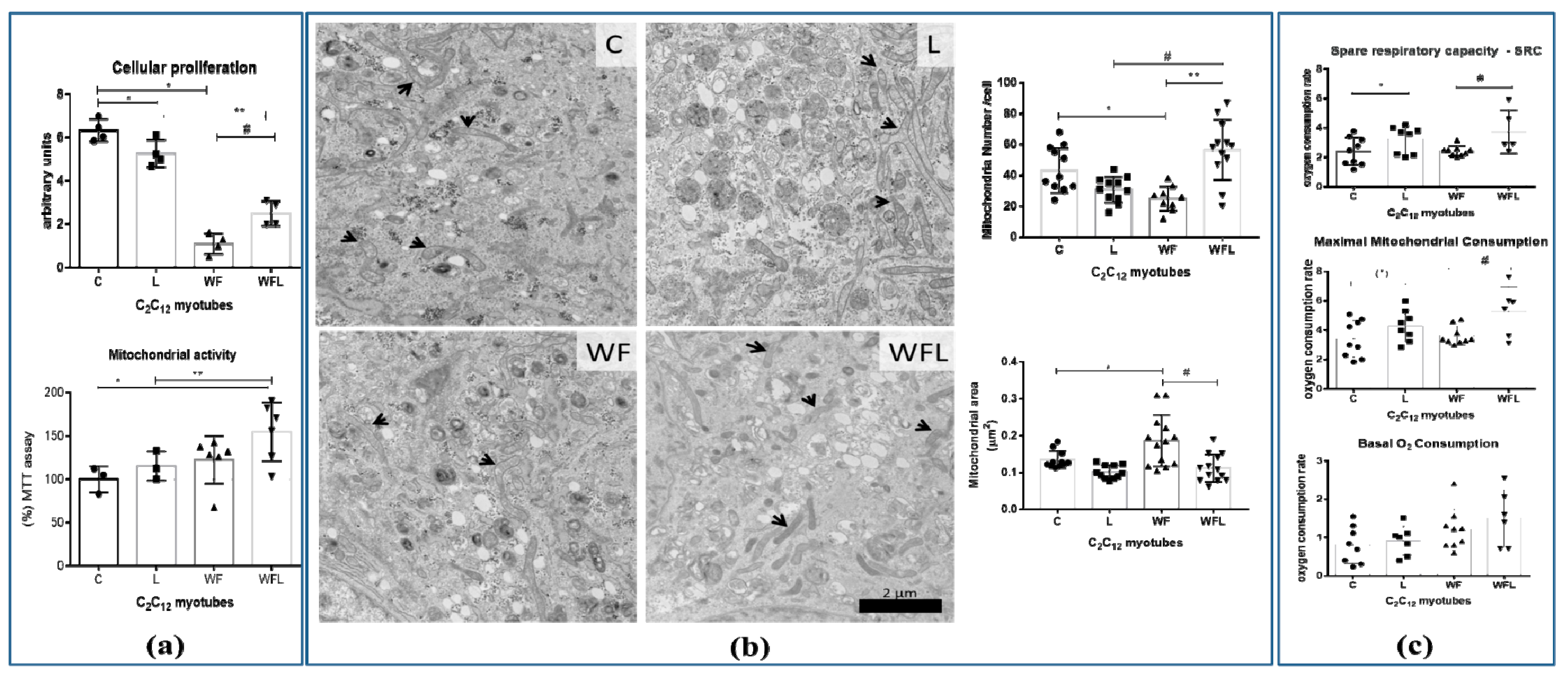
2.3. Walker Factor-Induced Damages in C2C12 Myotubes Were Modulated by Leucine Treatment
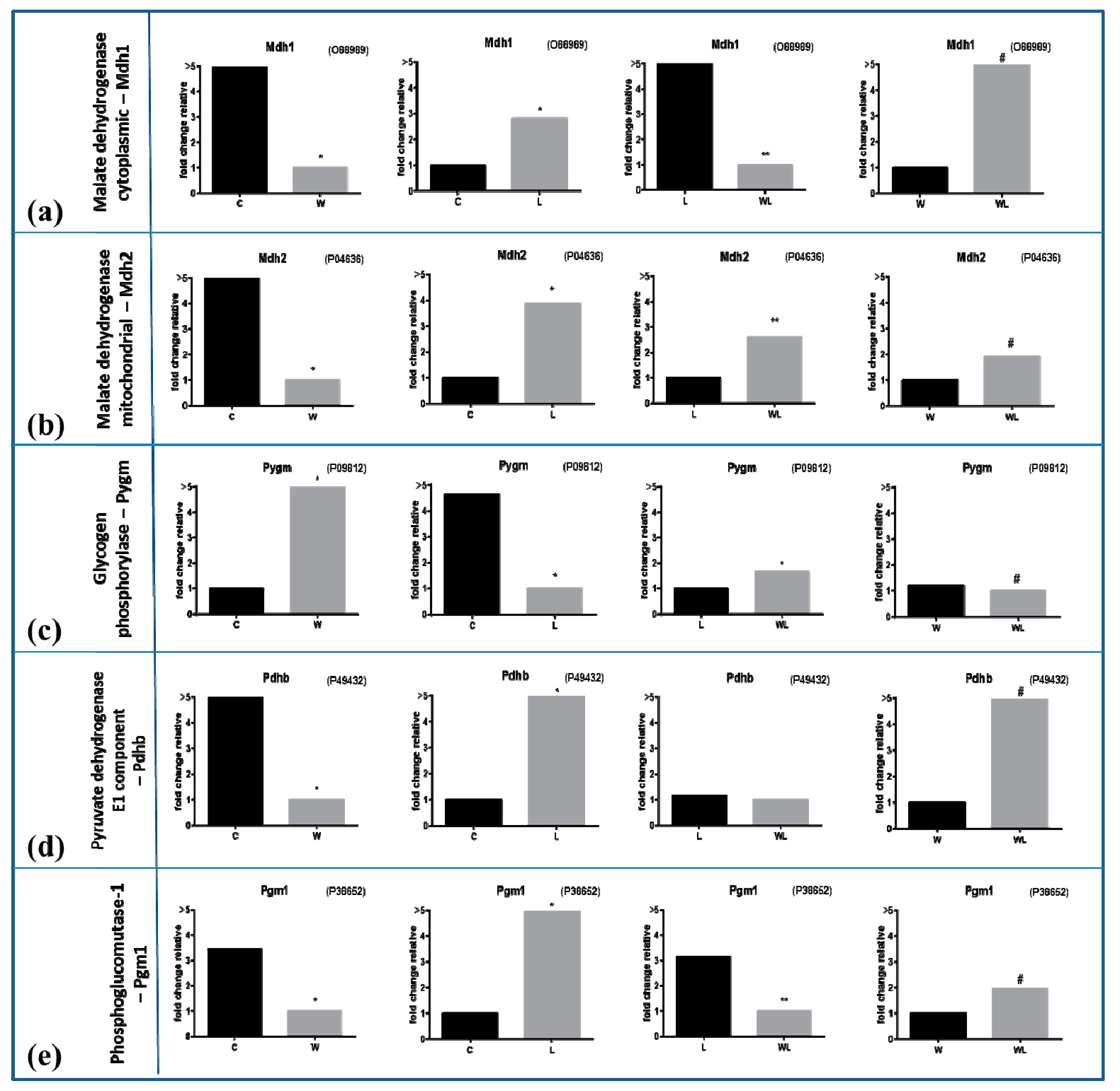
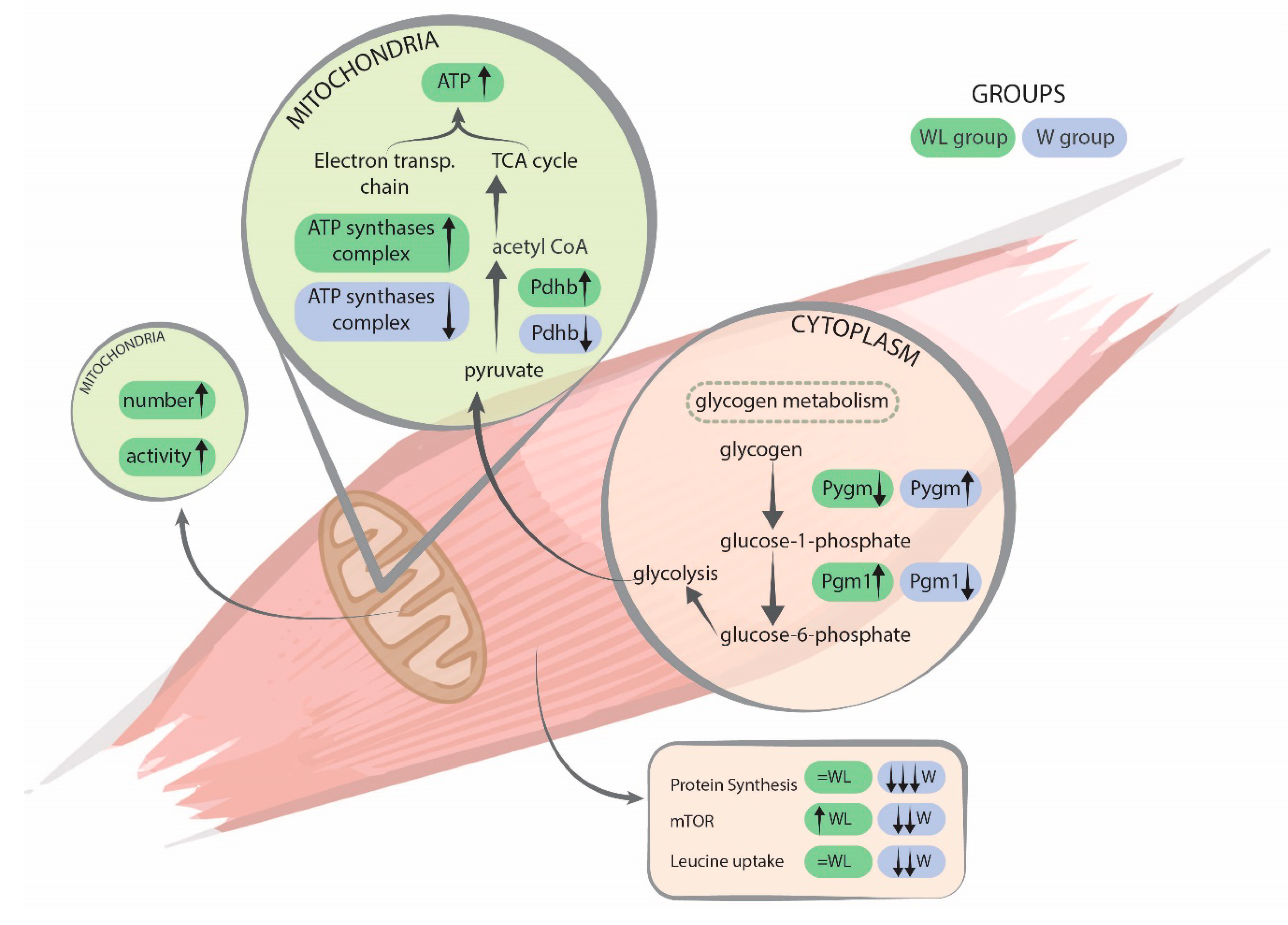
2.4. Muscle Proteomic Profile Changed by Tumour Evolution but Was Diverted by Leucine-Rich Diet
3. Discussion
4. Materials and Methods
4.1. In Vivo Assays
4.1.1. Diets
4.1.2. Animal and Experimental Procedures
4.2. In Vitro Assays
4.3. Metabolomics Analysis
4.4. Proteomics Analysis
4.5. Oxygen Consumption Rate
4.6. Mitochondrial Activity and C2C12 Proliferation
4.7. Electronic Microscopy C2C12 Myotubes
4.8. Western Blot
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arthur, S.; Noone, J.; Roy, D.; Blanchette, C.; Van Doren, B. One-year prevalence, comorbidities and cost of cachexia-related inpatient admissions in the USA. Drugs Context 2014, 3, 212265. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Arends, J.; Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013, 10, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Lok, C. Cachexia: The last illness. Nature 2015, 528, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; von Haehling, S. Efforts begin to sprout: Publications in JCSM on cachexia, sarcopenia and muscle wasting receive attention. J. Cachexia Sarcopenia Muscle 2014, 5, 171–176. [Google Scholar] [CrossRef]
- Vagnildhaug, O.M.; Blum, D.; Wilcock, A.; Fayers, P.; Strasser, F.; Baracos, V.E.; Hjermstad, M.J.; Kaasa, S.; Laird, B.; Solheim, T.S. The applicability of a weight loss grading system in cancer cachexia: A longitudinal analysis. J. Cachexia Sarcopenia Muscle 2017, 8, 789–797. [Google Scholar] [CrossRef]
- Inui, A. Cancer anorexia-cachexia syndrome: Current issues in research and management. CA Cancer J. Clin. 1999, 52, 72–91. [Google Scholar] [CrossRef]
- Garlick, P.J. The role of leucine in the regulation of protein metabolism. J. Nutr. 2005, 135, 1553S–1556S. [Google Scholar] [CrossRef]
- Escobar, J.; Frank, J.W.; Suryawan, A.; Nguyen, H.V.; Kimball, S.R.; Jefferson, L.S.; Davis, T.A. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E612–E621. [Google Scholar] [CrossRef]
- Wilson, F.A.; Suryawan, A.; Gazzaneo, M.C.; Orellana, R.A.; Nguyen, H.V.; Davis, T.A. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J. Nutr. 2010, 140, 264–270. [Google Scholar] [CrossRef]
- Ventrucci, G.; Mello, M.A.R.; Gomes-Marcondes, M.C.C. Proteasome activity is altered in skeletal muscle tissue of tumour-bearing rats a leucine-rich diet. Endocr. Relat. Cancer 2004, 11, 887–895. [Google Scholar] [CrossRef]
- Cruz, B.; Gomes-Marcondes, M.C.C. Leucine-rich diet supplementation modulates foetal muscle protein metabolism impaired by Walker-256 tumour. Reprod. Biol. Endocrinol. 2014, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Razi, M.D.; Aaseth, J.; Chirumbolo, S.; Pen, J.J. Cancer-associated cachexia, reactive oxygen species, and nutrition therapy. Curr. Med. Chem. 2019, 26, 5728–5744. [Google Scholar] [CrossRef] [PubMed]
- Schiessel, D.L.; Baracos, V.E. Barriers to cancer nutrition therapy: Excess catabolism of muscle and adipose tissues induced by tumour products and chemotherapy. Proc. Nutr. Soc. 2018, 77, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.; Oliveira, A.; Gomes-Marcondes, M.C.C. L-leucine dietary supplementation modulates muscle protein degradation and increases pro-inflammatory cytokines in tumour-bearing rats. Cytokine 2017, 96, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Van der Ende, M.; Grefte, S.; Plas, R.; Meijerink, J.; Witkamp, R.F.; Keijer, J.; van Norren, K. Mitochondrial dynamics in cancer-induced cachexia. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 137–150. [Google Scholar] [CrossRef]
- Hardee, J.P.; Montalvo, R.N.; Carson, J.A. Linking cancer cachexia-induced anabolic resistance to skeletal muscle oxidative metabolism. Oxid. Med. Cell. Longev. 2017, 2017, 8018197. [Google Scholar] [CrossRef]
- Brown, J.L.; Rosa-Caldwell, M.E.; Lee, D.E.; Blackwell, T.A.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Hardee, J.P.; Carson, J.A.; Wiggs, M.P.; et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2017, 8, 926–938. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Fix, D.K.; Carson, J.A. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: A role for inflammation. Oxid. Med. Cell. Longev. 2017, 2017, 3292087. [Google Scholar] [CrossRef]
- Chacon-Cabrera, A.; Mateu-Jimenez, M.; Langohr, K.; Fermoselle, C.; García-Arumí, E.; Andreu, A.L.; Yelamos, J.; Barreiro, E. Role of PARP activity in lung cancer-induced cachexia: Effects on muscle oxidative stress, proteolysis, anabolic markers, and phenotype. J. Cell. Physiol. 2017, 232, 3744–3761. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Busquets, S. Muscle wasting in cancer: The role of mitochondria. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 221–225. [Google Scholar] [CrossRef]
- Vitorino, R.; Moreira-Gonçalves, D.; Ferreira, R. Mitochondrial plasticity in cancer-related muscle wasting: Potential approaches for its management. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 226–233. [Google Scholar] [CrossRef]
- Petruzzelli, M.; Wagner, E.F. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016, 30, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Toneto, A.T.; Ferreira Ramos, L.A.; Salomão, E.M.; Tomasin, R.; Aereas, M.A.; Gomes-Marcondes, M.C.C. Nutritional leucine supplementation attenuates cardiac failure in tumour-bearing cachectic animals. J. Cachexia Sarcopenia Muscle 2016, 7, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Giles, K.; Guan, C.; Jagoe, T.R.; Mazurak, V. Diet composition as a source of variation in experimental animal models of cancer cachexia. J. Cachexia Sarcopenia Muscle 2016, 7, 110–125. [Google Scholar] [CrossRef][Green Version]
- Viana, L.R.; Canevarolo, R.; Luiz, A.C.; Soares, R.F.; Lubaczeuski, C.; Zeri, A.C.; Gomes-Marcondes, M.C. Leucine-rich diet alters the 1H-NMR based metabolomic profile without changing the Walker-256 tumour mass in rats. BMC Cancer 2016, 16, 764–778. [Google Scholar] [CrossRef]
- Nakano, M.; Nakashima, A.; Nagano, T.; Ishikawa, S.; Kikkawa, U.; Kamada, S. Branched-chain amino acids enhance premature senescence through mammalian target of rapamycin complex I-mediated upregulation of p21 protein. PLoS ONE 2013, 8, e80411. [Google Scholar] [CrossRef]
- Viana, L.R.; Tobar, N.; Busanello, E.N.B.; Marques, A.C.; de Oliveira, A.G.; Lima, T.I.; Machado, G.; Castelucci, B.G.; Ramos, C.D.; Brunetto, S.Q.; et al. Leucine-rich diet induces a shift in tumour metabolism from glycolytic towards oxidative phosphorylation, reducing glucose consumption and metastasis in Walker-256 tumour-bearing rats. Sci. Rep. 2019, 9, 15529–15540. [Google Scholar] [CrossRef] [PubMed]
- Bajotto, G.; Sato, Y.; Kitaura, Y.; Shimomura, Y. Effect of branched-chain amino acid supplementation during unloading on regulatory components of protein synthesis in atrophied soleus muscles. Eur. J. Appl. Physiol. 2011, 111, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Ebert, S.M.; Adams, C.M.; Rasmussen, B.B. Amino acid sensing in skeletal muscle. Trends Endocrinol. Metab. 2016, 27, 796–806. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- Sheffield-Moore, M.; Dillon, E.L.; Randolph, K.M.; Casperson, S.L.; White, G.R.; Jennings, K.; Rathmacher, J.; Schuette, S.; Janghorbani, M.; Urban, R.J.; et al. Isotopic decay of urinary or plasma 3-methylhistidine as a potential biomarker of pathologic skeletal muscle loss. J. Cachexia Sarcopenia Muscle 2014, 5, 19–25. [Google Scholar] [CrossRef]
- Gaunitz, F.; Hipkiss, A.R. Carnosine and cancer: A perspective. Amino Acids 2012, 43, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Artioli, G.G.; Sale, C.; Jones, R.L. Carnosine in health and disease. Eur. J. Sport Sci. 2019, 19, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Perim, P.; Marticorena, F.M.; Ribeiro, F.; Barreto, G.; Gobbi, N.; Kerksick, C.; Dolan, E.; Saunders, B. Can the skeletal muscle carnosine response to beta-alanine supplementation be optimised? Front. Nutr. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Yano, C.L.; Ventrucci, G.; Field, W.N.; Tisdale, M.J.; Gomes-Marcondes, M.C.C. Metabolic and morphological alterations induced by proteolysis-inducing factor from Walker tumour-bearing rats in C2C12 myotubes. BMC Cancer 2008, 8, 24. [Google Scholar] [CrossRef]
- Mirza, K.A.; Tisdale, M.J. Functional identity of receptors for proteolysis-inducing factor on human and murine skeletal muscle. Br. J. Cancer 2014, 111, 903–908. [Google Scholar] [CrossRef]
- Dechant, R.; Peter, M. Nutrient signals driving cell growth. Curr. Opin. Cell Biol. 2008, 20, 678–687. [Google Scholar] [CrossRef]
- Wang, X.; Proud, C.G. Nutrient control of TORC1, a cell-cycle regulator. Trends Cell Biol. 2009, 19, 260–267. [Google Scholar] [CrossRef]
- Katz, A.; Westerblad, H. Regulation of glycogen breakdown and its consequences for skeletal muscle function after training. Mamm. Genome 2014, 25, 464–472. [Google Scholar] [CrossRef]
- Borges, F.H.; Marinello, P.C.; Cecchini, A.L.; Blegniski, F.P.; Guarnier, F.A.; Cecchini, R. Oxidative and proteolytic profiles of the right and left heart in a model of cancer-induced cardiac cachexia. Pathophysiology 2014, 21, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Garcia-Smith, R.; Gannon, N.P.; Bisoffi, M.; Trujillo, K.A.; Conn, C.A. Leucine treatment enhances oxidative capacity through complete carbohydrate oxidation and increased mitochondrial density in skeletal muscle cells. Amino Acids 2013, 45, 901–911. [Google Scholar] [CrossRef]
- Toneto, A.T.; Toneto, A.T.; Silva, G.O.; Gomes-Marcondes, M.C.C. Leucine-rich diet and light aerobic training modulate GLUT4 expression and increase the glycogen content in the gastrocnemius muscle of Walker-256 tumour-bearing rats. J. Physiobiochemical Metab. 2014, 3. [Google Scholar] [CrossRef]
- Carson, J.A.; Hardee, J.P.; VanderVeen, B.N. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Semin. Cell Dev. Biol. 2016, 54, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Arnould, T.; Vankoningsloo, S.; Renard, P.; Houbion, A.; Ninane, N.; Demazy, C.; Remacle, J.; Raes, M. CREB activation induced by mitochondrial dysfunction is a new signaling pathway that impairs cell proliferation. EMBO J. 2002, 21, 53–63. [Google Scholar] [CrossRef]
- Salomão, E.M.; Toneto, A.T.; Silva, G.O.; Gomes-Marcondes, M.C.C. Physical exercise and a leucine-rich diet modulate the muscle protein metabolism in Walker tumor-bearing rats. Nutr. Cancer 2010, 62, 1095–1104. [Google Scholar] [CrossRef]
- Peshavaria, M.; Day, I.N. Molecular structure of the human muscle-specific enolase gene (ENO3). Biochem. J. 1991, 275 Pt 2, 427–433. [Google Scholar] [CrossRef]
- Gao, S.; Li, H.; Cai, Y.; Ye, J.; Liu, Z.; Lu, J.; Huang, X.; Feng, X.; Gao, H.; Chen, S.; et al. Mitochondrial binding of α-enolase stabilises mitochondrial membrane: Its role in doxorubicin-induced cardiomyocyte apoptosis. Arch. Biochem. Biophys. 2014, 542, 46–55. [Google Scholar] [CrossRef]
- Cruz, B.; Oliveira, A.; Ventrucci, G.; Gomes-Marcondes, M.C.C. A leucine-rich diet modulates the mTOR cell signalling pathway in the gastrocnemius muscle under different Walker-256 tumour growth conditions. BMC Cancer 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Vale, C.; Stewart, L.; Tierney, J. Trends in UK cancer trials: Results from the UK coordinating committee for cancer research national register of cancer trials. Br. J. Cancer 2005, 92, 811–814. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonçalves, E.M. Gomes-Marcondes, M.C.C. Leucine affects the fibroblastic Vero cells stimulating the cell proliferation and modulating the proteolysis process. Amino Acids 2010, 38, 145–153. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Salomão, E.M.; Gomes-Marcondes, M.C.C. Leucine modulates the effect of Walker factor, a proteolysis-inducing factor-like protein from Walker tumours, on gene expression and cellular activity in C2C12 myotubes. Cytokine 2013, 64, 343–350. [Google Scholar] [CrossRef]
- Le Belle, J.E.; Harris, N.G.; Williams, S.R.; Bhakoo, K.K. A comparison of cell and tissue extraction techniques using high-resolution 1H-NMR spectroscopy. NMR Biomed. 2002, 15, 37–44. [Google Scholar] [CrossRef]
- Distler, U.; Kuharev, J.; Navarro, P.; Levin, Y.; Schild, H.; Tenzer, S. Drift time-specific collision energies enable deep-coverage data-independent acquisition proteomics. Nat. Methods 2014, 11, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; Miao, Y.; Wang, Q.; Jiang, W.; Xu, C.; Li, J.; Han, J.; Zhang, F.; Gong, B.; et al. SubpathwayMiner: A software package for flexible identification of pathways. Nucleic Acids Res. 2009, 37, e131. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2016, 44, D481–D487. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gad, S. Statistics for toxicologists. In Principles and Methods of Toxicology; Hayes, A.W., Ed.; CRC Press: New York, NY, USA, 2008; pp. 369–452. [Google Scholar]

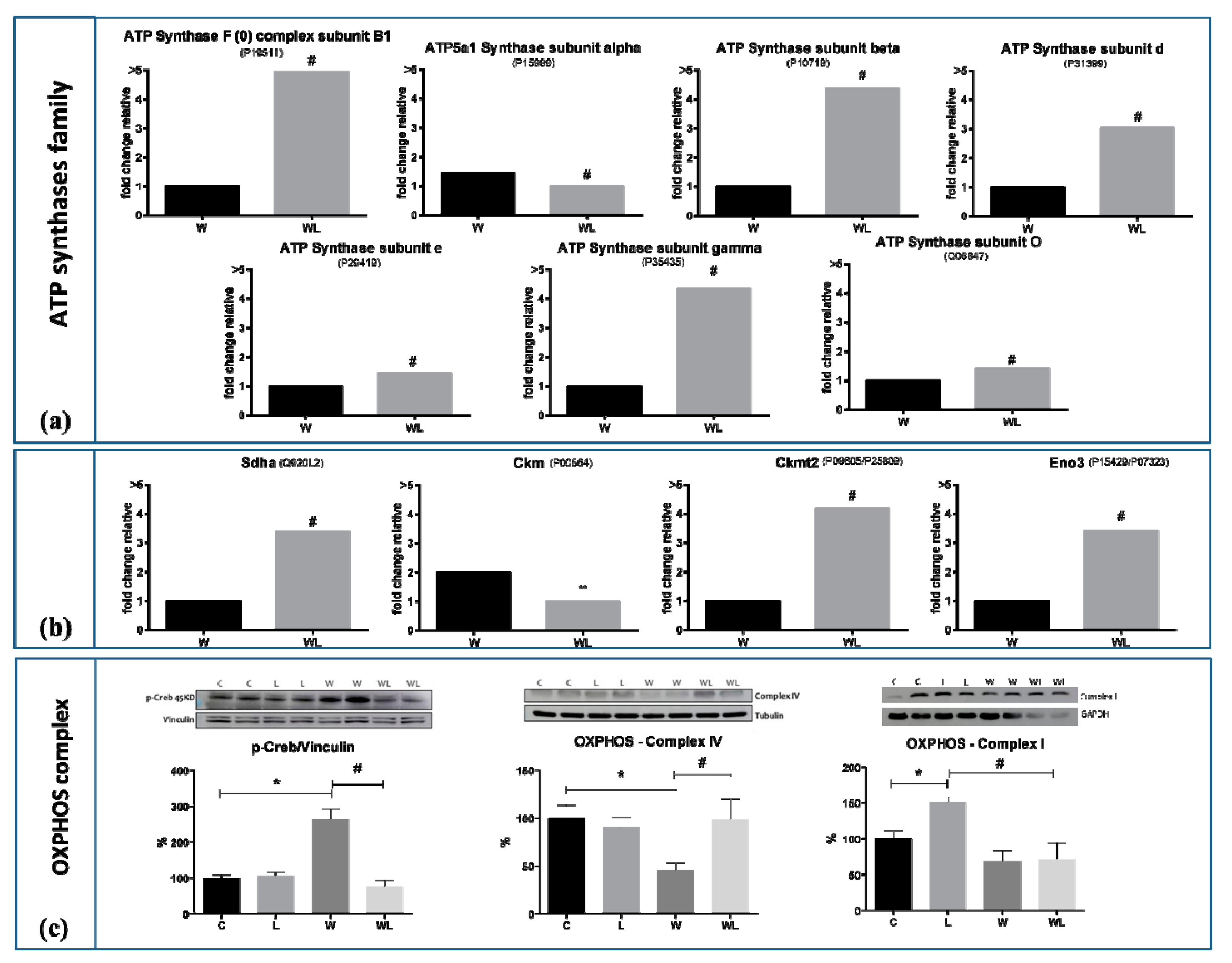
| Morphometric Parameters | Group | 7th Day | 14th Day | 21st Day |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Body Weight Gain (g) | C | 7.72 ± 0.13 | 14.45 ± 0.18 | 27.78 ± 2.78 |
| L | 7.84 ± 1.31 | 21.25 ± 2.78 | 34.10 ± 0.98 | |
| W | 7.00 ± 0.72 | 10.11 ± 1.96 * | 14.06 ± 1.92 * | |
| WL | 8.56 ± 0.67 | 13.67 ± 1.49 **# | 22.13 ± 2.79 **# | |
| Muscle Weight (g) | C | 2.20 ± 0.20 | 2.20 ± 0.10 | 2.20 ± 0.20 |
| L | 2.38 ± 0.33 | 2.31 ± 0.19 | 2.11 ± 0.13 | |
| W | 2.15 ± 0.19 | 1.78 ± 0.23 * | 1.70 ± 0.16 * | |
| WL | 2.48 ± 0.26 # | 2.04 ± 0.19 **# | 2.02 ± 0.13 # | |
| Lean Mass (%) | C | n/e | 23.39 ± 1.65 | 24.00 ± 1.41 |
| L | n/e | 20.49 ± 5.00 | 23.00 ± 2.64 | |
| W | n/e | 11.92 ± 2.93 * | 15.33 ± 3.56 * | |
| WL | n/e | 15.28 ± 7.18 ** | 19.25 ± 3.78 ** | |
| Body Fat (%) | C | n/e | 28.00 ± 1.56 | 29.70 ± 1.70 |
| L | n/e | 36.63 ± 1.50 | 35.83 ± 2.45 | |
| W | n/e | 25.15 ± 3.40 * | 23.75 ± 6.94 * | |
| WL | n/e | 28.20 ± 3.12 ** | 21.20 ± 6.27 ** | |
| Body Water (%) | C | n/e | 45.30 ± 2.20 | 43.00 ± 2.00 |
| L | n/e | 39.43 ± 2.86 | 37.67 ± 0.33 | |
| W | n/e | 59.38 ± 1.58 * | 56.50 ± 3.64 * | |
| WL | n/e | 53.00 ± 2.88 ** | 56.00 ± 3.48 ** | |
| Tumour Weight (g) | C | - | - | - |
| L | - | - | - | |
| W | 11.76 ± 1.89 | 22.14 ± 4.24 | 35.04 ± 3.49 | |
| WL | 15.22 ± 2.05 | 24.76 ± 2.89 | 38.8 ± 2.23 |
| Accession | Description | Max Fold Change | Highest Mean Condition | Lowest Mean Condition |
|---|---|---|---|---|
| Q5XI78 | 2-oxoglutarate dehydrogenase, mitochondrial GN = Ogdh | 1.805 | WL | W |
| 17764 | Acetyl-CoA acetyltransferase, mitochondrial GN = Acat1 | >5 | WL | W |
| Q9ER34 | Aconitate hydratase, mitochondria GN = Aco2 | 1.811 | WL | W |
| P00507 | Aspartate aminotransferase, mitochondrial GN = Got2 | 1.196 | WL | W |
| P19511 | ATP synthase F(0) complex subunit B1, mitochondrial GN = Atp5f1 | >5 | WL | W |
| P10719 | ATP synthase subunit beta, mitochondrial GN = Atp5b | 4.378 | WL | W |
| P31399 | ATP synthase subunit d, mitochondrial GN = Atp5h | 3.048 | WL | W |
| P29419 | ATP synthase subunit e, mitochondrial GN = Atp5i | 1.477 | WL | W |
| P35435 | ATP synthase subunit gamma, mitochondrial GN = Atp5c1 | 4.358 | WL | W |
| Q06647 | ATP synthase subunit O, mitochondrial GN = Atp5o | 1.420 | WL | W |
| Q8VHF5 | Citrate synthase, mitochondrial GN = Cs | 3.133 | WL | W |
| P09605; P25809 | Creatine kinase S-type, mitochondrial GN = Ckmt2 | 4.191 | WL | W |
| P32551 | Cytochrome b-c1 complex subunit 2, mitochondrial GN = Uqcrc2 | 1.853 | WL | W |
| P10888 | Cytochrome c oxidase subunit 4 isoform 1, mitochondrial GN = Cox4i1 | 3.775 | WL | W |
| P11240 | Cytochrome c oxidase subunit 5A, mitochondrial GN = Cox5a | 2.577 | WL | W |
| P12075 | Cytochrome c oxidase subunit 5B, mitochondrial GN = Cox5b | >5 | WL | W |
| P13803 | Electron transfer flavoprotein subunit alpha, mitochondrial GN = Etfa | 1.343 | WL | W |
| P14408 | Fumarate hydratase, mitochondrial GN = Fh | >5 | WL | W |
| P10860 | Glutamate dehydrogenase 1, mitochondrial GN = Glud1 | 2.046 | WL | W |
| P56574 | Isocitrate dehydrogenase [NADP], mitochondrial GN = Idh2 | 2.443 | WL | W |
| P15650 | Long-chain specific acyl-CoA dehydrogenase, mitochondrial GN = Acadl | 1.182 | WL | W |
| P04636 | Malate dehydrogenase, mitochondrial GN = Mdh2 | 1.922 | WL | W |
| P08503 | Medium-chain specific acyl-CoA dehydrogenase, mitochondrial GN = Acadm | >5 | WL | W |
| Q66HF1 | NADH-ubiquinone oxidoreductase 75 kDa subunit, mitochondrial GN = Ndufs1 | >5 | WL | W |
| Q9R063 | Peroxiredoxin-5, mitochondrial GN = Prdx5 | >5 | WL | W |
| P49432 | Pyruvate dehydrogenase E1 component subunit beta, mitochondrial GN = Pdhb | >5 | WL | W |
| P15651 | Short-chain specific acyl-CoA dehydrogenase, mitochondrial GN = Acads | 1.174 | WL | W |
| P48721 | Stress-70 protein, mitochondrial GN = Hspa9 | >5 | WL | W |
| Q920L2 | Succinate dehydrogenase [ubiquinone] flavoprotein subunit, mitochondrial GN = Sdha | 3.386 | WL | W |
| B2GV06 | Succinyl-CoA:3-ketoacid coenzyme A transferase 1, mitochondrial GN = Oxct1 | 1.576 | WL | W |
| Q9Z0V6 | Thioredoxin-dependent peroxide reductase, mitochondrial GN = Prdx3 | 1.458 | WL | W |
| Q64428 | Trifunctional enzyme subunit alpha, mitochondrial GN = Hadha | 1.682 | WL | W |
| Q60587 | Trifunctional enzyme subunit beta, mitochondrial GN = Hadhb | 2.138 | WL | W |
| P45953 | Very long-chain specific acyl-CoA dehydrogenase, mitochondrial GN = Acadvl | 3.580 | WL | W |
| Q99NA5 | Isocitrate dehydrogenase [NAD] subunit alpha, mitochondrial GN = Idh3a | 2.031 | W | WL |
| P15999 | ATP synthase subunit alpha, mitochondrial GN = Atp5a1 | 1.473 | W | WL |
| P13437 | 3-ketoacyl-CoA thiolase, mitochondrial GN = Acaa2 | 2.441 | W | WL |
| # Pathway ID | Molecular Function Pathway Description | Gene Count | FDR |
| GO.0003824 | Catalytic activity | 35 | 1.79 × 10−19 |
| GO.0016491 | Oxidoreductase activity | 17 | 2.23 × 10−14 |
| GO.0015078 | Hydrogen ion transmembrane transporter activity | 10 | 1.50 × 10−13 |
| GO.0043167 | Ion binding | 29 | 1.33 × 10−12 |
| GO.0048037 | Cofactor binding | 11 | 1.09 × 10−10 |
| GO.0009055 | Electron carrier activity | 7 | 1.71 × 10−09 |
| GO.0050662 | Coenzyme binding | 9 | 6.65 × 10−09 |
| GO.0003674 | Molecular function | 33 | 1.48 × 10−08 |
| GO.0046872 | Metal ion binding | 20 | 1.48 × 10−08 |
| GO.0005215 | Transporter activity | 14 | 2.68 × 10−08 |
| # Pathway ID | Biological Process Pathway Description | Gene Count | FDR |
| GO.0006091 | Generation of precursor metabolites and energy | 20 | 6.99 × 10−29 |
| GO.0044710 | Single-organism metabolic process | 34 | 3.18 × 10−23 |
| GO.0055114 | Oxidation-reduction process | 24 | 3.18 × 10−23 |
| GO.0019752 | Carboxylic acid metabolic process | 21 | 1.59 × 10−19 |
| GO.0072350 | Tricarboxylic acid metabolic process | 10 | 1.54 × 10−17 |
| GO.0044281 | Small molecule metabolic process | 23 | 2.07 × 10−17 |
| GO.0006099 | Tricarboxylic acid cycle | 9 | 3.87 × 10−16 |
| GO.0045333 | Cellular respiration | 11 | 8.45 × 10−16 |
| GO.0046496 | Nicotinamide nucleotide metabolic process | 11 | 8.45 × 10−16 |
| GO.0015980 | Energy derivation by oxidation of organic compounds | 12 | 1.31 × 10−15 |
| GO.0006732 | Coenzyme metabolic process | 13 | 2.82 × 10−15 |
| # Pathway ID | Cellular Component Pathway Description | Gene Count | FDR |
| GO.0005739 | Mitochondrion | 32 | 3.66 × 10−31 |
| GO.0031966 | Mitochondrial membrane | 23 | 1.17 × 10−26 |
| GO.0005740 | Mitochondrial envelope | 23 | 3.64 × 10−26 |
| GO.0044444 | Cytoplasmic part | 42 | 1.17 × 10−25 |
| GO.0005743 | Mitochondrial inner membrane | 19 | 1.29 × 10−23 |
| GO.0044429 | Mitochondrial part | 22 | 1.00 × 10−22 |
| GO.0043209 | Myelin sheath | 14 | 2.79 × 10−19 |
| GO.0005737 | Cytoplasm | 39 | 1.46 × 10−16 |
| GO.0043234 | Protein complex | 26 | 5.62 × 10−15 |
| GO.0070062 | Extracellular exosome | 20 | 6.93 × 10−15 |
| GO.0031090 | Organelle membrane | 24 | 1.04 × 10−14 |
| GO.0031988 | Membrane-bounded vesicle | 22 | 9.29 × 10−14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, B.; Oliveira, A.; Viana, L.R.; Lopes-Aguiar, L.; Canevarolo, R.; Colombera, M.C.; Valentim, R.R.; Garcia-Fóssa, F.; de Sousa, L.M.; Castelucci, B.G.; et al. Leucine-Rich Diet Modulates the Metabolomic and Proteomic Profile of Skeletal Muscle during Cancer Cachexia. Cancers 2020, 12, 1880. https://doi.org/10.3390/cancers12071880
Cruz B, Oliveira A, Viana LR, Lopes-Aguiar L, Canevarolo R, Colombera MC, Valentim RR, Garcia-Fóssa F, de Sousa LM, Castelucci BG, et al. Leucine-Rich Diet Modulates the Metabolomic and Proteomic Profile of Skeletal Muscle during Cancer Cachexia. Cancers. 2020; 12(7):1880. https://doi.org/10.3390/cancers12071880
Chicago/Turabian StyleCruz, Bread, André Oliveira, Lais Rosa Viana, Leisa Lopes-Aguiar, Rafael Canevarolo, Maiara Caroline Colombera, Rafael Rossi Valentim, Fernanda Garcia-Fóssa, Lizandra Maia de Sousa, Bianca Gazieri Castelucci, and et al. 2020. "Leucine-Rich Diet Modulates the Metabolomic and Proteomic Profile of Skeletal Muscle during Cancer Cachexia" Cancers 12, no. 7: 1880. https://doi.org/10.3390/cancers12071880
APA StyleCruz, B., Oliveira, A., Viana, L. R., Lopes-Aguiar, L., Canevarolo, R., Colombera, M. C., Valentim, R. R., Garcia-Fóssa, F., de Sousa, L. M., Castelucci, B. G., Consonni, S. R., Martins-de-Souza, D., de Jesus, M. B., Russell, S. T., & Gomes-Mardondes, M. C. C. (2020). Leucine-Rich Diet Modulates the Metabolomic and Proteomic Profile of Skeletal Muscle during Cancer Cachexia. Cancers, 12(7), 1880. https://doi.org/10.3390/cancers12071880









