Emerging Concepts and Novel Strategies in Radiation Therapy for Laryngeal Cancer Management
Abstract
:1. Introduction
2. Early-Stage Glottic Cancer (I–II)
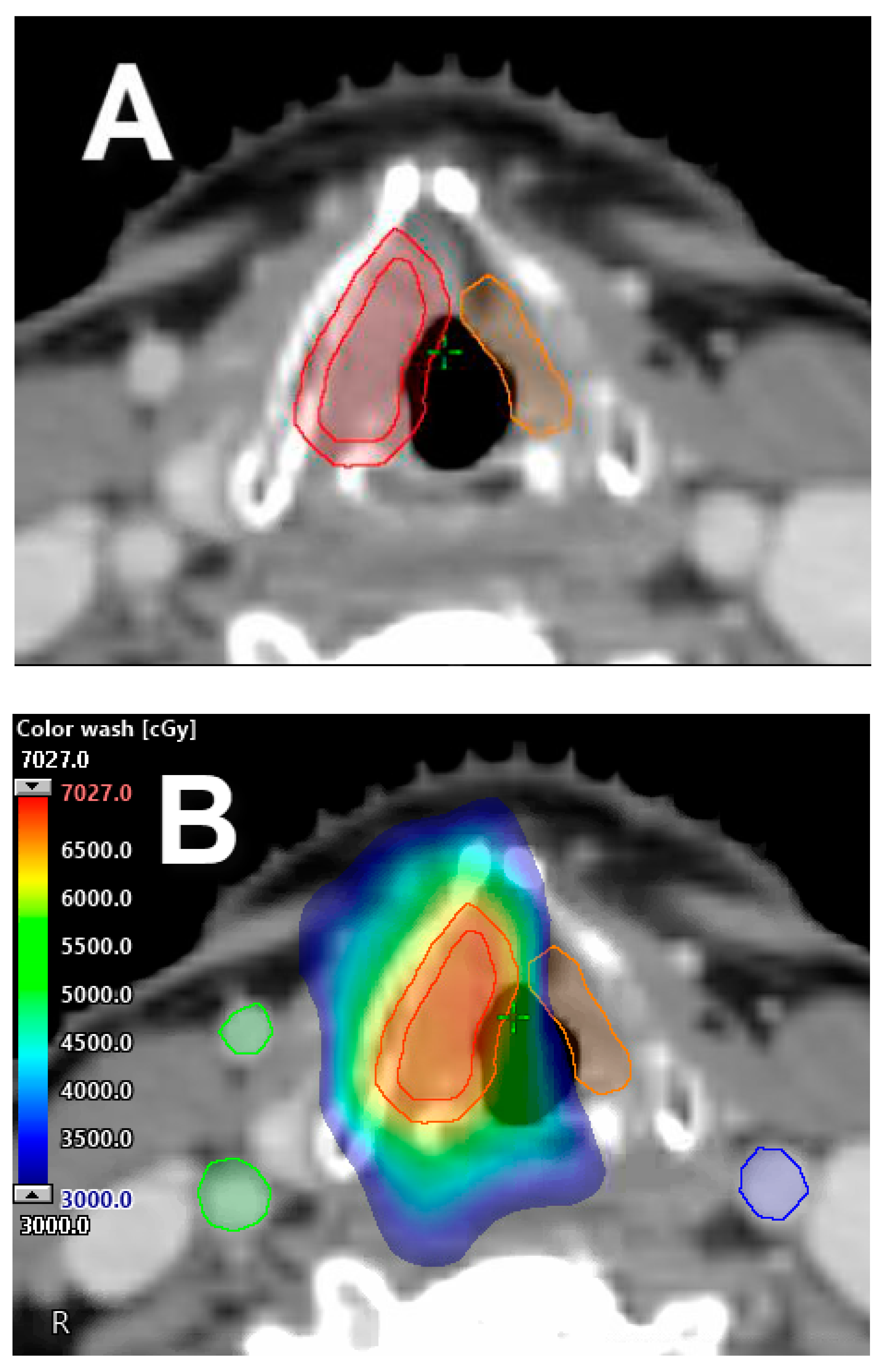
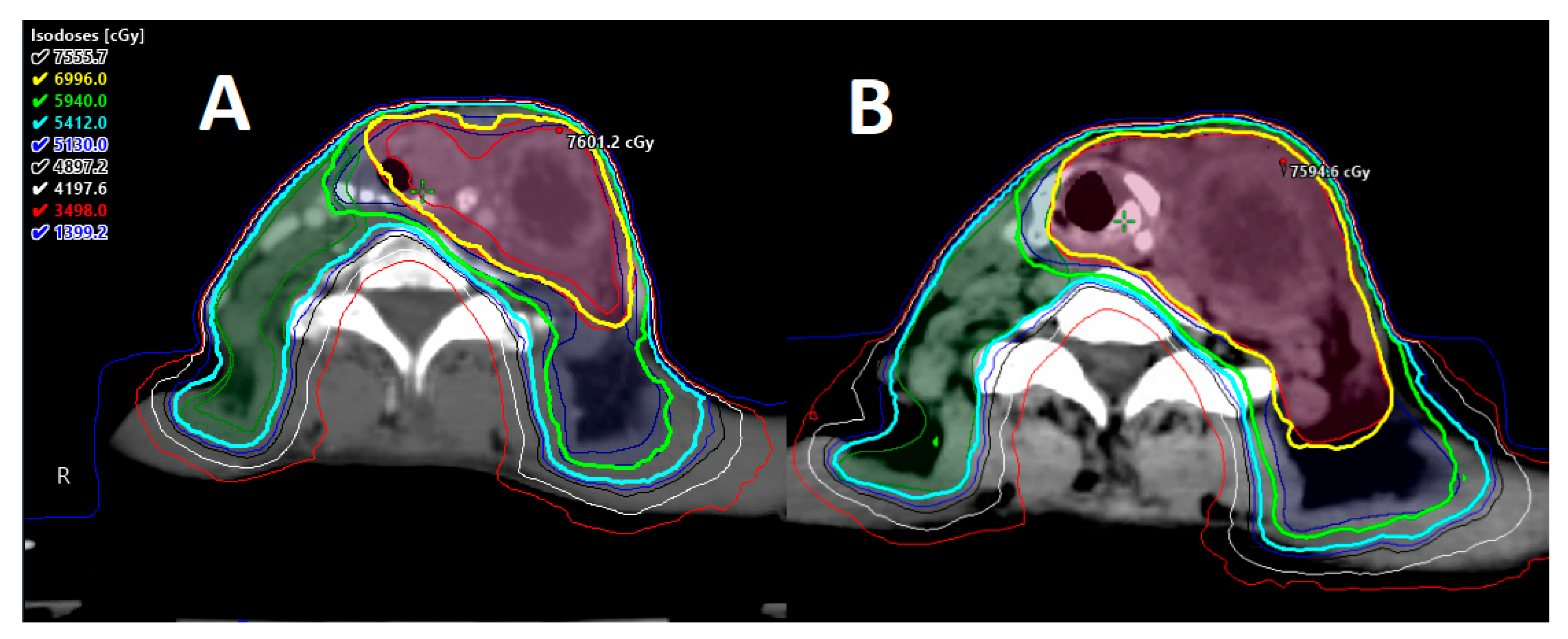
2.1. Carotid-Sparing IMRT
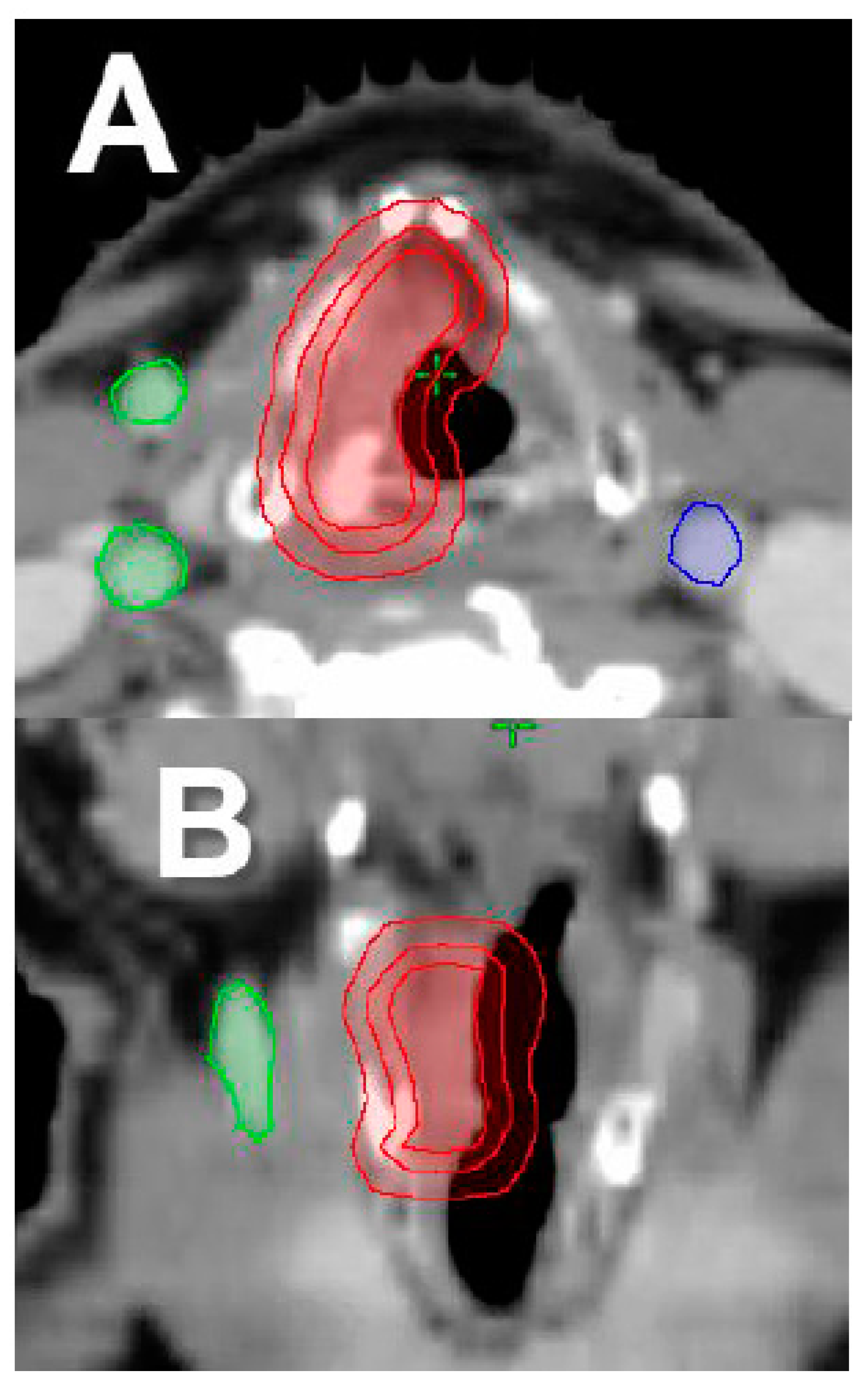
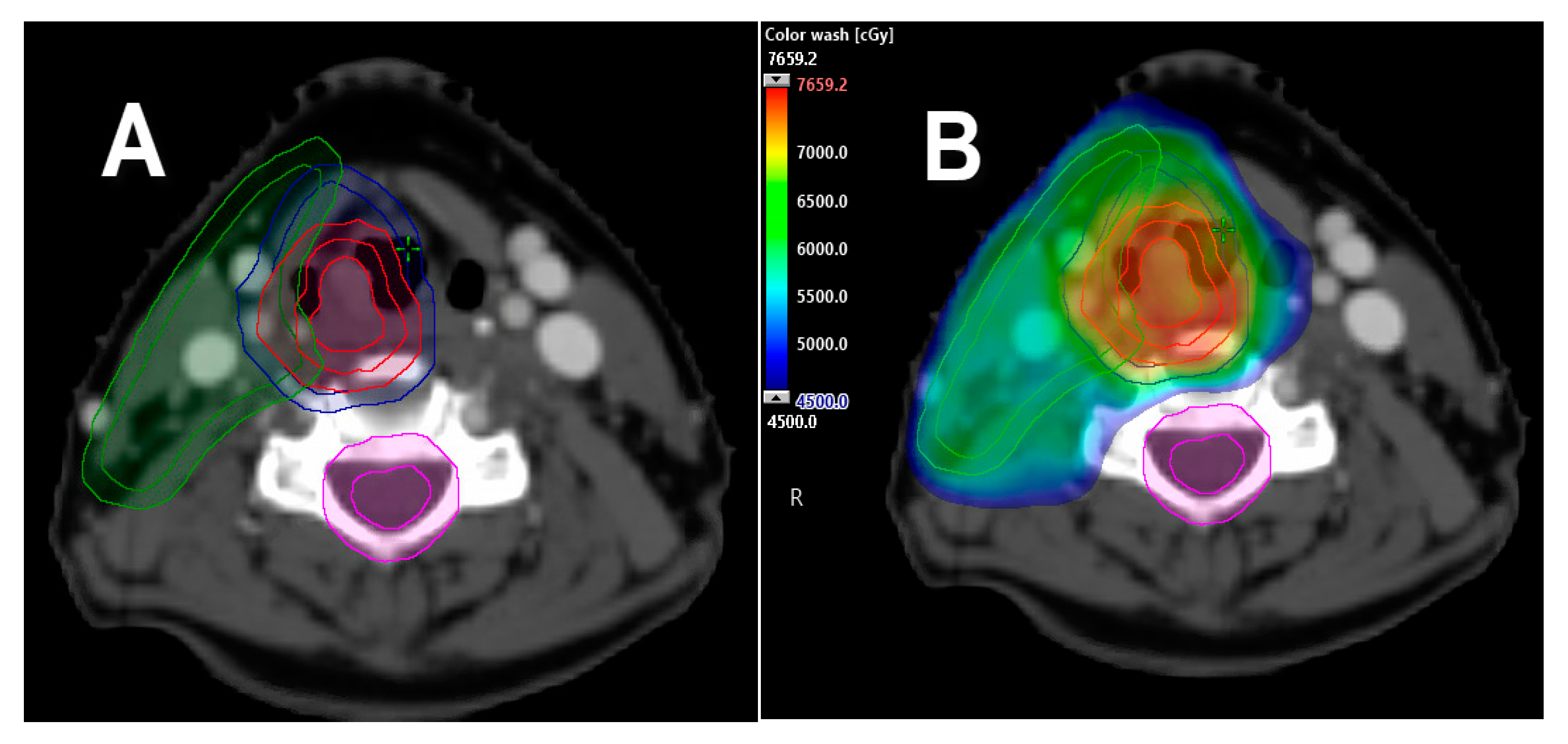
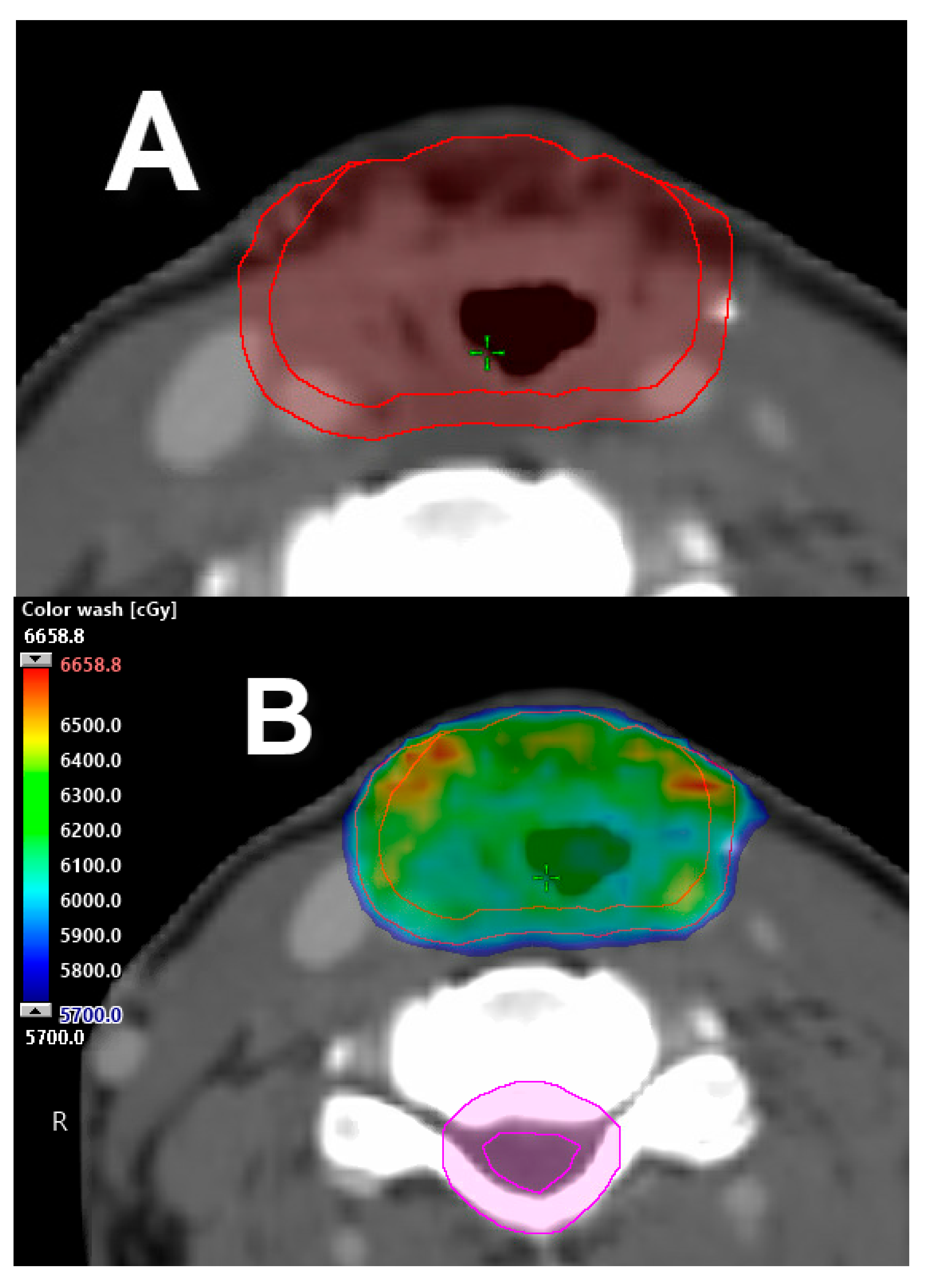
2.2. Single Vocal Cord Irradiation
2.3. Moderate–Extreme Hypofractionation
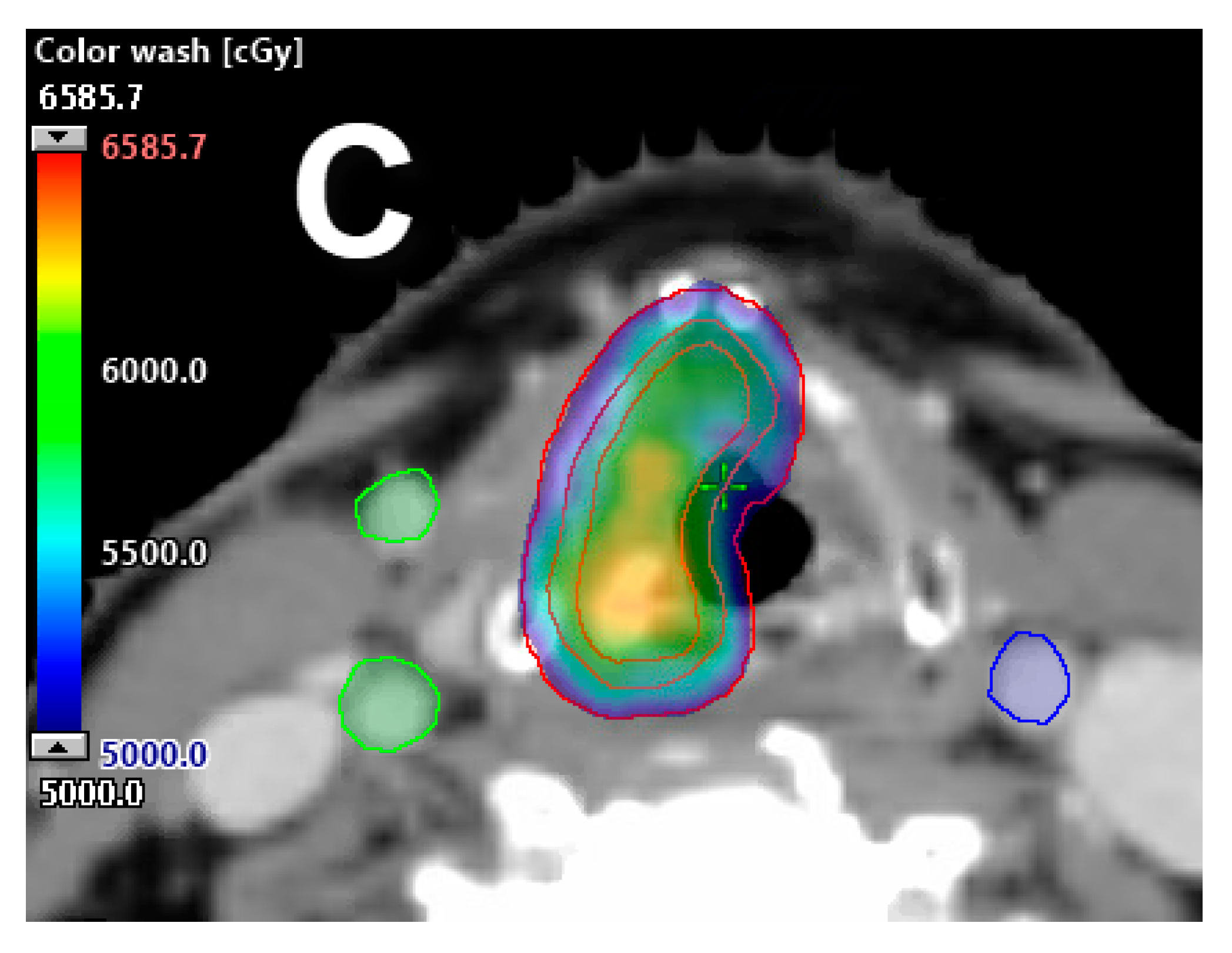
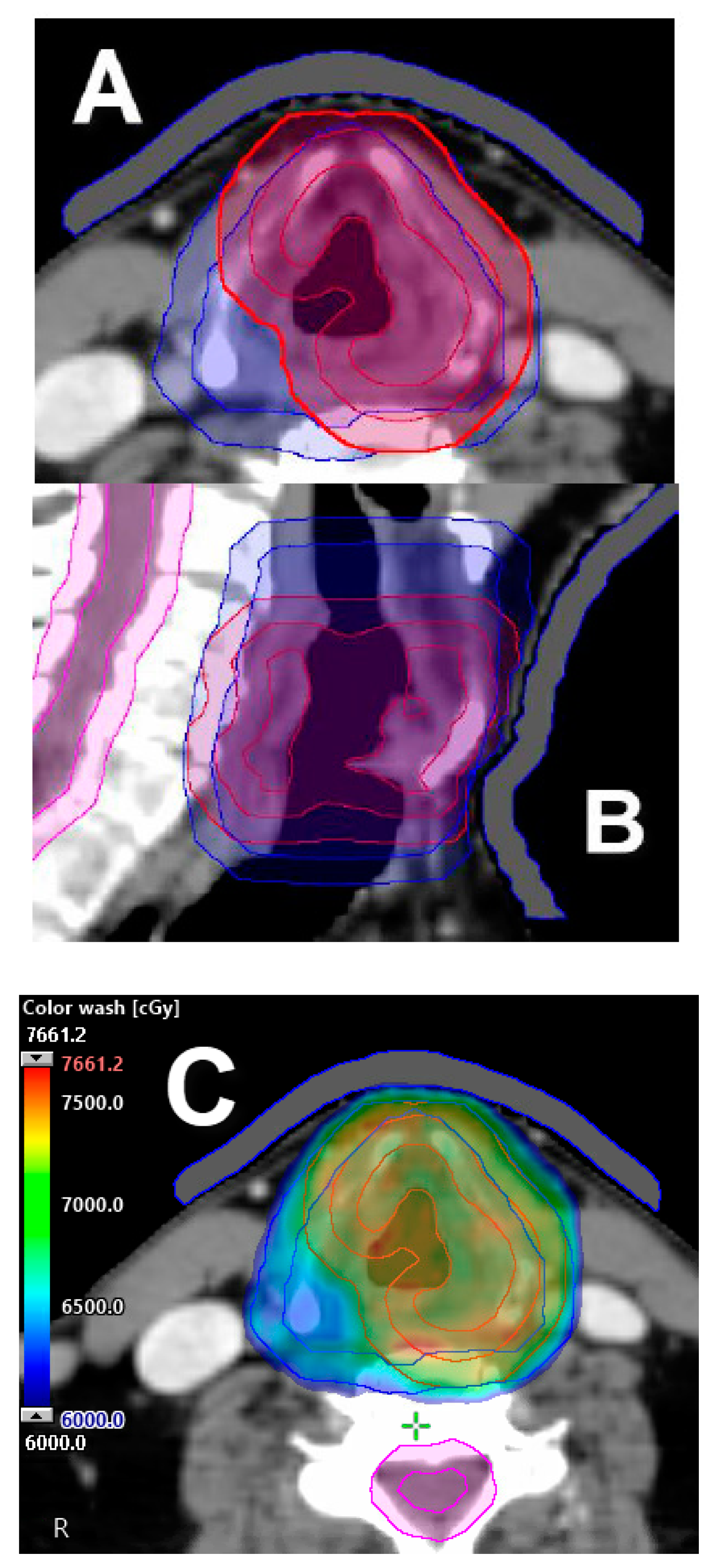
2.4. Partial Laryngeal IMRT
3. Locally Advanced Stage (III–IV)
3.1. Tumor Volume
3.2. Pretreatment Organ Function
3.3. Selective Nodal Irradiation
3.4. Adaptive Radiotherapy
3.5. Unilateral Neck Irradiation
3.6. Omission of Resected Neck—Radiation to the Primary Surgical Bed Only
3.7. Radiation to Neck(s) Only
3.8. Nanoparticle Therapy
3.9. Deep Machine Learning: Radiomics
3.10. Tumor Heterogeneity
3.11. MRI-Guided Radiotherapy
3.12. Ultra-High-Dose-Rate (FLASH) Radiotherapy
3.13. Radiotherapy Coupled with Biological Agents
4. Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef]
- Shin, J.Y.; Truong, M.T. Racial disparities in laryngeal cancer treatment and outcome: A population-based analysis of 24.069 patients. Laryngoscope 2015, 125, 1667–1674. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Miller, K.D.; Sauer, A.G.; Jemal, A.; Siegel, R.L. Cancer Statistics for African Americans, 2019. CA Cancer J. Clin. 2019, 69, 211–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wydner, E.L.; Bross, I.J.; Day, E. Epidemiological approach to the etiology of cancer of the larynx. J. Am. Med. Assoc. 1956, 160, 1384–1391. [Google Scholar]
- Hoffman, H.T.; Porter, K.; Karnell, L.H.; Copper, J.S.; Weber, R.S.; Langer, C.J.; Ang, K.K.; Gay, G.; Stewart, A.; Robinson, R.A. Laryngeal cancer in the United States: Changes in demographics, patterns of care and survival. Laryngoscope 2006, 116 (Suppl. 111), 1–13. [Google Scholar] [CrossRef] [Green Version]
- Megwalu, U.C.; Sikora, A.G. Survival outcomes in advanced laryngeal cancer. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 855–860. [Google Scholar] [CrossRef] [Green Version]
- Raitiola, H.; Pukander, J.; Laippala, P. Glottic and supraglottic laryngeal carcinoma: Differences in epidemiology, clinical characteristics and prognosis. Acta Otolaryngol. 1999, 119, 847–851. [Google Scholar]
- Dahm, J.D.; Sessions, D.G.; Paniello, R.C.; Harvey, J. Primary subglottic cancer. Laryngoscope 1998, 108, 741–746. [Google Scholar] [CrossRef]
- Lahav, Y.; Burns, J.A.; Feinberg, S.; Heaton, J.T.; Zeitels, S.M. Initial anatomic geographic presentation of glottal dysplasia. Ann. Otol. Rhinol. Laryngol. 2009, 118, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Shoffel-Havakuk, H.; Halperin, D.; Yosef, L.; Haimovich, Y.; Lahav, Y. The anatomic distribution of malignant and premalignant glottic lesions and its relations to smoking. Otolaryngol. Head Neck Surg. 2015, 152, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Epstein, B.E.; Lee, D.J.; Kashima, H.; Johns, M.E. Stage T1 glottic carcinoma: Results of radiation therapy or laser excision. Radiology 1990, 175, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.B.; Solomon, B.; Miller, S.; Fass, D.E.; Armstrong, J.; Sessions, R.B. Prospective computer-assisted voice analysis for patients with early stage glottic cancer: A preliminary report of the functional result of laryngeal irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 123–127. [Google Scholar] [CrossRef]
- Aaltonen, L.M.; Rautiainen, N.; Sellman, J.; Saarilahti, K.; Makitie, A.; Rihkanen, H.; Laranne, J.; Kleemola, L.; Wigren, T.; Sala, E.; et al. Voice quality after treatment of early vocal cord cancer: Randomized trial comparing laser surgery with RT therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 255–260. [Google Scholar] [CrossRef]
- Nunez, B.F.; Caminero Cueva, M.J.; Senaris Gonzalez, S.; Llorente Pendas, J.L.; Gorriz Gil, C.; Lopez Llames, A.; Alonso Pantiga, R.; Suarez Nieto, C. Voice quality after endoscopic laser surgery and radiotherapy for early glottic cancer: Objective measurements emphasizing the voice handicap index. Eur. Arch. Otorhinolaryngol. 2008, 265, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Regaud, C.; Coutard, H.; Hautant, A. Rapport sur la Curie thérapie et la roentgenthérapie dans le cancer du larynx. Ann. d. mal. de l’oreille, du larynx 1922, 41, 967. [Google Scholar]
- Coutard, H. Principles of x ray therapy of malignant diseases. Lancet 1934, 2, 1–12. [Google Scholar] [CrossRef]
- Harwood, A.R.; Hawkins, N.V.; Rider, W.D.; Bryce, D.P. Radiotherapy of early glottic cancer—I. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 473–476. [Google Scholar] [CrossRef]
- Harwood, A.R.; Tierie, A. Radiotherapy of early glottic cancer—II. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 477–482. [Google Scholar] [CrossRef]
- Yamazaki, H.; Nishiyama, K.; Tanaka, E.; Koizumi, M.; Chatani, M. Radiotherapy for early glottic carcinoma (T1N0M0): Results of prospective randomized study of radiation fraction size and overall treatment time. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 77–82. [Google Scholar] [CrossRef]
- Gomez, D.; Cahlon, O.; Mechalakos, J.; Lee, N. An investigation of intensity-modulated radiation therapy versus conventional two-dimensional and 3D-conformal radiation therapy for early stage larynx cancer. Radiat. Oncol. 2010, 5, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chera, B.S.; Amdur, R.J.; Morris, C.G.; Mendenhall, W.M. Carotid-sparing intensity-modulated radiotherapy for early-stage squamous cell carcinoma of the true vocal cord. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.R.; Smith, B.D.; Smith, J.B.; Sevak, P.; Malek, J.S.; Kanwar, A.; Browne, T.; Gunn, G.B.; Garden, A.S.; Frank, S.J.; et al. Outcomes of carotid-sparing IMRT for T1 glottic cancer: Comparison with conventional radiation. Laryngoscope 2020, 130, 146–153. [Google Scholar] [CrossRef]
- Korpics, M.C.; Turchan, W.T.; Rooney, M.K.; Koshy, M.; Spiotto, M.T. Patterns of care and outcomes of intensity-modulated radiotherapy and 3D conformal radiotherapy for early stage glottic cancer: A national cancer database analysis. Cancers 2019, 11, 1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, S.O.; De Boer, H.C.; Heijmen, B.J.; Levendag, P.C. Four-dimensional CT analysis of vocal cords mobility for highly focused single vocal cord irradiation. Radiother. Oncol. 2008, 89, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.O.; De Boer, H.C.; Astreinidou, E.; Gangsaas, A.; Heijmen, B.J.M.; Levendag, P.C. On-line cone beam CT image guidance for vocal cord tumor targeting. Radiother. Oncol. 2009, 93, 8–13. [Google Scholar] [CrossRef]
- Osman, S.O.; Astreinidou, E.; De Boer, H.C.; Keskin-Cambay, F.; Breedveld, S.; Voet, P.; Al-Mamgani, A.; Heijmen, B.J.M.; Levendag, P.C. IMRT for Image-guided single vocal cord irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 989–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levendag, P.C.; Teguh, D.N.; Keskin-Cambay, F.; Al-Mamgani, A.; Van Rooij, P.; Astreinidou, E.; Kwa, S.L.S.; Heijmen, B.; Monserez, D.A.; Osman, S.O.S. Single vocal cord irradiation: A competitive treatment strategy in early glottic cancer. Radiother. Oncol. 2011, 101, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Al-Mamgani, A.; Kwa, S.L.S.; Tans, L.; Moring, M.; Fransen, D.; Mehilal, R.; Verduijn, G.M.; Battenburg de Jong, R.J.; Heijmen, B.J.M.; Levendag, P.C. Single vocal cord irradiation: Image guided intensity modulated hypofractionated radiation therapy for T1a glottic cancer: Early clinical results. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 337–343. [Google Scholar] [CrossRef]
- Sher, D.J.; Timmerman, R.D.; Nedzi, L.; Ding, C.; Pham, N.L.; Zhao, B.; Sumer, B.D. Phase 1 fractional dose-escalation study of equipotent stereotactic radiation therapy regimens for early-stage glottic larynx cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Yu, T.; Kim, J.H.; Park, J.M.; Kim, J.I.; Chung, E.J.; Kwon, S.K.; Kim, J.H.; Wu, H.G. Early closure of a phase 1 clinical trial for SABR in early-stage glottic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.; Patel, M.; Khan, M.; McLean, S.; Dragovic, J.; Jin, J.Y.; Movsas, B.; Ryu, S. Stereotactic body radiation therapy for primary, recurrent, and metastatic tumors in the head-and-neck region. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Trotti, I.I.I.A.; Zhang, Q.; Bentzen, S.M.; Emami, B.; Hammond, M.E.; Jones, C.U.; Morrison, W.H.; Sagar, S.M.; Ridge, J.A.; Fu, K.K.; et al. Randomized trial of hyperfractionation versus conventional fractionation in T2 squamous cell carcinoma of the vocal cord (RTOG 9512). Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 958–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rock, K.; Huang, S.H.; Tiong, A.; Lu, L.; Xu, W.; Ringash, J.; Bratman, S.V.; Tong, L.; Chan, B.; Cho, J.; et al. Partial laryngeal IMRT for T2N0 glottic cancer: Impact of image guidance and radiation therapy intensification. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Chera, B.S.; Amdur, R.J.; Morris, C.G.; Kirwan, J.M.; Mendenhall, W.M. T1N0 to T2N0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 461–466. [Google Scholar] [CrossRef]
- Garden, A.S.; Forster, K.; Wong, P.F.; Morrison, W.H.; Schechter, N.R.; Ang, K.K. Results of radiotherapy for T2N0 glottic carcinoma: Does the “2” stand for twice-daily treatment? Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 322–328. [Google Scholar] [CrossRef]
- Le, Q.T.; Fu, K.K.; Kroll, S.; Ryu, J.K.; Quivey, J.M.; Meyler, T.S.; Krieg, R.M.; Phillips, T.L. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 115–126. [Google Scholar] [CrossRef]
- Akimoto, T.; Nonaka, T.; Kitamoto, Y.; Ishikawa, H.; Ninomiya, H.; Chikamatsu, K.; Furuya, N.; Hayakawa, K.; Mitsuhashi, N.; Nakano, T. Radiation therapy for T2N0 laryngeal cancer: A retrospective analysis for the impact of concurrent chemotherapy on local control. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 995–1001. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Riggs, C.E.; Vaysberg, M.; Amdur, R.J.; Werning, J.W. Altered fractionation and adjuvant chemotherapy for head and neck squamous cell carcinoma. Head Neck 2010, 32, 939–945. [Google Scholar] [CrossRef]
- Furusaka, T.; Matsuda, A.; Saito, T.; Katsura, Y.; Ikeda, M. Concurrent chemoradiation therapy with docetaxel for laryngeal preservation in T2N0M0 glottic squamous cell carcinomas. Acta Oto-Laryngol. 2013, 133, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Dagan, R.; Bryant, C.M.; Amdur, R.J.; Mancuso, A.A. Definitive radiotherapy for squamous cell carcinoma of the glottic larynx. Cancer Control 2016, 23, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forastiere, A.A.; Ismaila, N.; Lewin, J.S.; Nathan, C.A.; Adelstein, D.J.; Eisbruch, A.; Fass, G.; Fisher, S.G.; Laurie, S.A.; Le, Q.T.; et al. Use of larynx-preservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2017, 36, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, M.R.; Blakaj, A.; Blakaj, D. Organ preservation for advanced larynx cancer: A review of chemotherapy and radiation combination strategies. Oral Oncol. 2018, 86, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Light, T.; El Rassi, E.; Maggiore, R.J.; Holland, J.; Reed, J.; Suriano, K.; Stooksbury, M.; Tobin, N.; Gross, N.; Clayburgh, D. Improving outcomes in veterans with oropharyngeal squamous cell carcinoma trough implementation of a multidisciplinary clinic. Head Neck 2017, 39, 1106–1112. [Google Scholar] [CrossRef]
- Boero, I.J.; Paravati, A.J.; Xu, B.; Cohen, E.E.W.; Mell, L.K.; Le, Q.T.; Murphy, J.D. Importance of radiation oncologist experience among patients with head and neck cancer treated with intensity-modulated radiation therapy. J. Clin. Oncol. 2016, 34, 684–690. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, A.O.; Echevarria, M.I.; Strom, T.J.; Abuodeh, Y.A.; Venkat, P.S.; Ahmed, K.A.; Demetriou, S.; Frakes, J.M.; Kim, Y.; Kish, J.A.; et al. Patient choice for high-volume center radiation impacts head and neck cancer outcome. Cancer Med. 2018, 7, 4964–4979. [Google Scholar] [CrossRef]
- Salvador-Coloma, C.; Cohen, E. Multidisciplinary care of laryngeal cancer. J. Oncol. Pract. 2016, 12, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.R.; Thames, H.D.; Huang, D.T.; Schmidt-Ullrich, R.K. The tumor volume and clonogen number relationship: Tumor control predictions based upon tumor volume estimates derived from computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 281–287. [Google Scholar] [CrossRef]
- Lee, W.R.; Mancusso, A.A.; Saleh, E.M.; Mendenhall, W.M.; Parsons, J.T.; Million, R.R. Can pretreatment computed tomography findings predict local control in T3 squamous cell carcinoma of the glottic larynx treated with radiotherapy alone? Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 683–687. [Google Scholar] [CrossRef]
- Mukherji, S.K.; O’Brien, S.M.; Gerstle, R.J.; Weissler, M.; Shockley, W.; Castillo, M. Tumor volume: An independent predictor of outcome for laryngeal cancer. J. Comput. Assist. Tomogr. 1999, 23, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.; Amdur, R.J.; Mendenhall, W.M.; Morris, C.G.; Mancuso, A.A.; Yeung, A. Tumor volume as a predictor of treatment success in patients with laryngeal cancer treated with primary chemoradiotherapy. Hong Kong J. Radiol. 2013, 16, 198–202. [Google Scholar] [CrossRef]
- Issa, M.R.; Samuels, S.E.; Bellile, E.; Shalabi, F.L.; Eisbruch, A.; Wolf, G. Tumor volumes and prognosis in laryngeal cancer. Cancers 2015, 7, 2236–2261. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bai, J.; Duan, P. Prognostic value of 18F-FDG PET/CT functional parameters in patients with head and neck cancer: A meta-analysis. Nucl. Med. Commun. 2019, 40, 361–369. [Google Scholar] [CrossRef]
- Bradford, C.R.; Ferlito, A.; Devaney, K.O.; Makitie, A.A.; Rinaldo, A. Prognostic factors in laryngeal squamous cell carcinoma. Laryngoscope Investig. Otolaryngol. 2020, 5, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Pera, E.; Moreno, A.; Galindo, L. Prognostic factors in laryngeal carcinoma: A multifactorial study of 416 cases. Cancer 1986, 58, 928–934. [Google Scholar] [CrossRef]
- McCoul, E.D.; Har-El, G. Meta-analysis of impaired vocal cord mobility as a prognostic factor in T2 glottic carcinoma. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Sher, D.J.; Sen, N.; Shah, J.L.; Pham, N.L., Jr.; Subramaniam, R.; Moore, W.; Williams, K.; Khan, S. Recurrence and quality-of-life following elective nodal volume and dose de-escalation for oropharyngeal and laryngeal cancer: Initial results from the Infield trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, S53–S54. [Google Scholar] [CrossRef]
- Bhide, S.A.; Davies, M.; Burke, K.; McNair, H.A.; Hansen, V.; Barbachano, Y.; El-Hariry, A.; Newbold, K.; Harrington, K.J.; Nutting, C.M. Weekly volume and dosimetric changes during chemoradiotherapy with intensity-modulated radiation therapy for head and neck cancer: A prospective observational study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1360–1368. [Google Scholar] [CrossRef]
- Morgan, H.E.; Sher, D.J. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck 2020, 5, 1. [Google Scholar] [CrossRef]
- De Veij Mestdagh, P.D.; Jonker, M.C.J.; Vogel, W.V.; Schreuder, W.H.; Donswijk, M.L.; Klop, W.M.C.; Al-Mamgani, A. SPECT/CT-guided lymph drainage mapping for the planning of unilateral elective nodal irradiation in head and neck squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2018, 275, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- De Veij Mestdagh, P.D.; Janssen, T.; Lamers, E.; Carbaat, C.; Hamming-Vrieze, O.; Vogel, W.V.; Sonke, J.J.; Al-Mamgani, A. SPECT/CT-guided elective nodal irradiation for head and neck cancer: Estimation of clinical benefits using NTCP models. Radiother. Oncol. 2019, 130, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Veij Mestdagh Walraven, I.; Vogel, W.V.; Schreuder, W.H.; Van Werkhoven, E.; Carbaat, C.; Donswijk, M.L.; Van Den Brekel, M.W.M.; Al-Mamgani, A. SPECT/CT-guided elective nodal irradiation for head and neck cancer is oncologically safe and less toxic: A potentially practice-changing approach. Radiother. Oncol. 2020, 147, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.C.; Frank, S.J.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Gunn, G.B.; Reddy, J.P.; Morrison, W.H.; Williamson, T.D.; Holliday, E.B.; et al. Intensity modulated proton therapy (IMPT)—The future of IMRT for head and neck cancer. Oral Oncol. 2019, 88, 66–74. [Google Scholar] [CrossRef]
- Anand, A.; Bues, M.; Gamez, M.E.; Stefan, C.; Patel, S.H. Individual field simultaneous optimization (IFSO) in spot scanning proton therapy of head and neck cancers. Med. Dosim. 2019, 44, 375–378. [Google Scholar] [CrossRef]
- Contreras, J.A.; Spencer, C.; DeWees, T.; Haughey, B.; Henke, L.E.; Chin, R.I.; Paniello, R.; Rich, J.; Jackson, R.; Oppelt, P.; et al. Eliminating postoperative radiation to the pathologically node-negative neck: Long-term results of a prospective phase II study. J. Clin. Oncol. 2019, 37, 2548–2555. [Google Scholar] [CrossRef]
- Sinha, P.; Pipkorn, P.; Thorstad, W.L.; Gay, H.A.; Haughey, B.H. Dose elimination of planned postoperative radiation to the primary bed in p16-positive, transorally-resected oropharyngeal carcinoma associate with poorer outcomes? Oral Oncol. 2016, 61, 127–134. [Google Scholar] [CrossRef]
- Gamez, M.E.; Halyard, M.Y.; Hinni, M.L.; Hayden, R.E.; Nagel, T.H.; Vargas, C.E.; Wong, W.W.; Curtis, K.K.; Zarka, M.A.; Ma, D.; et al. Mucosal sparing radiation therapy in resected oropharyngeal cancer. Ann. Otol. Rhinol. Laryngol. 2017, 126, 185–191. [Google Scholar] [CrossRef]
- Swisher-McClure, S.; Lukens, J.N.; Aggarwal, C.; Ahn, P.; Basu, D.; Bauml, M.; Brody, R.; Chalian, A.; Cohen, R.B.; Fotouhi-Ghiam, A.; et al. A phase 2 trial of alternative volumes of oropharyngeal irradiation for de-intensification (AVOID): Omission of the resected primary tumor bed after transoral robotic surgery for human papilloma virus-related squamous cell carcinoma of the oropharynx. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 725–732. [Google Scholar] [CrossRef]
- Calugaru, V.; Magne, N.; Herault, J.; Bonvalot, S.; Tourneau, C.L.; Thariat, J. Nanoparticles and radiation therapy. Bull. Cancer 2015, 102, 83–91. [Google Scholar] [CrossRef]
- Tourneau, C.L.; Calugaru, V.; Borcoman, E.; Moreno, V.; Calvo, E.; Liem, X.; Salas, S.; Doger, B.; Jouffroy, T.; Mirabel, X.; et al. Hafnium oxide nanoparticles (NBTXR3) activated by radiotherapy for the treatment of frail and/or elderly patients with locally advanced HNSCC: A phase I/II study. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 1142–1143. [Google Scholar] [CrossRef]
- Giraud, P.; Giraud, P.; Gasnier, A.; El Ayachy, R.; Kreps, S.; Foy, J.P.; Durdux, C.; Huguet, F.; Burgun, A.; Bibault, J.E. Radiomics and machine learning for radiotherapy in head and neck cancers. Front. Oncol. 2019, 9, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kann, B.H.; Hicks, D.F.; Payabvash, S.; Mahajan, A.; Du, J.; Gupta, V.; Park, H.S.; Yu, J.B.; Yarbrough, W.G.; Burtness, B.A.; et al. Multi-institutional validation of deep learning for pretreatment identification of extranodal extension in head and neck squamous cell carcinoma. J. Clin. Oncol. 2020, 38, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Scalco, E.; Moriconi, S.; Rizzo, G. Texture analysis to assess structural modifications induced by radiotherapy. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 2015, 5219–5222. [Google Scholar] [PubMed]
- Scalco, E.; Fiorino, C.; Cattaneo, G.M.; Sanguineti, G.; Rizzo, G. Texture analysis for the assessment of structural changes in parotid glands induced by radiotherapy. Radiother. Oncol. 2013, 109, 384–387. [Google Scholar] [CrossRef]
- Jiang, W.; Lakshminarayanan, P.; Hui, X.; Han, P.; Cheng, Z.; Bowers, M.; Shpitser, I.; Siddiqui, S.; Taylor, R.H.; Quon, H.; et al. Machine learning methods uncover radiomorphologic dose patterns in salivary glands that predict xerostomia in patients with head and neck cancer. Adv. Radiat. Oncol. 2019, 4, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Jiang, W.; Lakshminarayanan, P.; Han, P.; Cheng, Z.; Bowers, M.; Hui, X.; Shpitser, I.; Siddiqui, S.; Taylor, R.H.; et al. Spatial radiation dose influence on xerostomia recovery and its comparison to acute incidence in patients with head and neck cancer. Adv. Radiat. Oncol. 2020, 5, 221–230. [Google Scholar] [CrossRef]
- Caudell, J.J.; Torres-Roca, J.F.; Gillies, R.J.; Enderling, H.; Kim, S.; Rishi, A.; Moros, E.G.; Harrison, L.B. The future of personalized radiotherapy for head and neck cancer. Lancet Oncol. 2017, 18, e266–e273. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossman, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Wong, A.J.; Kanwar, A.; Mohamed, A.S.; Fuller, C.D. Radiomics in head and neck cancer: From exploration to application. Transl. Cancer Res. 2016, 5, 371–382. [Google Scholar] [CrossRef]
- Mroz, E.A.; Rocco, J.W. Intra-tumor heterogeneity in head and neck cancer and its clinical implications. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.M.; Hsu, S.; Lamb, J.; Yang, Y.; Agazaryan, N.; Steinberg, M.L.; Low, D.A.; Cao, M. MRI-guided radiotherapy for head and neck cancer: Initial clinical experience. Clin. Transl. Oncol. 2018, 20, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Cao, M.; Hsu, S.; Lamb, J.; Mikaeilian, A.; Yang, Y.; Agazaryan, N.; Low, D.A.; Steinberg, M.L. Magnetic resonance imaging guided reirradiation of recurrent and second primary head and neck cancer. Adv. Radiat. Oncol. 2017, 2, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Chen, H.S.; Dolly, S.; Li, H.; Fischer-Valuck, B.; Victoria, J.; Dempsey, J.; Ruan, S.; Anastasio, M.; Mazur, T.; et al. An integrated model-driven method for in-treatment upper airway motion tracking using cine MRI in head and neck radiation therapy. Med. Phys. 2016, 43, 4700. [Google Scholar] [CrossRef] [PubMed]
- Bruijnen, T.; Stemkens, B.; Terhaard, C.H.J.; Lagendijk, J.J.W.; Raaijmakers, C.P.J.; Tijssen, R.H.N. Intrafraction motion quantification and planning target volume margin determination of head-and-neck tumors using cine magnetic resonance imaging. Radiother. Oncol. 2019, 130, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Corradini, S.; Alongi, F.; Andratschke, N.; Belka, C.; Boldrini, L.; Cellini, F.; Debus, J.; Guckenberger, M.; Horner-Rieber, J.; Lagerwaard, F.J.; et al. MR-guidance in clinical reality: Current treatment challenges and future perspectives. Radiat. Oncol. 2019, 14, 92. [Google Scholar] [CrossRef] [Green Version]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupe, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Vozenin, M.C.; Hendry, J.H.; Limoli, C.L. Biological benefits of ultra-high dose rate flash radiotherapy: Sleeping beauty awoken. Clin. Oncol. 2019, 31, 407–415. [Google Scholar] [CrossRef]
- Bourhis, J.; Montay-Gruel, P.; Goncalves, J.P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Goncalves, J.P.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef]
- Argiris, A.; Bauman, J.E.; Ohr, J.; Gooding, W.E.; Heron, D.E.; Duvvuri, U.; Kubicek, G.J.; Posluszny, D.M.; Vassilakopoulou, M.; Kim, S.; et al. Phase II randomized trial of radiation therapy, cetuximab, and pemetrexed with or without bevacizumab in patients with locally advanced head and neck cancer. Ann. Oncol. 2016, 27, 1594–1600. [Google Scholar] [CrossRef]
- Kubicek, G.J.; Axelrod, R.S.; Machtay, M.; Ahn, P.H.; Anne, P.R.; Fogh, S.; Cognetti, D.; Myers, T.J.; Curran, W.J., Jr.; Dicker, A.P. Phase I trial using the proteasome inhibitor bortezomib and concurrent chemoradiotherapy for head-and-neck malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manukian, G.; Bar-Ad, V.; Lu, B.; Argiris, A.; Johnson, J.M. Combining radiation and immune checkpoint blockade in the treatment of head and neck squamous cell carcinoma. Front. Oncol. 2019, 9, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiwert, T.Y.; Darga, T.; Haraf, D.; Blair, E.A.; Stenson, K.; Cohen, E.E.W.; Salama, J.K.; Villaflor, V.; Witt, M.E.; Lingen, M.W.; et al. A phase I dose escalation study of ad GV.EGR.TNF.11 D (TNFeradeTM Biologic) with concurrent chemoradiotherapy in patients with recurrent head and neck cancer undergoing reirradiation. Ann. Oncol. 2013, 24, 769–776. [Google Scholar] [CrossRef]
- Lee, J.W.; Parameswaran, J.; Sandoval-Schaefer, T.; Eoh, K.J.; Yang, D.H.; Zhu, F.; Mehra, R.; Sharma, R.; Gaffney, S.G.; Perry, E.B.; et al. Combined aurora kinase A (AURKA) and WEE1 inhibition demonstrates synergistic antitumor effect in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2019, 25, 3430–3442. [Google Scholar] [CrossRef] [Green Version]
- Karam, S.D.; Reddy, K.; Blatchford, P.J.; Waxweiler, T.; DeLouize, A.M.; Oweida, A.; Somerset, H.; Marshall, C.; Young, C.; Davies, K.D.; et al. Final report of a phase I trial of olaparib with cetuximab and radiation therapy for heavy smoker patients with locally advanced head and neck cancer. Clin. Cancer Res. 2016, 24, 4949–4959. [Google Scholar]
- Eytan, D.F.; Snow, G.E.; Carlson, S.; Derakhshan, A.; Saleh, A.; Schiltz, S.; Chen, H.; Mohan, S.; Cornelius, S.; Coupar, J.; et al. SMAC mimetic birinapant plus radiation eradicates human head and neck cancers with genomic amplifications of cell death genes FADD and BIRC2. Cancer Res. 2016, 76, 5442–5454. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamez, M.E.; Blakaj, A.; Zoller, W.; Bonomi, M.; Blakaj, D.M. Emerging Concepts and Novel Strategies in Radiation Therapy for Laryngeal Cancer Management. Cancers 2020, 12, 1651. https://doi.org/10.3390/cancers12061651
Gamez ME, Blakaj A, Zoller W, Bonomi M, Blakaj DM. Emerging Concepts and Novel Strategies in Radiation Therapy for Laryngeal Cancer Management. Cancers. 2020; 12(6):1651. https://doi.org/10.3390/cancers12061651
Chicago/Turabian StyleGamez, Mauricio E., Adriana Blakaj, Wesley Zoller, Marcelo Bonomi, and Dukagjin M. Blakaj. 2020. "Emerging Concepts and Novel Strategies in Radiation Therapy for Laryngeal Cancer Management" Cancers 12, no. 6: 1651. https://doi.org/10.3390/cancers12061651
APA StyleGamez, M. E., Blakaj, A., Zoller, W., Bonomi, M., & Blakaj, D. M. (2020). Emerging Concepts and Novel Strategies in Radiation Therapy for Laryngeal Cancer Management. Cancers, 12(6), 1651. https://doi.org/10.3390/cancers12061651




