Transitions of Liver and Biliary Enzymes during Proton Beam Therapy for Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Patients Characteristics
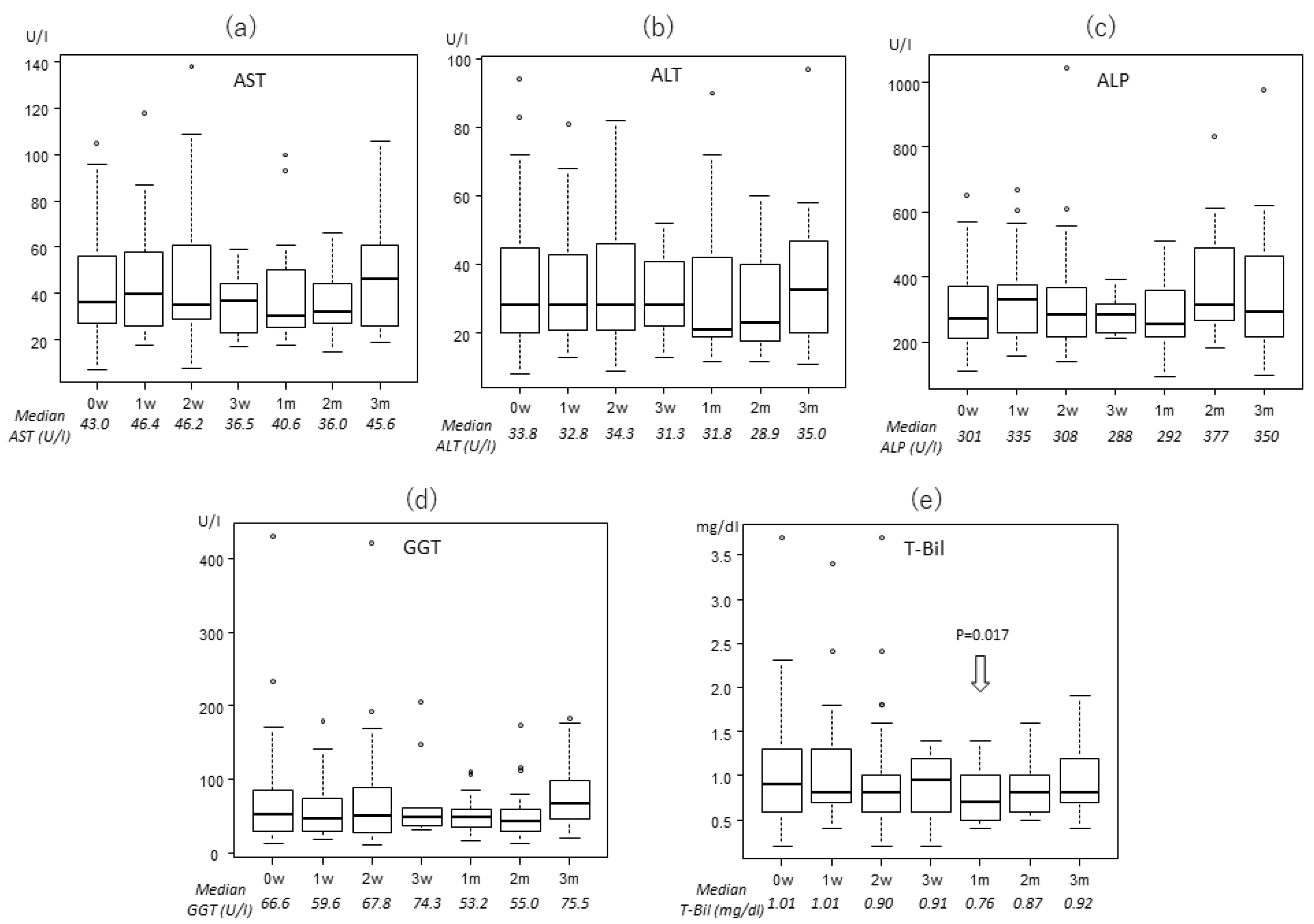
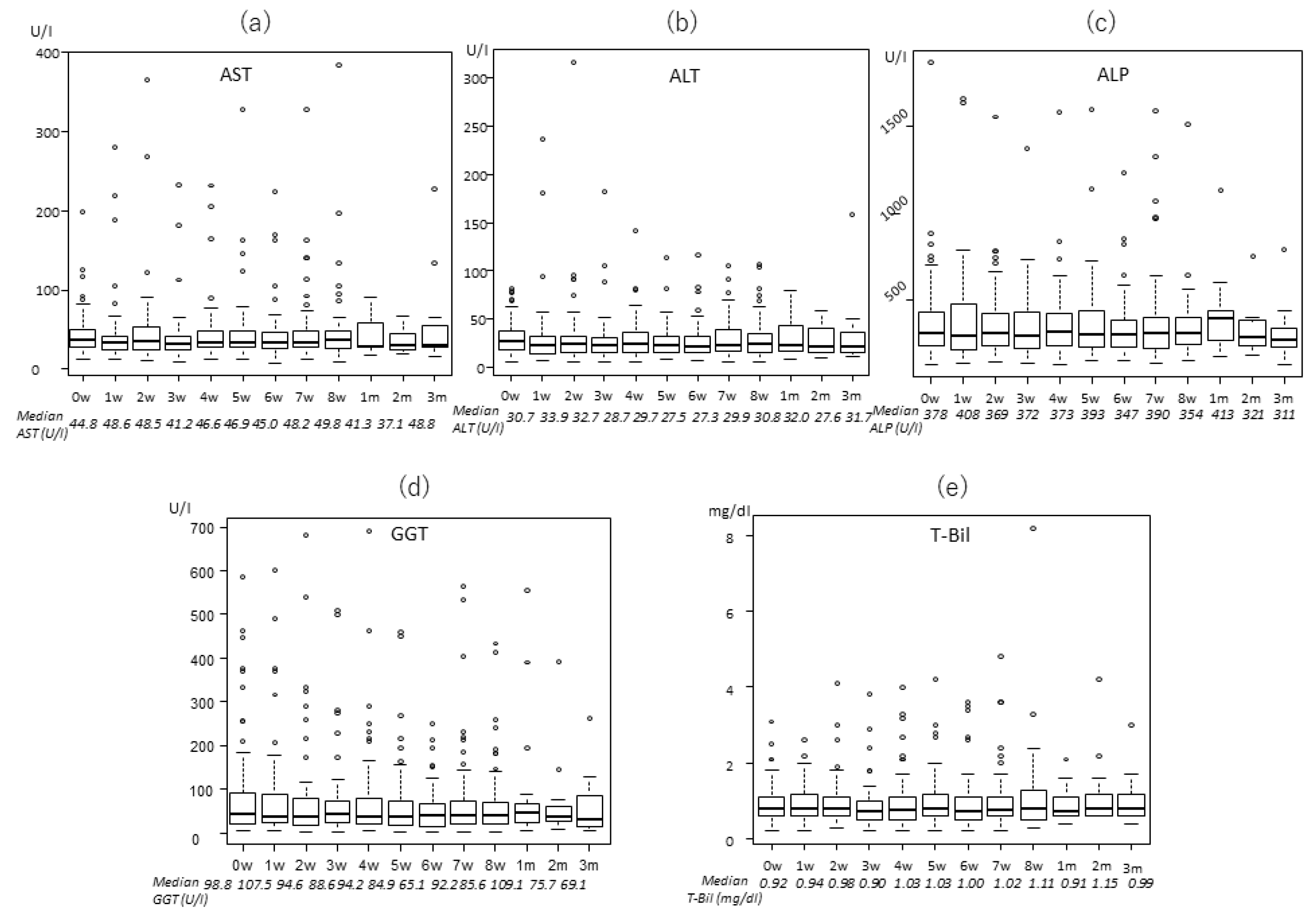
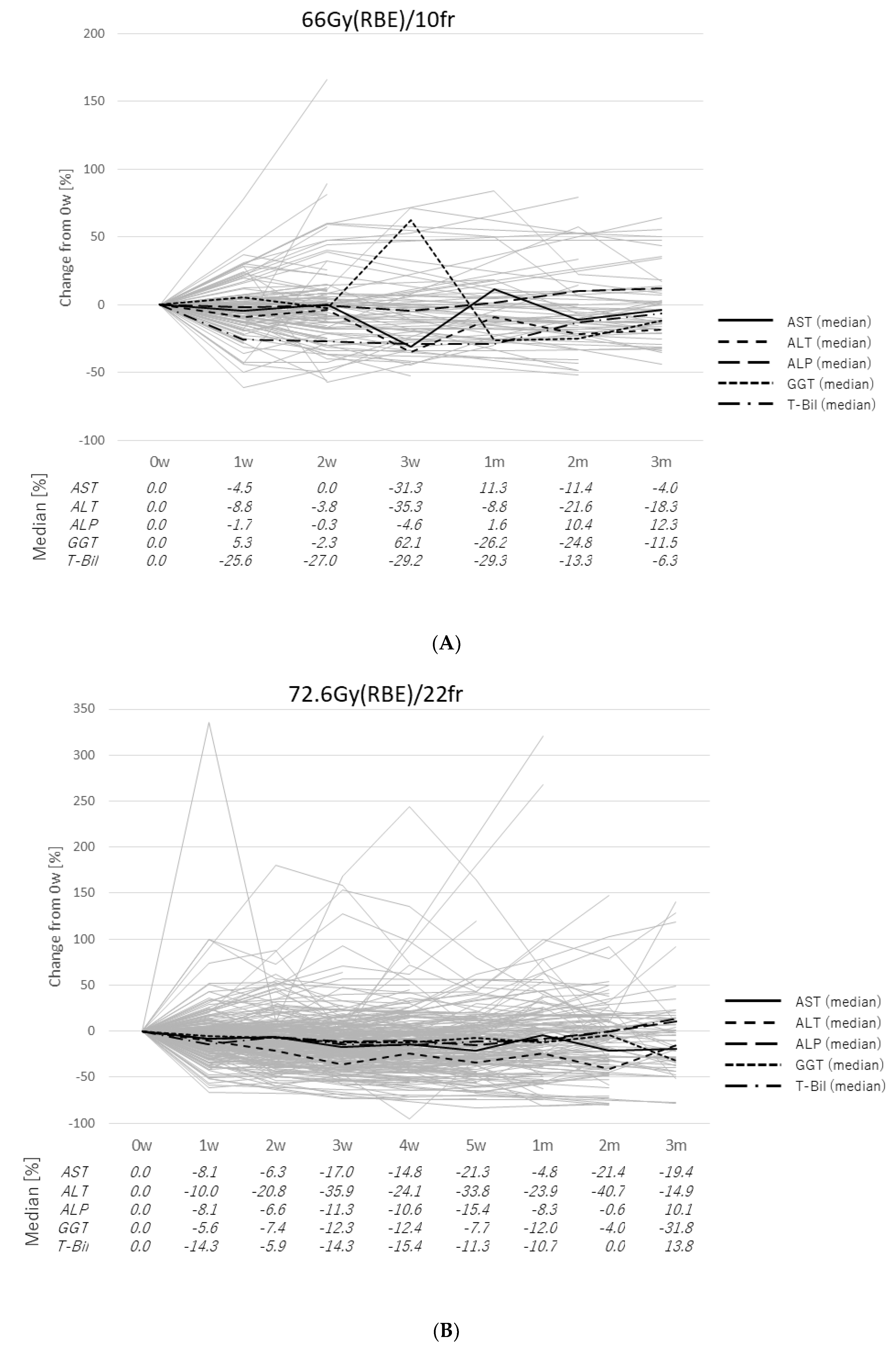
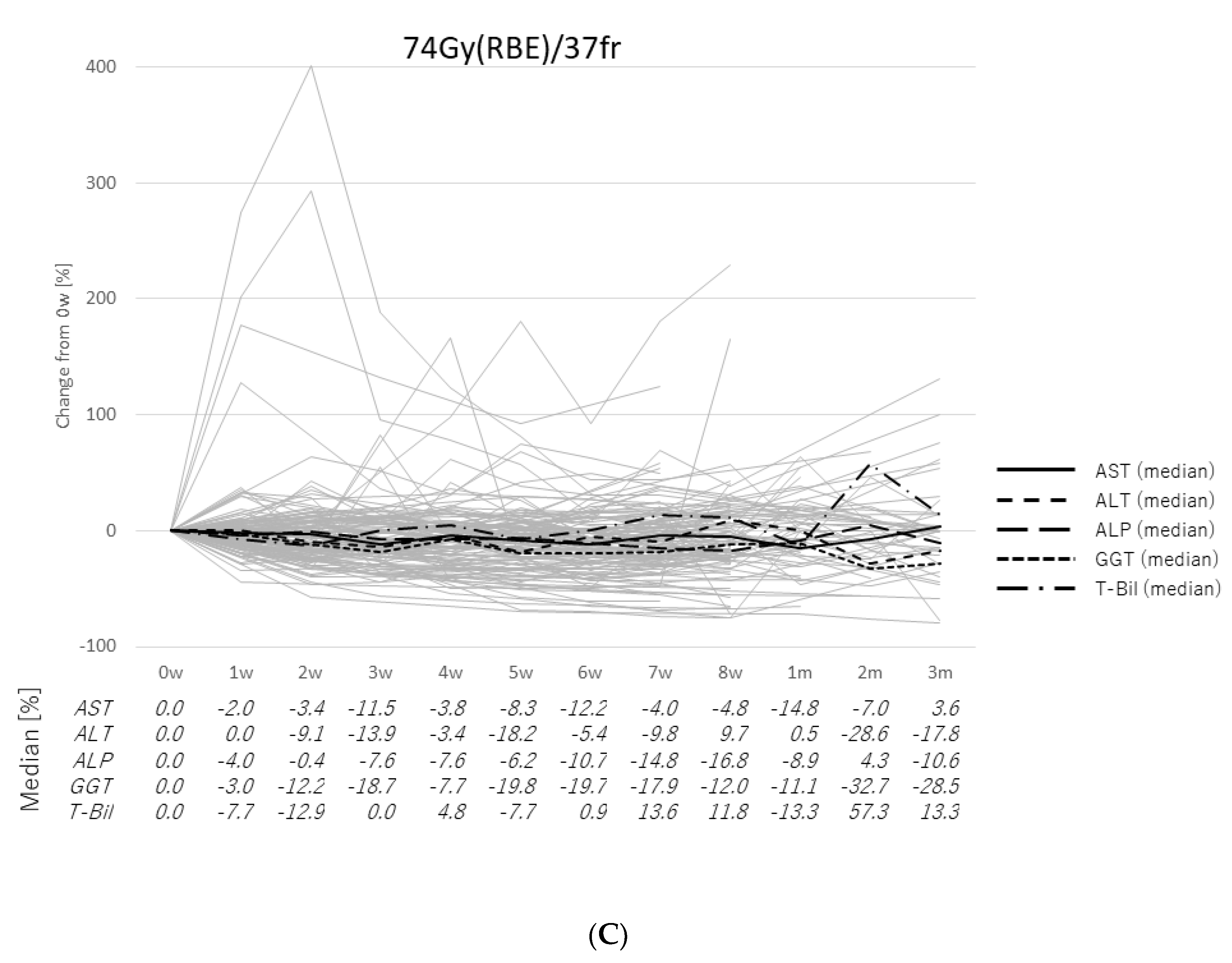
2.2. Transitions of the Laboratory Data
2.3. Case Presentation
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Proton Beam Therapy
4.3. Follow-Up Procedures
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghouri, Y.A.; Mian, I.; Rowe, J.H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16. [Google Scholar] [CrossRef]
- Mittal, S.; El-Serag, H.B. Epidemiology of HCC: Consider the Population. J. Clin. Gastroenterol. 2013, 47, S2–S6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El–Serag, H.B.; Rudolph, K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [CrossRef] [PubMed] [Green Version]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, K.; Okumura, T.; Abei, M.; Fukumitsu, N.; Ishige, K.; Mizumoto, M.; Hasegawa, N.; Numajiri, H.; Ohnishi, K.; Ishikawa, H.; et al. Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci. 2017, 108, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Fukumitsu, N.; Okumura, T.; Numajiri, H.; Murofushi, K.; Ohnishi, K.; Mizumoto, M.; Ishikawa, H.; Tsuboi, K.; Sakurai, H. Three cases of hepatocellular carcinoma treated 4 times with proton beams. Mol. Clin. Oncol. 2020, 12, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Sekino, Y.; Okumura, T.; Fukumitsu, N.; Iizumi, T.; Numajiri, H.; Mizumoto, M.; Nakai, K.; Nonaka, T.; Ishikawa, H.; Sakurai, H. Proton beam therapy for hepatocellular carcinoma associated with inferior vena cava tumor thrombus. J. Cancer Res. Clin. Oncol. 2020, 146, 711–720. [Google Scholar] [CrossRef]
- Nakamura, M.; Fukumitsu, N.; Kamizawa, S.; Numajiri, H.; Nemoto Murofushi, K.; Ohnishi, K.; Aihara, T.; Ishikawa, H.; Okumura, T.; Tsuboi, K.; et al. A validated proton beam therapy patch-field protocol for effective treatment of large hepatocellular carcinoma. J. Radiat. Res. 2018, 59, 632–638. [Google Scholar] [CrossRef]
- Fukumitsu, N.; Takahashi, S.; Okumura, T.; Ishida, T.; Murofushi, K.N.; Ohnishi, K.; Aihara, T.; Ishikawa, H.; Tsuboi, K.; Sakurai, H. Normal liver tissue change after proton beam therapy. Jpn. J. Radiol. 2018, 36, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, M.; Oshiro, Y.; Okumura, T.; Fukumitsu, N.; Numajiri, H.; Ohnishi, K.; Aihara, T.; Ishikawa, H.; Tsuboi, K.; Sakurai, H. Proton Beam Therapy for Hepatocellular Carcinoma: A Review of the University of Tsukuba Experience. Int. J. Part. Ther. 2016, 2, 570–578. [Google Scholar] [CrossRef]
- Mizumoto, M.; Okumura, T.; Hashimoto, T.; Fukuda, K.; Oshiro, Y.; Fukumitsu, N.; Abei, M.; Kawaguchi, A.; Hayashi, Y.; Ookawa, A.; et al. Proton Beam Therapy for Hepatocellular Carcinoma: A Comparison of Three Treatment Protocols. Int. J. Radiat. Oncol. 2011, 81, 1039–1045. [Google Scholar] [CrossRef]
- Sugahara, S.; Nakayama, H.; Fukuda, K.; Mizumoto, M.; Tokita, M.; Abei, M.; Shoda, J.; Matsuzaki, Y.; Thono, E.; Tsuboi, K.; et al. Proton-Beam Therapy for Hepatocellular Carcinoma Associated with Portal Vein Tumor Thrombosis. Strahlenther. Onkol. 2009, 185, 782. [Google Scholar] [CrossRef]
- Sugahara, S.; Oshiro, Y.; Nakayama, H.; Fukuda, K.; Mizumoto, M.; Abei, M.; Shoda, J.; Matsuzaki, Y.; Thono, E.; Tokita, M.; et al. Proton Beam Therapy for Large Hepatocellular Carcinoma. Int. J. Radiat. Oncol. 2010, 76, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Fukumitsu, N.; Sugahara, S.; Nakayama, H.; Fukuda, K.; Mizumoto, M.; Abei, M.; Shoda, J.; Thono, E.; Tsuboi, K.; Tokuuye, K. A Prospective Study of Hypofractionated Proton Beam Therapy for Patients With Hepatocellular Carcinoma. Int. J. Radiat. Oncol. 2009, 74, 831–836. [Google Scholar] [CrossRef]
- Mizumoto, M.; Tokuuye, K.; Sugahara, S.; Nakayama, H.; Fukumitsu, N.; Ohara, K.; Abei, M.; Shoda, J.; Tohno, E.; Minami, M. Proton Beam Therapy for Hepatocellular Carcinoma Adjacent to the Porta Hepatis. Int. J. Radiat. Oncol. 2008, 71, 462–467. [Google Scholar] [CrossRef]
- Hata, M.; Tokuuye, K.; Sugahara, S.; Tohno, E.; Nakayama, H.; Fukumitsu, N.; Mizumoto, M.; Abei, M.; Shoda, J.; Minami, M.; et al. Proton Beam Therapy for Aged Patients With Hepatocellular Carcinoma. Int. J. Radiat. Oncol. 2007, 69, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Tokuuye, K.; Sugahara, S.; Fukumitsu, N.; Hashimoto, T.; Ohnishi, K.; Nemoto, K.; Ohara, K.; Matsuzaki, Y.; Akine, Y. Proton Beam Therapy for Hepatocellular Carcinoma Patients with Severe Cirrhosis. Strahlenther. Onkol. 2006, 182, 713. [Google Scholar] [CrossRef] [Green Version]
- Mizumoto, M.; Oshiro, Y.; Okumura, T.; Fukuda, K.; Fukumitsu, N.; Abei, M.; Ishikawa, H.; Ohnishi, K.; Numajiri, H.; Tsuboi, K.; et al. Association between pretreatment retention rate of indocyanine green 15min after administration and life prognosis in patients with HCC treated by proton beam therapy. Radiother. Oncol. 2014, 113, 54–59. [Google Scholar] [CrossRef]
- Mizumoto, M.; Okumura, T.; Hashimoto, T.; Fukuda, K.; Oshiro, Y.; Fukumitsu, N.; Abei, M.; Kawaguchi, A.; Hayashi, Y.; Ohkawa, A.; et al. Evaluation of Liver Function After Proton Beam Therapy for Hepatocellular Carcinoma. Int. J. Radiat. Oncol. 2012, 82, e529–e535. [Google Scholar] [CrossRef]
- Oshiro, Y.; Mizumoto, M.; Okumura, T.; Fukuda, K.; Fukumitsu, N.; Abei, M.; Ishikawa, H.; Takizawa, D.; Sakurai, H. Analysis of repeated proton beam therapy for patients with hepatocellular carcinoma. Radiother. Oncol. 2017, 123, 240–245. [Google Scholar] [CrossRef]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef]
- Dufour, D.R.; Lott, J.A.; Nolte, F.S.; Gretch, D.R.; Koff, R.S.; Seeff, L.B. Diagnosis and Monitoring of Hepatic Injury. I. Performance Characteristics of Laboratory Tests. Clin. Chem. 2000, 46, 2027–2049. [Google Scholar] [CrossRef]
- Dufour, D.R.; Lott, J.A.; Nolte, F.S.; Gretch, D.R.; Koff, R.S.; Seeff, L.B. Diagnosis and Monitoring of Hepatic Injury. II. Recommendations for Use of Laboratory Tests in Screening, Diagnosis, and Monitoring. Clin. Chem. 2000, 46, 2050–2068. [Google Scholar] [CrossRef]
- Vroon, D.H.; Israili, Z. Alkaline Phosphatase and Gamma Glutamyltransferase. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Fevery, J.; Blanckaert, N. What can we learn from analysis of serum bilirubin? J. Hepatol. 1986, 2, 113–121. [Google Scholar] [CrossRef]
- Igaki, H.; Mizumoto, M.; Okumura, T.; Hasegawa, K.; Kokudo, N.; Sakurai, H. A systematic review of publications on charged particle therapy for hepatocellular carcinoma. Int. J. Clin. Oncol. 2018, 23, 423–433. [Google Scholar] [CrossRef]
- Wang, X.; Krishnan, S.; Zhang, X.; Dong, L.; Briere, T.; Crane, C.H.; Martel, M.; Gillin, M.; Mohan, R.; Beddar, S. Proton Radiotherapy for Liver Tumors: Dosimetric Advantages Over Photon Plans. Med. Dosim. 2008, 33, 259–267. [Google Scholar] [CrossRef]
- Gandhi, S.J.; Liang, X.; Ding, X.; Zhu, T.C.; Ben-Josef, E.; Plastaras, J.P.; Metz, J.M.; Both, S.; Apisarnthanarax, S. Clinical decision tool for optimal delivery of liver stereotactic body radiation therapy: Photons versus protons. Pract. Radiat. Oncol. 2015, 5, 209–218. [Google Scholar] [CrossRef]
- Andolino, D.L.; Johnson, C.S.; Maluccio, M.; Kwo, P.; Tector, A.J.; Zook, J.; Johnstone, P.A.S.; Cardenes, H.R. Stereotactic Body Radiotherapy for Primary Hepatocellular Carcinoma. Int. J. Radiat. Oncol. 2011, 81, e447–e453. [Google Scholar] [CrossRef]
- Culleton, S.; Jiang, H.; Haddad, C.R.; Kim, J.; Brierley, J.; Brade, A.; Ringash, J.; Dawson, L.A. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother. Oncol. 2014, 111, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.S.; Robertson, J.M.; Anscher, M.S.; Jirtle, R.L.; Ensminger, W.D.; Fajardo, L.F. Hepatic toxicity resulting from cancer treatment. Int. J. Radiat. Oncol. 1995, 31, 1237–1248. [Google Scholar] [CrossRef]
- Bush, D.A.; Kayali, Z.; Grove, R.; Slater, J.D. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: A phase 2 prospective trial. Cancers 2011, 117, 3053–3059. [Google Scholar] [CrossRef] [PubMed]
- Shirai, S.; Sato, M.; Noda, Y.; Kishi, K.; Kawai, N.; Minamiguchi, H.; Nakai, M.; Sanda, H.; Sahara, S.; Ikoma, A.; et al. Distribution of Functional Liver Volume in Hepatocellular Carcinoma Patients with Portal Vein Tumor Thrombus in the 1st Branch and Main Trunk Using Single Photon Emission Computed Tomography—Application to Radiation Therapy. Cancers 2011, 3, 4114–4126. [Google Scholar] [CrossRef]
- Ohara, K.; Okumura, T.; Akisada, M.; Inada, T.; Mori, T.; Yokota, H.; Calaguas, M.J.B. Irradiation synchronized with respiration gate. Int. J. Radiat. Oncol. 1989, 17, 853–857. [Google Scholar] [CrossRef]
- Tsunashima, Y.; Sakae, T.; Shioyama, Y.; Kagei, K.; Terunuma, T.; Nohtomi, A.; Akine, Y. Correlation between the respiratory waveform measured using a respiratory sensor and 3D tumor motion in gated radiotherapy. Int. J. Radiat. Oncol. 2004, 60, 951–958. [Google Scholar] [CrossRef]
- Fisher, R.A. On the Interpretation of χ2 from Contingency Tables, and the Calculation of P. J. R. Stat. Soc. 1922, 85, 87–94. [Google Scholar] [CrossRef]
- Welch, B.L. The generalization of students’ problem when several different population variances are involved. Biometrika 1947, 34, 28–35. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]


| Proton Therapy | Total | 66 Gy (RBE)/10 Fractions | 72.6 Gy (RBE)/22 Fractions | 74 Gy (RBE)/37 Fractions |
|---|---|---|---|---|
| Patient number (%) | 300 | 77 (25.7%) | 148 (49.3%) | 75 (25%) |
| Median age (range) | 73 (27–91) | 70 (42–88) | 72.5 (33–91) | 77 (27–91) |
| Gender | ||||
| Male | 219 (73%) | 53 (68.8%) | 114 (77%) | 52 (69.3%) |
| Female | 81 (27%) | 24 (31.2%) | 34 (23%) | 23 (30.7%) |
| Alcoholic liver cirrhosis | ||||
| No | 256 (85.3%) | 69 (89.6% | 121 (81.8%) | 66 (88%) |
| Yes | 44 (14.7%) | 8 (10.4%) | 27 (18.2%) | 9 (12%) |
| Hepatitis B | ||||
| No | 249 (83%) | 65 (84.4%) | 120 (81.1%) | 64 (85.3%) |
| Yes | 51 (17%) | 12 (15.6%) | 28 (18.9%) | 11 (14.7%) |
| Hepatitis C | ||||
| No | 160 (53.3%) | 35 (45.5%) | 86 (58.1%) | 39 (52%) |
| Yes | 140 (46.7%) | 42 (54.5%) | 62 (41.9%) | 36 (48%) |
| Pugh score | ||||
| 5 | 165 (55%) | 48 (62.3%) | 79 (53.4%) | 38 (50.7%) |
| 6 | 72 (24%) | 16 (20.8%) | 35 (23.6%) | 21 (28%) |
| 7 | 36 (12%) | 7 (9.1%) | 17 (11.5%) | 12 (16%) |
| 8 | 19 (6.3% | 5 (6.5%) | 12 (8.1%) | 2 (2.7%) |
| 9 | 7 (2.3%) | 1 (1.3%) | 4 (2.7%) | 2 (2.7%) |
| 10 | 1 (0.3%) | 0 (0%) | 1 (0.7%) | 0 (0%) |
| Portal vein thrombosis (Vp) | ||||
| 0 | 252 (84%) | 77 (100%) | 111 (75%) | 64 (85.3%) |
| 1 | 3 (1%) | 0 (0%) | 3 (2%) | 0 (0%) |
| 2 | 16 (5.3%) | 0 (0%) | 12 (8.1%) | 4 (5.3%) |
| 3 | 18 (6%) | 0 (0%) | 13 (8.8%) | 5 (6.7%) |
| 4 | 11 (3.7%) | 0 (0%) | 9 (6.1%) | 2 (2.7%) |
| Median CTV (cc) | 84.5 (3–3236) | 23.5 (7–337) | 93 (3–3236) | 113 (6–1681) |
| Median pretreatment values (range) | ||||
| AST (U/l) | 36 (7–199) | 36 (7–105) | 35.5 (13–171) | 37 (13–199) |
| ALT (U/l) | 27 (4–145) | 28 (8–94) | 26 (4–145) | 26 (6–82) |
| ALP (U/l) | 294 (78–1869) | 269 (110–652) | 292.5 (78–1162) | 308 (129–1869) |
| GGT (U/l) | 55 (13–1435) | 51 (13–431) | 59 (13–1435) | 54 (16–599) |
| T-Bil (mg/dl) | 0.8 (0.2–4.9) | 0.9 (0.2–3.7) | 0.9 (0.2–4.9) | 0.8 (0.2–3.1) |
| Laboratory Data | CTCAE Grade | Fractionation of PBT | p-Value | Number of Patients | ||
|---|---|---|---|---|---|---|
| 10fr | 22fr | 37fr | ||||
| AST | 0 | 32 (80%) | 68 (81.9%) | 27 (67.5%) | 0.204 | 127 (77.9%) |
| 1 | 8 (20%) | 15 (18.1%) | 11 (27.5%) | 34 (20.9%) | ||
| 2 | 0 (0%) | 0 (0%) | 1 (2.5%) | 1 (0.6%) | ||
| 3 | 0 (0%) | 0 (0%) | 1 (2.5%) | 1 (0.6%) | ||
| Total | 40 | 83 | 40 | 163 | ||
| ALT | 0 | 51 (89.5%) | 102 (87.9%) | 49 (81.7%) | 0.429 | 202 (86.7%) |
| 1 | 6 (10.5%) | 14 (12.1%) | 10 (16.7%) | 30 (12.9%) | ||
| 2 | 0 (0%) | 0 (0%) | 1 (1.7%) | 1 (0.4%) | ||
| Total | 57 | 116 | 60 | 233 | ||
| ALP | 0 | 42 (76.4%) | 73 (76.8%) | 32 (69.6%) | 0.636 | 147 (75%) |
| 1 | 13 (23.6%) | 22 (23.2%) | 14 (30.4%) | 49 (25%) | ||
| Total | 55 | 95 | 46 | 196 | ||
| GGT | 0 | 44 (91.7%) | 65 (79.3%) | 36 (87.8%) | 0.130 | 145 (84.8%) |
| 1 | 3 (6.2%) | 16 (19.5%) | 5 (12.2%) | 24 (14%) | ||
| 2 | 1 (2.1%) | 0 (0%) | 0 (0%) | 1 (0.6%) | ||
| 3 | 0 (0%) | 1 (1.2%) | 0 (0%) | 1 (0.6%) | ||
| Total | 48 | 82 | 41 | 171 | ||
| T-Bil | 0 | 50 (86.2%) | 85 (78%) | 49 (81.7%) | 0.105 | 184 (81.1%) |
| 1 | 8 (13.8%) | 17 (15.6%) | 10 (16.7%) | 35 (15.4%) | ||
| 2 | 0 (0%) | 7 (6.4%) | 0 (0%) | 7 (3.1%) | ||
| 3 | 0 (0%) | 0 (0%) | 1 (1.7%) | 1 (0.4%) | ||
| Total | 58 | 109 | 60 | 227 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumiya, T.; Mizumoto, M.; Oshiro, Y.; Baba, K.; Murakami, M.; Shimizu, S.; Nakamura, M.; Hiroshima, Y.; Ishida, T.; Iizumi, T.; et al. Transitions of Liver and Biliary Enzymes during Proton Beam Therapy for Hepatocellular Carcinoma. Cancers 2020, 12, 1840. https://doi.org/10.3390/cancers12071840
Sumiya T, Mizumoto M, Oshiro Y, Baba K, Murakami M, Shimizu S, Nakamura M, Hiroshima Y, Ishida T, Iizumi T, et al. Transitions of Liver and Biliary Enzymes during Proton Beam Therapy for Hepatocellular Carcinoma. Cancers. 2020; 12(7):1840. https://doi.org/10.3390/cancers12071840
Chicago/Turabian StyleSumiya, Taisuke, Masashi Mizumoto, Yoshiko Oshiro, Keiichiro Baba, Motohiro Murakami, Shosei Shimizu, Masatoshi Nakamura, Yuichi Hiroshima, Toshiki Ishida, Takashi Iizumi, and et al. 2020. "Transitions of Liver and Biliary Enzymes during Proton Beam Therapy for Hepatocellular Carcinoma" Cancers 12, no. 7: 1840. https://doi.org/10.3390/cancers12071840





