Presenting Features and Early Mortality from SARS-CoV-2 Infection in Cancer Patients during the Initial Stage of the COVID-19 Pandemic in Europe
Abstract
1. Introduction
2. Results
2.1. Demographics and Oncological Features
2.2. Features of COVID-19 Disease
2.3. Outcomes
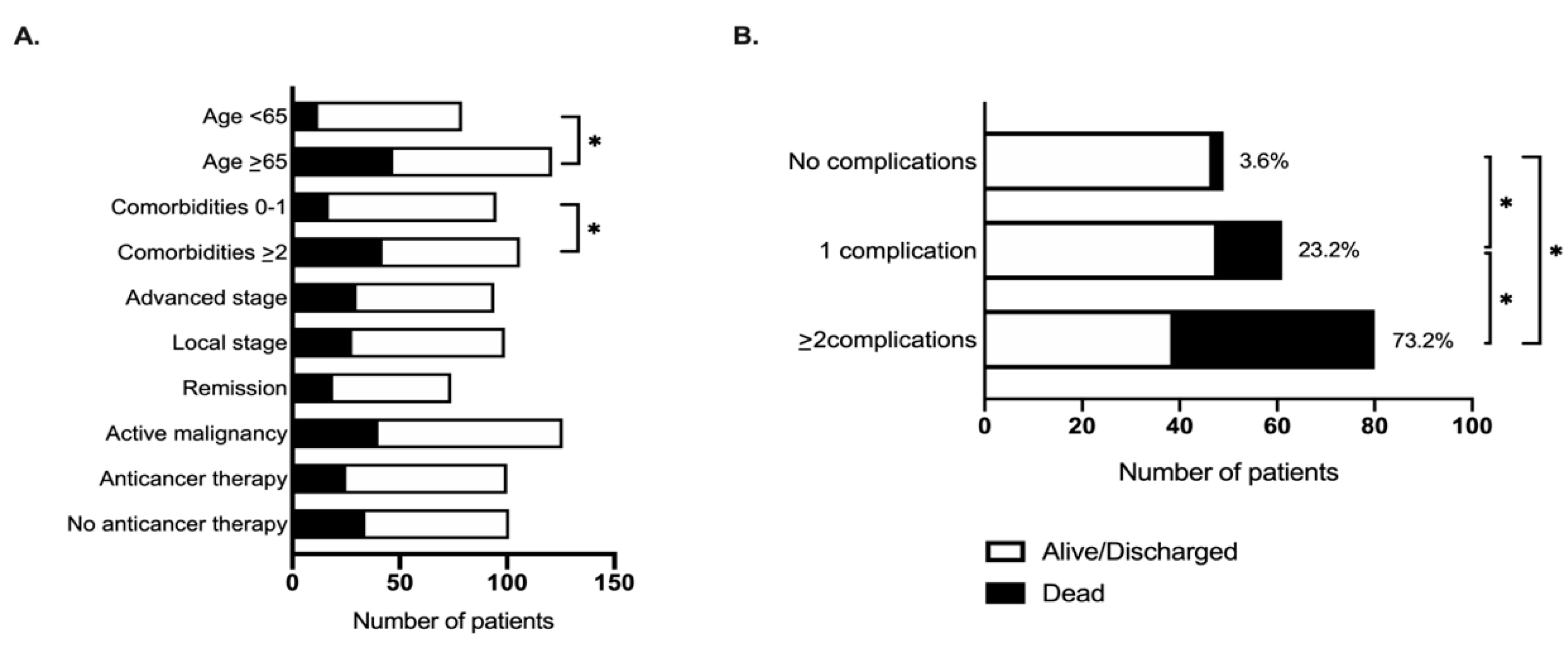
2.4. Factors Associated with Mortality from COVID-19 in Cancer Patients
3. Discussion
4. Materials and Methods
4.1. Study Population, Setting, and Data Collection
4.2. Study Definitions
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Lake, M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin. Med. (Lond.) 2020, 20, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Garassino, M.C.; Whisenant, J.G.; Huang, L.C.; Trama, A.; Torri, V.; Agustoni, F.; Baena, J.; Banna, G.; Berardi, R.; Bettini, A.C.; et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort study. Lancet Oncol. 2020. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer Appear More Vulnerable to SARS-COV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Mehta, V.; Goel, S.; Kabarriti, R.; Cole, D.; Goldfinger, M.; Acuna-Villaorduna, A.; Pradhan, K.; Thota, R.; Reissman, S.; Sparano, J.A.; et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020. [Google Scholar] [CrossRef]
- Robilotti, E.V.; Babady, N.E.; Mead, P.A.; Rolling, T.; Perez-Johnston, R.; Bernardes, M.; Bogler, Y.; Caldararo, M.; Figueroa, C.J.; Glickman, M.S.; et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020. [Google Scholar] [CrossRef]
- Luo, J.; Rizvi, H.; Egger, J.V.; Preeshagul, I.R.; Wolchok, J.D.; Hellmann, M.D. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020. [Google Scholar] [CrossRef]
- World Health Organization. Novel Coronavirus (COVID-19) Situation. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 28 June 2020).
- Flaxman, S.; Mishra, S.; Gandy, A.; Unwin, H.J.T.; Mellan, T.A.; Coupland, H.; Whittaker, C.; Zhu, H.; Berah, T.; Eaton, J.W.; et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 2020. [Google Scholar] [CrossRef]
- Ueda, M.; Martins, R.; Hendrie, P.C.; McDonnell, T.; Crews, J.R.; Wong, T.L.; McCreery, B.; Jagels, B.; Crane, A.; Byrd, D.R.; et al. Managing Cancer Care during the COVID-19 Pandemic: Agility and Collaboration toward a Common Goal. J. Natl. Compr. Cancer Netw. 2020, 1–4. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, R.; Zhao, J.; Li, W.; Shen, H. Risk of COVID-19 for cancer patients. Lancet Oncol. 2020. [Google Scholar] [CrossRef]
- Public Health England. Guidance on Shielding and Protecting People Who Are Clinically Extremely Vulnerable from COVID-19. Available online: https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19 (accessed on 28 June 2020).
- National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Delivery of Systemic Anticancer Treatments NICE Guideline [NG161]. Available online: https://www.nice.org.uk/guidance/ng161 (accessed on 28 June 2020).
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. N. Engl. J. Med. 2020, 382, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Hanna, T.P.; Evans, G.A.; Booth, C.M. Cancer, COVID-19 and the precautionary principle: Prioritizing treatment during a global pandemic. Nat. Rev. Clin. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020, 21, e181. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Cazier, J.B.; Starkey, T.; Turnbull, C.D.; Kerr, R.; Middleton, G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Chen, Y.; et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Henry, B.M. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur. J. Intern. Med. 2020, 75, 107–108. [Google Scholar] [CrossRef]
- Williamson, E.; Walker, A.J.; Bhaskaran, K.J.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. OpenSAFELY: Factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Sehatzadeh, S. Cardiopulmonary Resuscitation in Patients with Terminal Illness: An Evidence-Based Analysis. Ont. Health Technol. Assess. Ser. 2014, 14, 1–38. [Google Scholar] [PubMed]
- Lewis, M.A. Between Scylla and Charybdis—Oncologic Decision Making in the Time of Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- WHO. Clinical Management of Severe Acuite Respiratory Infection (SARI) when Covid-19 Disease is Supected: Interim Guidance V 1.2.; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brunink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eur. Surveill. 2020, 25. [Google Scholar] [CrossRef]
| Characteristic | Italy (n = 56) | Spain (n = 51) | UK (n = 97) | Total (n = 204) |
|---|---|---|---|---|
| Age (Years), mean ± SD | 66.3 ± 14.0 | 69.4 ± 10.2 | 71.0 ± 13.3 | 69.3 ± 13.0 |
| Age ≥ 65 years No. (%) | 34 (60.7) | 32 (62.7) | 63 (65.0) | 129 (63.2) |
| Sex No. (%) | ||||
| Male | 32 (57.1) | 31 (60.8) | 64 (66.0) | 127 (62.3) |
| Female | 24 (42.9) | 20 (39.2) | 33 (34.0) | 77 (37.7) |
| Smoking history No. (%) | ||||
| Never smoker | 24 (42.9) | 21 (41.2) | 34 (35.0) | 79 (38.7) |
| Current/former smoker | 25 (44.6) | 28 (54.9) | 59 (60.8) | 112 (54.9) |
| Unknown | 7 (12.5) | 2 (3.9) | 4 (4.1) | 13 (6.4) |
| Cancer type No. (%) | ||||
| Genito-urinary | 7 (12.5) | 11 (21.6) | 25 (25.8) | 43 (21.1) |
| Lung | 6 (10.7) | 12 (23.5) | 18 (18.6) | 36 (17.7) |
| Gastrointestinal | 8 (14.3) | 6 (11.8) | 14 (14.4) | 28 (13.7) |
| Breast | 8 (14.3) | 8 (15.7) | 11(11.3) | 27 (13.2) |
| Gynecological | 8 (14.3) | 3 (5.9) | 7 (7.2) | 13 (6.4) |
| Gastro-esophageal | 3 (5.4) | 2 (3.9) | 5 (5.1) | 10 (4.9) |
| Hepatobiliary | 2 (3.6) | 1 (2.0) | 6 (6.2) | 9 (4.4) |
| Head and neck | 1 (1.8) | 2 (3.9) | 4 (4.1) | 7 (3.4) |
| Skin | - | 2 (3.9) | 1 (1.0) | 3 (1.5) |
| Other | 2 (3.6) | 3 (5.9) | 1 (1.0) | 6 (2.9) |
| Hematological malignancies | 16 (28.6) | - | 3 (3.1) | 19 (9.8) |
| Tumor Stage * No. (%) | ||||
| Localized | 16 (43.2) | 15 (29.4) | 63 (64.9) | 94 (50.8) |
| Metastatic | 20 (54.0) | 30 (58.8) | 32 (33.0) | 82 (44.3) |
| Unknown | 1 (2.7) | 6 (11.8) | 2 (2.1) | 9 (4.9) |
| Prior treatments No. (%) | ||||
| Surgery | 19 (33.9) | 28 (54.9) | 49 (50.5) | 96 (47.1) |
| Adjuvant/neoadjuvant chemotherapy | 7 (12.5) | 17 (17.5) | 25 (25.8) | 49 (24.0) |
| Palliative systemic therapy No. (%) | 21 (37.5) | 18 (35.3) | 22 (22.7) | 61 (29.9) |
| Chemotherapy | 18 (85.7) | 7 (38.9) | 13 (59.1) | 38 (62.3) |
| Immunotherapy | 4 (19.0) | - | 1 (4.5) | 5 (8.2) |
| Endocrine therapy | 2 (9.5) | 6 (33.3) | 5 (22.7) | 13 (21.3) |
| Target therapy | 2 (9.5) | 7 (38.9) | 4 (18.2) | 13 (21.3) |
| Curative systemic therapy ** No. (%) | 11 (68.7) | - | 2 (66.7) | 13 (68.4) |
| Radiotherapy No. (%) | 18 (32.1) | 22 (43.1) | 25 (25.8) | 65 (31.9) |
| Prior lines of palliative therapy * No. (%) | ||||
| 1 | 13 (65.0) | 8 (50.0) | 9 (50.0) | 30 (55.6) |
| 2 | 3 (15.0) | 1 (6.3) | 3 (16.7) | 7 (13.0) |
| ≥3 | 4 (20.0) | 7 (43.7) | 6 (33.3) | 17 (31.4) |
| Co-morbidities No. (%) | ||||
| Hypertension | 20 (35.7) | 23 (45.1) | 45 (46.4) | 88 (43.1) |
| Diabetes | 10 (17.9) | 10 (19.6) | 26 (26.8) | 46 (22.6) |
| Cardiovascular disease | 8 (14.3) | 11 (22.5) | 25 (25.8) | 44 (21.5) |
| Chronic pulmonary disease | 7 (12.5) | 12 (23.5) | 15 (15.5) | 34 (16.7) |
| Chronic kidney disease | 2 (3.6) | 12 (23.5) | 18 (18.6) | 32 (15.7) |
| Cerebrovascular disease | 1 (1.8) | 2 (3.9) | 13 (13.4) | 16 (7.8) |
| Dementia | - | 3 (5.9) | 9 (9.3) | 12 (5.9) |
| Peripheral vascular disease | 1 (1.8) | 3 (5.9) | 4 (4.1) | 8 (3.9) |
| Liver impairment | - | 3 (5.9) | 3 (3.1) | 6 (2.9) |
| Immunosuppression | - | 2 (3.9) | 3 (3.1) | 5 (2.5) |
| Other | 6 (10.7) | 11 (21.6) | 32 (33.0) | 49 (24.0) |
| Number of comorbidities No. (%) | ||||
| 0 | 17 (30.4) | 9 (17.7) | 17 (17.5) | 43 (21.1) |
| 1 | 16 (28.6) | 18 (35.3) | 20 (20.6) | 54 (26.5) |
| 2 | 16 (28.6) | 7 (13.7) | 24 (24.7) | 47 (23.0) |
| ≥3 | 7 (12.5) | 17 (33.3) | 36 (37.1) | 60 (29.4) |
| Ongoing anticancer therapy at COVID-19 Diagnosis No. (%) | 35 (62.5) | 31 (60.8) | 36 (37.1) | 102 (50.0) |
| COVID-19 symptoms No. (%) | ||||
| Fever | 40 (71.4) | 41 (80.4) | 55 (56.7) | 136 (66.7) |
| Cough | 22 (39.3) | 34 (66.7) | 63 (65.0) | 119 (58.3) |
| Dyspnea | 20 (35.7) | 14 (27.5) | 47 (48.5) | 81 (39.7) |
| Fatigue | 9 (16.1) | 24 (47.1) | 18 (18.6) | 51 (25.0) |
| Myalgia | 4 (7.1) | 9 (17.7) | 11 (11.3) | 24 (11.8) |
| Diarrhea | 4 (7.1) | 8 (15.7) | 8 (8.3) | 20 (9.8) |
| Coryzal symptoms | - | 6 (12.0) | 5 (5.2) | 11 (5.4) |
| Nausea or vomiting | 3 (5.4) | 2 (3.9) | 5 (5.2) | 10 (4.9) |
| Sore throat | - | 2 (3.9) | 4 (4.1) | 6 (3.0) |
| Headache | - | 3 (5.9) | 2 (2.1) | 5 (2.5) |
| Dysgeusia | 1 (1.8) | 1 (2.0) | 1 (1.0) | 3 (1.5) |
| Anosmia | 2 (3.6) | - | 1 (1.0) | 3 (1.5) |
| Other (confusion, delirium) | 9 (16.1) | 5 (9.8) | 23 (23.7) | 37 (18.1) |
| Hospitalization rate No. (%) | 44 (78.6) | 50 (98.0) | 92 (94.8) | 186 (91.2) |
| Admission to ICU No. (%) | 13/44 (30.0) | 2/50 (4.0) | 21/92 (22.8) | 36/186 (19.4) |
| COVID-19 Specific Treatments No. (%) | ||||
| Antibiotics | 33 (58.9) | 47 (92.2) | 58 (59.8) | 138 (67.7) |
| Hydroxychloroquine | 20 (60.6) | 38 (80.9) | 49 (84.5) | 107 (77.5) |
| Lopinavir/ritonavir | 27 (81.8) | 43 (91.5) | 14 (24.1) | 84 (60.9) |
| Corticosteroids | 3 (9.1) | 31 (66.0) | 7 (12.1) | 41 (29.7) |
| Remdesivir | 3 (9.1) | 5 (10.6) | 7 (12.1) | 15 (10.9) |
| Tocilizumab | 8 (24.2) | 1 (2.1) | - | 9 (6.5) |
| Others | - | 5 (10.6) | - | 5 (3.6) |
| Oxygen therapy No. (%) | 3 (9.1) | 22 (46.8) | 3 (5.2) | 28 (20.3) |
| Mechanical ventilation No. (%) | 32 (57.1) 3 (5.4) | 31 (60.8) - | 65 (67.0) 15 (15.5) | 128 (62.8) 18 (8.8) |
| COVID-19 complications No. (%) | 32 (57.1) | 31 (60.7) | 78 (81.2) | 141 (69.1) |
| Acute respiratory failure | 32 (57.1) | 31 (60.7) | 65 (67.0) | 128 (62.7) |
| ARDS § | 11 (19.6) | 17 (33.3) | 21 (20.7) | 49 (24.0) |
| Acute kidney injury | 1 (1.8) | 10 (19.6) | 14 (14.4) | 25 (12.3) |
| Secondary infection | 4 (7.1) | 2 (3.9) | 14 (14.4) | 20 (9.8) |
| Acute cardiac injury | - | - | 3 (3.1) | 3 (1.5) |
| Acute liver injury | - | 1 (2.0) | 2 (2.1) | 3 (1.5) |
| Others | - | 3 (5.9) | 12 (12.4) | 15 (7.4) |
| Laboratory Findings | COVID-19 Diagnosis 1 Median (IQR) n = 193 | Pre COVID-19 2 Median (IQR) n = 128 | p Value |
|---|---|---|---|
| Albumin | 32.0 | 40.0 | p < 0.0001 |
| (g/L) | (28.0–37.0) | (37.0–43.0) | |
| Bilirubin | 8.8 | 9.7 | p = 0.13 |
| (µmol/L) | (5.2–13.8) | (6.6–13.8) | |
| Alanine aminotransferase | 30.5 | 21.0 | p < 0.0001 |
| (IU/L) | (20.0–47.0) | (16.0–33.0) | |
| Sodium | 136.0 | 139.0 | p < 0.0001 |
| (mEq/L) | (134.0–139.0) | (137.0–141.0) | |
| Urea | 7.2 | 6.8 | p = 0.29 |
| (mmol/L) | (4.8–10.7) | (4.8–9.6) | |
| Creatinine | 80.5 | 74.3 | p = 0.13 |
| (µmol/L) | (56.6–109.7) | (61.1–92.0) | |
| Hemoglobin | 115.0 | 118.0 | p = 0.002 |
| (g/L) | (101.0–132.0) | (102.0–133.0) | |
| Leukocyte count | 6.8 | 6.7 | p = 0.34 |
| (cells 103/mm3) | (4.3–10.0) | (4.9–8.9) | |
| Neutrophil count | 5.4 | 4.5 | p = 0.06 |
| (cells 103/mm3) | (3.0–8.0) | (2.8–6.4) | |
| Lymphocyte count | 0.7 | 1.4 | p < 0.0001 |
| (cells 103/mm3) | (0.5–1.1) | (1.0–1.9) | |
| Platelet count | 188.0 | 234.0 | p < 0.0001 |
| (cells 106/mm3) | (141.0–273.0) | (182.0–295.0) | |
| C-reactive protein | 91.0 | 8.1 | p < 0.0001 |
| (mg/L) | (36.9–181.4) | (1.6–25.9) |
| Characteristic | Alive/Discharged | Dead | HR (95% CI) |
|---|---|---|---|
| Age, years No. (%) | |||
| <65 | 62 (42.8) | 12 (20.3) | 1.0 |
| ≥65 | 83 (57.2) | 47 (79.7) | 2.6 (1.4–5.0) |
| Comorbidities No. (%) | |||
| 0–1 | 80 (55.2) | 17 (28.8) | 1.0 |
| ≥2 | 65 (44.8) | 42 (71.2) | 2.4 (1.3–4.2) |
| Smoking history No. (%) | 1.0 1.3 (0.8–2.3) | ||
| Lifetime non-smoker | 59 (40.7) | 20 (33.9) | |
| Ex/active smoker | 75 (51.7) | 37 (62.7) | |
| Unknown | 11 (7.6) | 2 (3.4) | |
| Tumor stage No. (%) | 1.0 1.4 (0.8–2.6) | ||
| Local/loco-regional | 67 (52.3) | 27 (47.4) | |
| Advanced | 53 (41.4) | 29 (50.9) | |
| Unknown | 8 (6.2) | 1 (1.8) | |
| Tumor status No. (%) | |||
| Remission/no measurable disease | 57 (39.3) | 19 (32.2) | 1.0 |
| Active malignancy | 88 (60.7) | 40 (67.8) | 1.4 (0.8–2.5) |
| Anticancer therapy No. (%) | |||
| Yes | 77 (53.1) | 25 (42.4) | 1.0 |
| No | 68 (46.9) | 34 (57.6) | 1.1 (0.6–1.9) |
| Cytotoxic chemotherapy No. (%) | |||
| Yes | 46 (31.7) | 16 (27.1) | 1.0 |
| No | 99 (68.3) | 43 (72.9) | 1.0 (0.6–1.9) |
| COVID-19 therapy No. (%) | |||
| Yes | 103 (71.0) | 35 (59.3) | 1.0 |
| No | 42 (29.0) | 24 (40.7) | 1.6 (0.9–2.8) |
| Temperature at COVID-19 diagnosis (median, IQR) | 37.9 (36.9–38.3) | 38.0 (36.9–38.6) | p = 0.8 |
| Duration of symptoms prior to COVID-19 diagnosis (median, IQR) | 2.5 (1.0–6.0) | 2.5 (0–4.0) | p = 0.8 |
| Interval from last anticancer treatment to COVID-19 diagnosis (median, IQR) | 12.0 (7.0–22.5) | 16.0 (8.0–34.0) | p = 0.7 |
| Laboratory findings at COVID-19 diagnosis (median, IQR) | |||
| Albumin (g/L) | 3 (27–36) | 34 (29–38) | p = 0.4 |
| Bilirubin (µmol/L) | 8.6 (5.2–13.0) | 9 (6–15.5) | p = 0.6 |
| Alanine aminotransferase (IU/L) | 28 (18–42) | 32 (23–51) | p = 0.06 |
| Sodium (mEq/L) | 136 (134–139) | 136 (133–138) | p = 0.4 |
| Urea (mmol/L) | 6.7 (4.3–9.8) | 10 (6.3–16) | p = 0.4 |
| Creatinine (µmol/L) | 72.6 (54.9–98.2) | 95.1 (72–141) | p < 0.001 |
| Hemoglobin (g/L) | 116 (101.5–132) | 109 (99–129) | p = 0.2 |
| Leukocyte count (cells 103/mm3) | 6.1 (4.0–9.5) | 8.2 (5.3–11.3) | p = 0.7 |
| Neutrophil count (cells 103/mm3) | 4.5 (2.8–7.4) | 6.5 (3.6–10.0) | p = 0.7 |
| Lymphocyte count (cells 103/mm3) | 0.8 (0.5–1.1) | 0.6 (0.5–1.0) | p = 0.3 |
| Platelet count (cells 106/mm3) | 190 (145–273) | 173 (139–278) | p = 0.9 |
| C-reactive protein (mg/L) | 69 (22.9–154.3) | 143.3 (70–260.6) | p = 0.002 |
| Lactate dehydrogenase (IU/L) | 363 (251–524) | 447 (276–596) | p = 0.04 |
| D-dimer (ng/mL) | 655.5 (463.5–1514) | 2100 (761–3387) | p = 0.2 |
| Ferritin (ng/mL) | 650 (215–1297) | 421 (289–1010) | p = 0.7 |
| Characteristic | Alive/Discharged | Dead | HR (95%CI) |
|---|---|---|---|
| Age No. (%) | |||
| <65 | 62 (42.8) | 12 (20.3) | 1.0 |
| ≥65 | 83 (57.2) | 47 (79.7) | 2.2 (1.0–4.6) |
| Comorbidities No. (%) | |||
| 0–1 | 80 (55.2) | 17 (28.8) | 1.0 |
| ≥2 | 65 (44.8) | 42 (71.2) | 1.9 (1.0–3.6) |
| Tumor stage No. (%) | 1.0 1.5 (0.7–3.2) | ||
| Local/loco-regional | 67 (52.3) | 27 (47.4) | |
| Advanced | 53 (41.4) | 29 (50.9) | |
| Unknown | 8 (6.2) | 1 (1.8) | |
| Tumor status No. (%) | |||
| Remission/no measurable disease | 57 (39.3) | 19 (32.2) | 1.0 |
| Active malignancy | 88 (60.7) | 40 (67.8) | 1.3 (0.6–2.8) |
| Anticancer therapy No. (%) | |||
| Yes | 77 (53.1) | 25 (42.4) | 1.0 |
| No | 68 (46.9) | 34 (57.6) | 1.3 (0.7–2.6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinato, D.J.; Lee, A.J.X.; Biello, F.; Seguí, E.; Aguilar-Company, J.; Carbó, A.; Bruna, R.; Bower, M.; Rizzo, G.; Benafif, S.; et al. Presenting Features and Early Mortality from SARS-CoV-2 Infection in Cancer Patients during the Initial Stage of the COVID-19 Pandemic in Europe. Cancers 2020, 12, 1841. https://doi.org/10.3390/cancers12071841
Pinato DJ, Lee AJX, Biello F, Seguí E, Aguilar-Company J, Carbó A, Bruna R, Bower M, Rizzo G, Benafif S, et al. Presenting Features and Early Mortality from SARS-CoV-2 Infection in Cancer Patients during the Initial Stage of the COVID-19 Pandemic in Europe. Cancers. 2020; 12(7):1841. https://doi.org/10.3390/cancers12071841
Chicago/Turabian StylePinato, David J., Alvin J. X. Lee, Federica Biello, Elia Seguí, Juan Aguilar-Company, Anna Carbó, Riccardo Bruna, Mark Bower, Gianpiero Rizzo, Sarah Benafif, and et al. 2020. "Presenting Features and Early Mortality from SARS-CoV-2 Infection in Cancer Patients during the Initial Stage of the COVID-19 Pandemic in Europe" Cancers 12, no. 7: 1841. https://doi.org/10.3390/cancers12071841
APA StylePinato, D. J., Lee, A. J. X., Biello, F., Seguí, E., Aguilar-Company, J., Carbó, A., Bruna, R., Bower, M., Rizzo, G., Benafif, S., Carmona, C., Chopra, N., Cruz, C. A., D’Avanzo, F., Evans, J. S., Galazi, M., Garcia-Fructuoso, I., Dalla Pria, A., Newsom-Davis, T., ... Gennari, A. (2020). Presenting Features and Early Mortality from SARS-CoV-2 Infection in Cancer Patients during the Initial Stage of the COVID-19 Pandemic in Europe. Cancers, 12(7), 1841. https://doi.org/10.3390/cancers12071841











