Prognostic Effect of Comorbid Disease and Immune Gene Expression on Mortality in Kidney Cancer—A Population Based Study
Abstract
1. Introduction
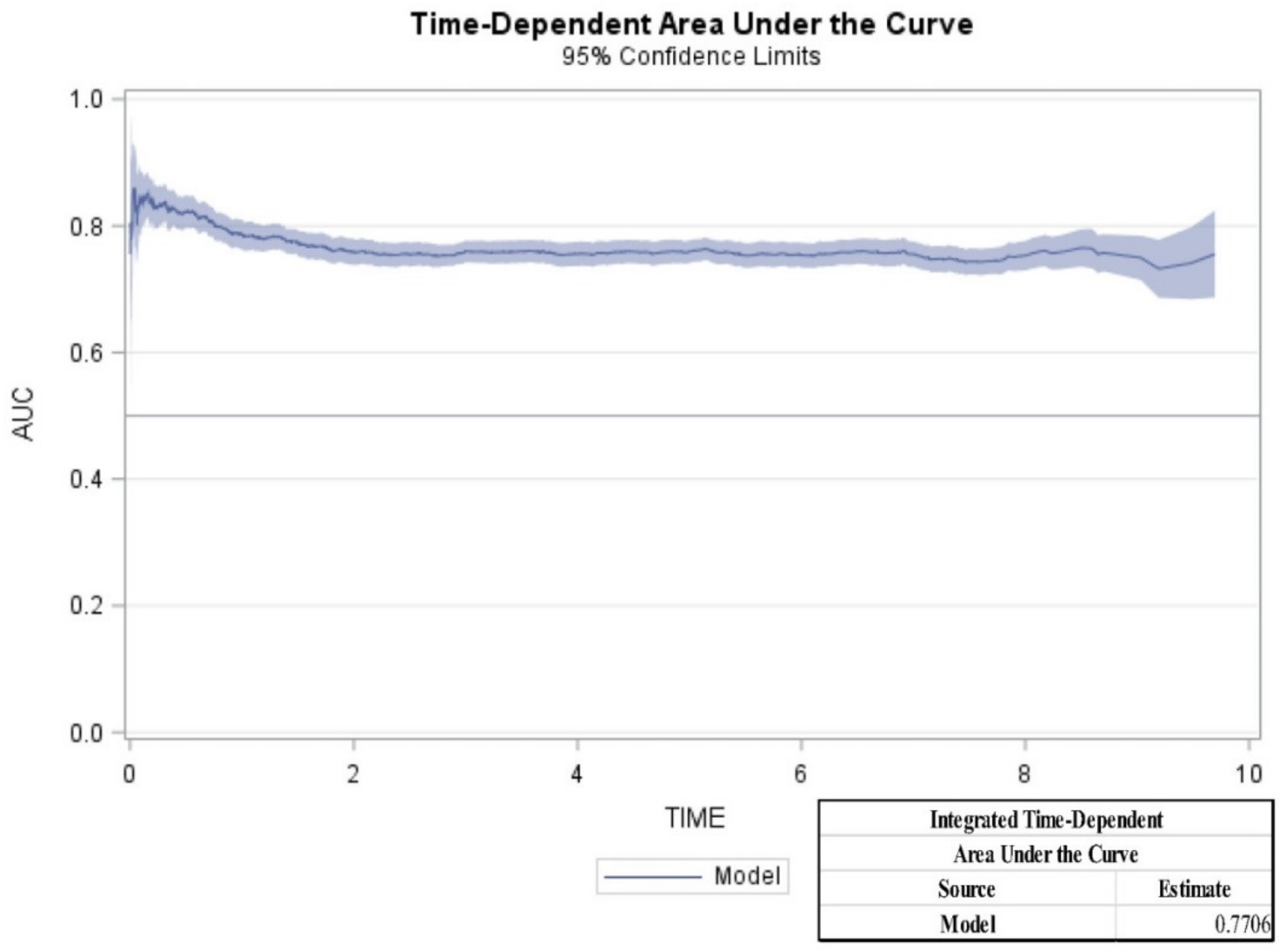
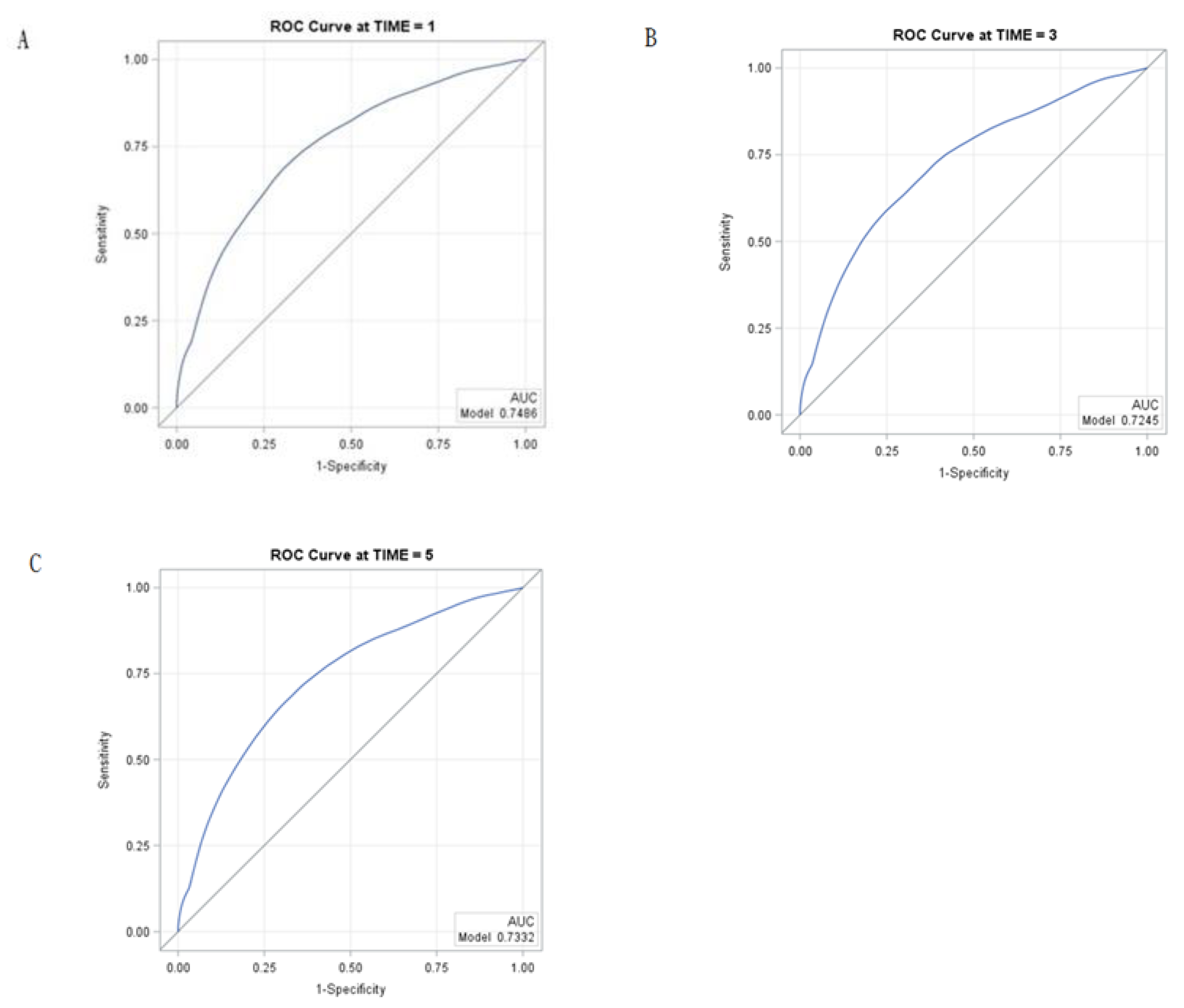
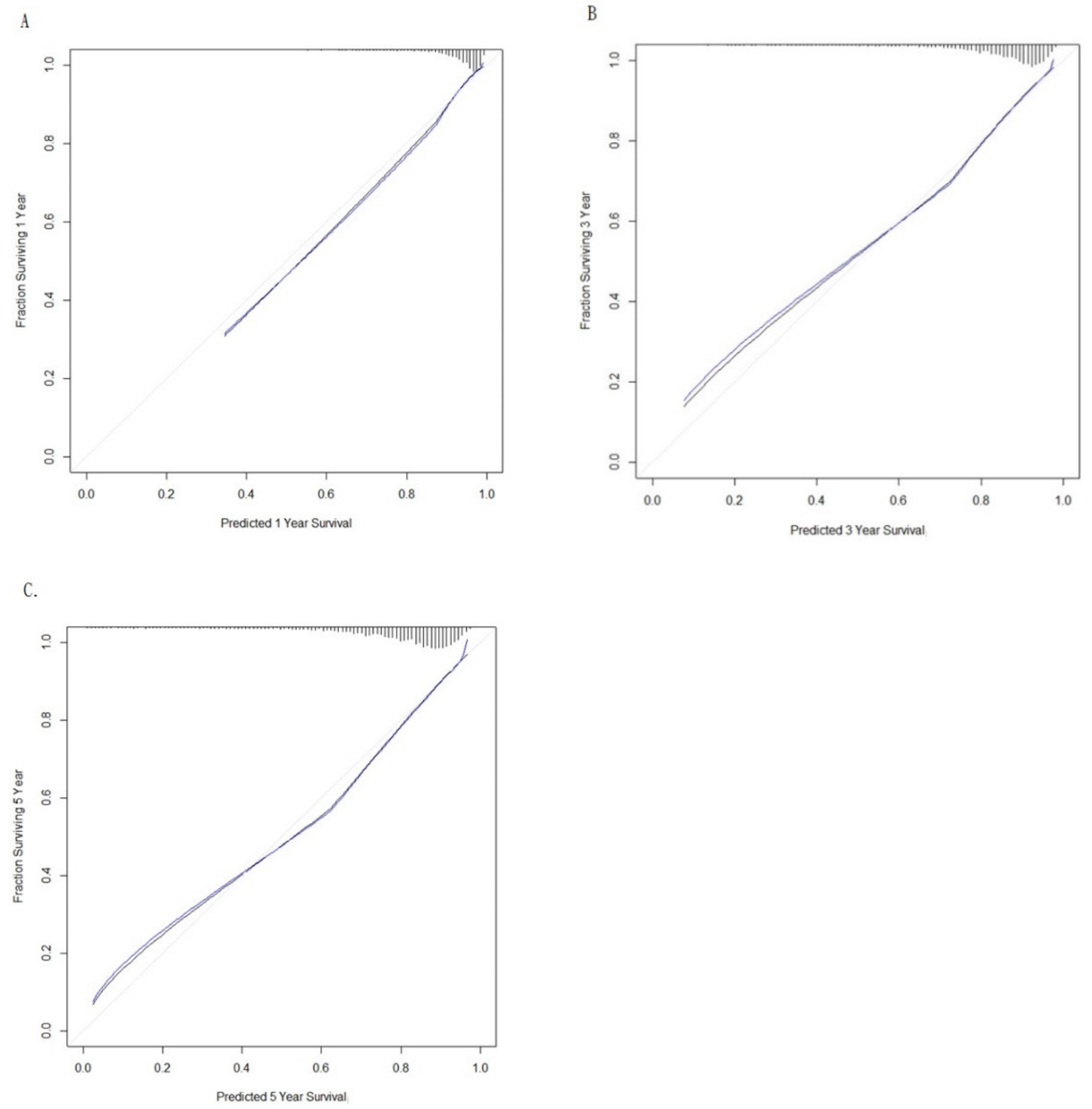
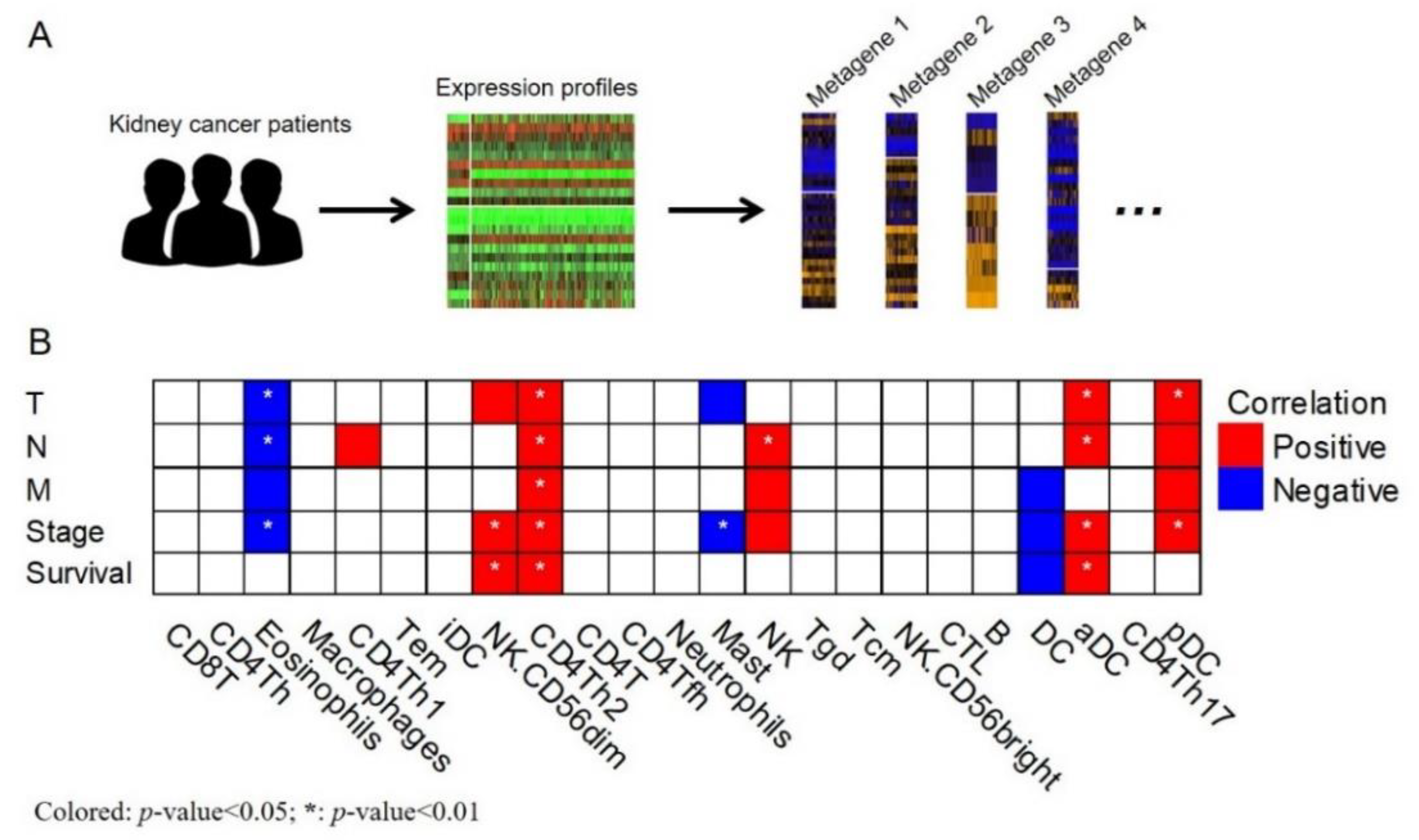
2. Results
Main Findings
3. Discussion
4. Materials and Methods
4.1. Longitudinal Health Insurance Database
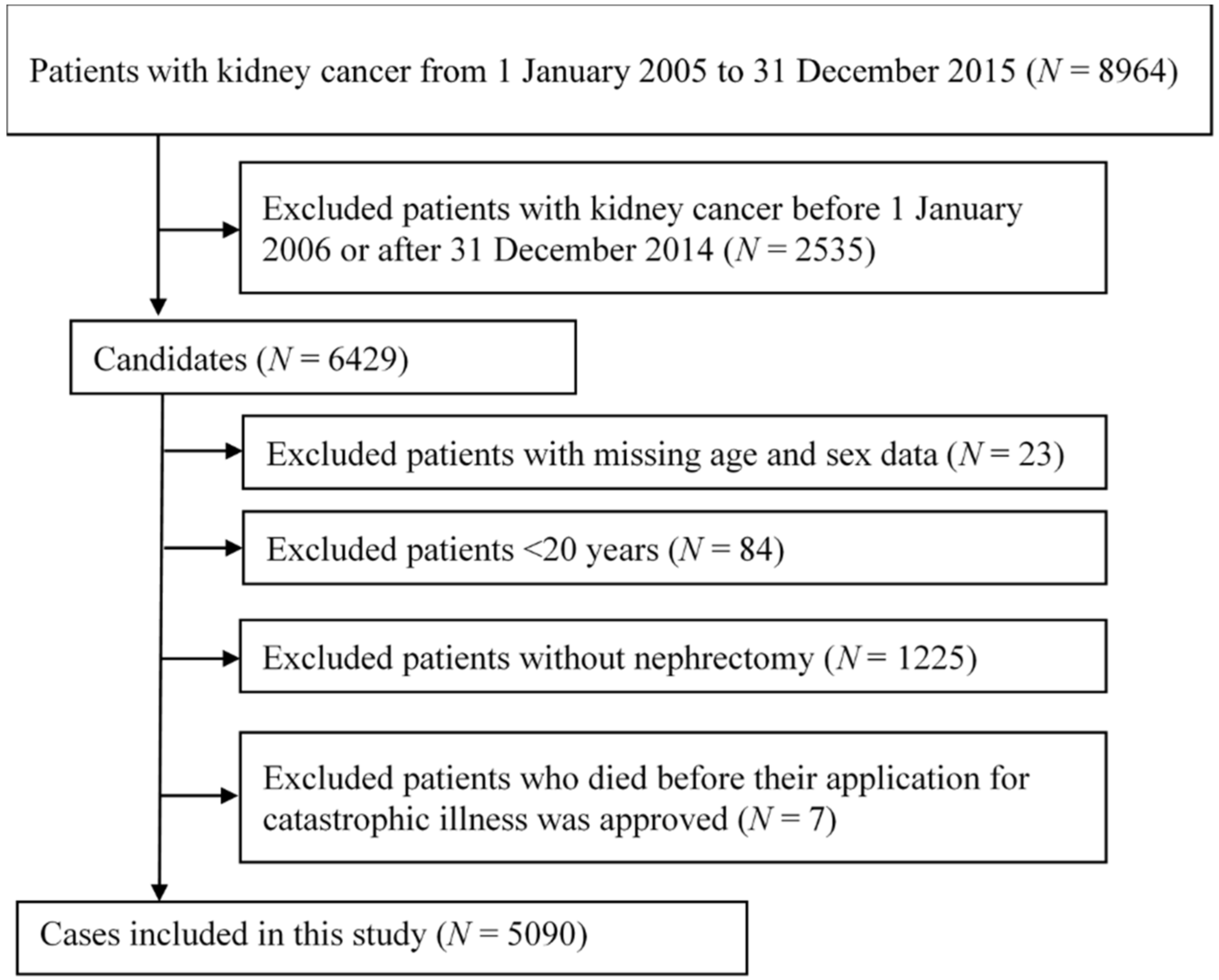
4.2. Study Design and Patient Population
4.3. Measurement of Covariates and Comorbidities
4.4. Analysis of Immune Metagenes
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sanchez-Martin, F.M.; Millan-Rodriguez, F.; Urdaneta-Pignalosa, G.; Rubio-Briones, J.; Villavicencio-Mavrich, H. Small renal masses: Incidental diagnosis, clinical symptoms, and prognostic factors. Adv. Urol. 2008, 2018, 310694. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Karakiewicz, P.I.; Trinh, Q.D.; Bhojani, N.; Bensalah, K.; Salomon, L.; De La Taille, A.; Tostain, J.; Cindolo, L.; Altieri, V.; Ficarra, V.; et al. Renal cell carcinoma with nodal metastases in the absence of distant metastatic disease: Prognostic indicators of disease-specific survival. Eur. Urol. 2007, 51, 1616–2164. [Google Scholar] [CrossRef]
- Berger, D.A.; Megwalu, I.; Vlahiotis, A.; Radwan, M.H.; Serrano, M.F.; Humphrey, P.A.; Piccirillo, J.F.; Kibel, A.S. Impact of comorbidity on overall survival in patients surgically treated for renal cell carcinoma. Urology 2008, 72, 359–363. [Google Scholar] [CrossRef]
- Sorbellini, M.; Kattan, M.W.; Snyder, M.E.; Reuter, V.; Motzer, R.; Goetzl, M.; Mckiernan, J.; Russo, P. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J. Urol. 2005, 173, 48–51. [Google Scholar] [CrossRef]
- Shinohara, N.S.; Nonomura, K.; Abe, T.; Maruyama, S.; Kamai, T.; Takahashi, M.; Tatsugami, K.; Yokoi, S.; Deguchi, T.; Kanayama, H.; et al. A new prognostic classification for overall survival in Asian patients with previously untreated metastatic renal cell carcinoma. Cancer Sci. 2012, 103, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Manola, J.; Royston, P.; Elson, P.; Mccormack, J.B.; Mazumdar, M.; Negrier, S.; Escudier, B.; Eisen, T.; Dutcher, J.; Atkins, M.; et al. Prognostic model for survival in patients with metastatic renal cell carcinoma: Results from the international kidney cancer working group. Clin. Cancer Res. 2011, 17, 5443–5450. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A.; et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [PubMed]
- Austin, P.C.; Steyerberg, E.W. Interpreting the concordance statistic of a logistic regression model: Relation to the variance and odds ratio of a continuous explanatory variable. BMC Med. Res. Methodol. 2012, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Guinan, P.; Sobin, L.H.; Algaba, F.; Badellino, F.; Kameyama, S.; Maclennan, G.; Novick, A. TNM staging of renal cell carcinoma: Workgroup No. 3. Union International Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 1997, 80, 992–993. [Google Scholar] [CrossRef]
- Gettman, M.T.; Blute, M.L.; Spotts, B.; Bryant, S.C.; Zincke, H. Pathologic staging of renal cell carcinoma: Significance of tumor classification with the 1997 TNM staging system. Cancer 2001, 91, 354–361. [Google Scholar] [CrossRef]
- Van Poppel, H.; Da Pozzo, L.; Albrecht, W.; Matveev, V.; Bono, A.; Borkowski, A.; Marechal, J.M.; Klotz, L.; Skinner, E.; Keane, T.; et al. A prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur. Urol. 2007, 51, 1606–1615. [Google Scholar] [CrossRef]
- Lane, B.R.; Abouassaly, R.; Gao, T.; Weight, C.J.; Hernandez, A.V.; Larson, B.T.; Kaouk, J.H.; Gill, I.S.; Campbell, S.C. Active treatment of localized renal tumors may not impact overall survival in patients aged 75 years or older. Cancer 2010, 116, 3119–3126. [Google Scholar] [CrossRef] [PubMed]
- Ather, M.H.; Nazim, S.M. Impact of Charlson’s comorbidity index on overall survival following tumor nephrectomy for renal cell carcinoma. Int. Urol. Nephrol. 2010, 42, 299–303. [Google Scholar] [CrossRef]
- Patnaik, J.L.; Byers, T.; Diguiseppi, C.; Denberg, T.D.; Dabelea, D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J. Natl. Cancer Inst. 2011, 103, 1101–1111. [Google Scholar] [CrossRef]
- De Groot, V.; Beckerman, H.; Lankhorst, G.J.; Bouter, L.M. How to measure comorbidity: A critical review of available methods. J. Clin. Epidemiol. 2003, 56, 221–229. [Google Scholar] [CrossRef]
- Zhe, M.; Hang, Z. Nephrolithiasis as a risk factor of chronic kidney disease: A meta-analysis of cohort studies with 4,770,691 participants. Urolithiasis 2017, 45, 441–448. [Google Scholar] [CrossRef]
- Shoag, J.; Halpern, J.; Goldfarb, D.S.; Eisner, B.H. Risk of chronic and end stage kidney disease in patients with nephrolithiasis. J. Urol. 2014, 192, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.J.; Chen, Y.T.; Ou, S.M.; Yang, W.C.; Chen, T.J.; Tarng, D.C. Urinary calculi and risk of cancer: A nationwide population-based study. Medicine 2014, 93, e342. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Levey, A.S.; Serio, A.M.; Snyder, M.; Vickers, A.J.; Raj, G.V.; Scardino, P.T.; Russo, P. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol. 2006, 7, 735–740. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; Mcculloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Weng, P.H.; Hung, K.Y.; Huang, H.L.; Chen, J.H.; Sung, P.K.; Huang, K.C. Cancer-specific mortality in chronic kidney disease: Longitudinal follow-up of a large cohort. Clin. J. Am. Soc. Nephrol. 2011, 6, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Ortel, T.L.; Francis, C.W. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J. Clin. Oncol. 2009, 27, 4902–4911. [Google Scholar] [CrossRef] [PubMed]
- Nierodzik, M.; Karpatkin, S. Hypercoagulability preceding cancer. Does hypercoagulability awaken dormant tumor cells in the host? J. Thromb. Haemost. 2005, 3, 577–580. [Google Scholar] [CrossRef]
- Sorensen, H.T.; Mellemkjaer, L.; Olsen, J.H.; Baron, J.A. Prognosis of cancers associated with venous thromboembolism. N. Engl. J. Med. 2000, 343, 1846–1850. [Google Scholar] [CrossRef]
- Chew, H.K.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch. Intern. Med. 2006, 166, 458–464. [Google Scholar] [CrossRef]
- Yancik, R.; Havlik, R.J.; Wesley, M.N.; Ries, L.; Long, S.; Rossi, W.K.; Edwards, B.K. Cancer and comorbidity in older patients: A descriptive profile. Ann. Epidemiol. 1996, 6, 399–412. [Google Scholar] [CrossRef]
- Zeber, J.E.; Copeland, L.A.; Hosek, B.J.; Karnad, A.B.; Lawrence, V.A.; Sanchez-Reilly, S.E. Cancer rates, medical comorbidities, and treatment modalities in the oldest patients. Crit. Rev. Oncol. Hematol. 2008, 67, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Ghatalia, P.; Gordetsky, J.; Kuo, F.; Dulaimi, E.; Cai, K.Q.; Devarajan, K.; Bae, S.; Naik, G.; Chan, T.A.; Uzzo, R.; et al. Prognostic impact of immune gene expression signature and tumor infiltrating immune cells in localized clear cell renal cell carcinoma. J. Immunother. Cancer 2019, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, C.J.; De Cubas, A.A.; Fan, H.; Smith, C.C.; Lang, M.; Reznik, E.; Bowlby, R.; Gibb, E.A.; Akbani, R.; Beroukhim, R.; et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep. 2018, 23, 313–326. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [PubMed]
| Death | Survivor | |
|---|---|---|
| N | 1181 | 3909 |
| Age, mean ± SD, y | 65.89 ± 12.68 | 58.93 ± 13.05 |
| Age group, N (%), y | ||
| 20–39 | 38 (3.2) | 327 (8.4) |
| 40–59 | 337 (28.5) | 1754 (44.9) |
| 60–79 | 657 (55.6) | 1628 (41.6) |
| 80–99 | 149 (12.6) | 200 (5.1) |
| Male, N (%) | 795 (67.3) | 2515 (64.3) |
| Comorbid conditions for 1 year prior to the kidney cancer, N (%) | ||
| Diabetes mellitus | 356 (30.1) | 910 (23.3) |
| Hypertension | 696 (58.9) | 2099 (53.7) |
| Chronic kidney disease | 289 (24.5) | 576 (14.7) |
| Dialysis requirements | 148 (12.5) | 228 (5.8) |
| Polycystic kidney disease | 9 (0.8) | 33 (0.8) |
| Renal Stone | 87 (7.4) | 220 (5.6) |
| Myocardial infarction | 22 (1.9) | 31 (0.8) |
| Congestive heart failure | 86 (7.3) | 152 (3.9) |
| Peripheral vascular disease | 41 (3.5) | 89 (2.3) |
| Cerebrovascular disease | 113 (9.6) | 195 (5.0) |
| Dementia | 17 (1.4) | 22 (0.6) |
| Chronic pulmonary disease | 133 (11.3) | 252 (6.4) |
| Peptic ulcer disease | 185 (15.7) | 504 (12.9) |
| Moderate or severe liver disease | 5 (0.4) | 11 (0.3) |
| Metastatic tumor | 310 (26.2) | 201 (5.1) |
| Charlson comorbidity index | ||
| 0–1 | 17 (1.4) | 60 (1.5) |
| 2 | 269 (22.8) | 1688 (43.2) |
| 3 | 208 (17.6) | 976 (25.0) |
| 4 | 161 (13.6) | 510 (13.0) |
| ≥5 | 526 (44.5) | 675 (17.3) |
| mean ± SD | 5.03 ± 2.94 | 3.30 ± 1.87 |
| The year for newly diagnosis of kidney cancer. N (%) | ||
| 2006 | 124 (10.5) | 217 (5.6) |
| 2007 | 159 (13.5) | 262 (6.7) |
| 2008 | 178 (15.1) | 354 (9.1) |
| 2009 | 158 (13.4) | 342 (8.7) |
| 2010 | 135 (11.4) | 372 (9.5) |
| 2011 | 125 (10.6) | 495 (12.7) |
| 2012 | 119 (10.1) | 549 (14.0) |
| 2013 | 100 (8.5) | 592 (15.1) |
| 2014 | 83 (7.0) | 726 (18.6) |
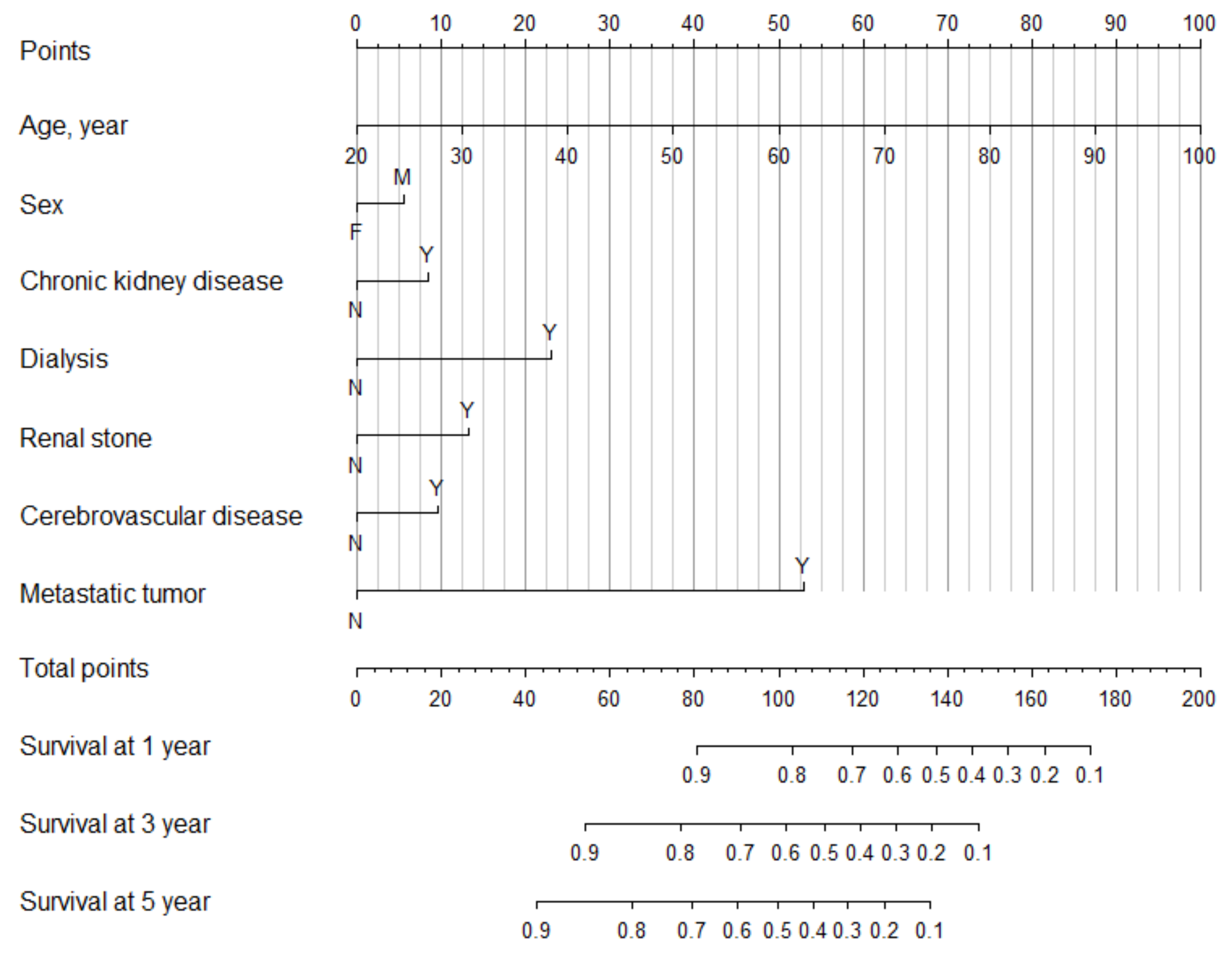
| Risk factor | Hazard Ratio | P-Value |
|---|---|---|
| Age, y | 1.04 (1.04,1.05) | <0.0001 |
| Male | 1.20 (1.06,1.36) | 0.0037 |
| Chronic kidney disease | 1.32 (1.10,1.58) | 0.0023 |
| Dialysis requirements | 2.14 (1.70,2.71) | <0.0001 |
| Renal stones | 1.55 (1.24,1.93) | 0.0001 |
| Cerebrovascular disease | 1.37 (1.12,1.67) | 0.0018 |
| Metastatic tumor | 5.75 (5.04,6.57) | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.-S.; Chen, T.-T.; Chang, W.-P.; Wong, H.S.-C.; Wu, M.-Y.; Adikusuma, W.; Lin, Y.-F.; Chang, W.-C. Prognostic Effect of Comorbid Disease and Immune Gene Expression on Mortality in Kidney Cancer—A Population Based Study. Cancers 2020, 12, 1654. https://doi.org/10.3390/cancers12061654
Wong C-S, Chen T-T, Chang W-P, Wong HS-C, Wu M-Y, Adikusuma W, Lin Y-F, Chang W-C. Prognostic Effect of Comorbid Disease and Immune Gene Expression on Mortality in Kidney Cancer—A Population Based Study. Cancers. 2020; 12(6):1654. https://doi.org/10.3390/cancers12061654
Chicago/Turabian StyleWong, Chung-Shun, Tzu-Ting Chen, Wei-Pin Chang, Henry Sung-Ching Wong, Mei-Yi Wu, Wirawan Adikusuma, Yuh-Feng Lin, and Wei-Chiao Chang. 2020. "Prognostic Effect of Comorbid Disease and Immune Gene Expression on Mortality in Kidney Cancer—A Population Based Study" Cancers 12, no. 6: 1654. https://doi.org/10.3390/cancers12061654
APA StyleWong, C.-S., Chen, T.-T., Chang, W.-P., Wong, H. S.-C., Wu, M.-Y., Adikusuma, W., Lin, Y.-F., & Chang, W.-C. (2020). Prognostic Effect of Comorbid Disease and Immune Gene Expression on Mortality in Kidney Cancer—A Population Based Study. Cancers, 12(6), 1654. https://doi.org/10.3390/cancers12061654







