Abstract
Radiation-induced lung injury (RILI), including acute radiation pneumonitis and chronic radiation-induced lung fibrosis, is the most common side effect of radiation therapy. RILI is a complicated process that causes the accumulation, proliferation, and differentiation of fibroblasts and, finally, results in excessive extracellular matrix deposition. Currently, there are no approved treatment options for patients with radiation-induced pulmonary fibrosis (RIPF) partly due to the absence of effective targets. Current research advances include the development of small animal models reflecting modern radiotherapy, an understanding of the molecular basis of RIPF, and the identification of candidate drugs for prevention and treatment. Insights provided by this research have resulted in increased interest in disease progression and prognosis, the development of novel anti-fibrotic agents, and a more targeted approach to the treatment of RIPF.
1. Introduction
Radiation therapy (RT) is performed in about 50% of all cancer patients at least once during their treatment course. Recently, with the development of computer and mechanical engineering, it has become increasingly important through effective treatment. Clinical RT involves irradiation with high radiation doses to remove tumors. Surrounding normal tissue adjacent to the tumor is prone to side-effects. The most common side effects after ionizing radiation (IR) treatment of thoracic tumors are pneumonitis and pulmonary fibrosis (PF). While pneumonitis occurs early following treatment and may be reversible, PF is considered to be irreversible delayed toxicity [1]. Pneumonitis can occur in as many as 50% of lung cancer patients, and rates of PF can be as high as 70–80% in high-dose regions of the lung [2]. Although it affects fewer people than idiopathic pulmonary fibrosis (IPF) per se, similar pathological changes are seen in a minority of adult cancer survivors exposed to lung irradiation. Currently, there are no approved treatment options for patients with radiation-induced PF (RIPF), which may be mediated by the absence of effective targets. However, regimens for IPF, such as pirfenidone and nintedanib, help to reduce clinical exacerbations that affect pulmonary function [3,4]. In this review, to emphasize the importance of small animal models, we first described the characteristics of radiation-induced lung injury (RILI) including radiation pneumonitis and RIPF. In addition, we discussed the pathological mechanisms of RIPF including the cytokine secretion, and the epithelial and endothelial-to-mesenchymal (EMT/EndMT) process. Lastly, we also described the antifibrotic agents used in the clinic or under the investigations.
2. Radiation-Induced Lung Injury (RILI)
When IR passes through the lung tissue, energy not only directly induces double-strand break (DSBs) of the DNA molecule, but also has sufficient strength to hydrolyze water and other molecules. This hydrolysis produces reactive oxygen species (ROS), which can interact with DNA and other cellular components of extracellular matrix (ECM) [5]. Most DNA damage is repaired, but incorrect repair can lead to cell deficiency and apoptosis for a much longer time, and may even initiate a strong immune response even before tissue damage is induced [6]. IR exposure can lead to apoptosis of epithelial and endothelial cells within hours and this apoptosis has been experimentally demonstrated to occur in the lung parenchyma after injury [7]. Moreover, ROS can also be produced by cascades of pro-inflammatory cytokines, and the release of cytokines and other products of activated transcription factors play an important role in the progression of RILI [8] (Figure 1).
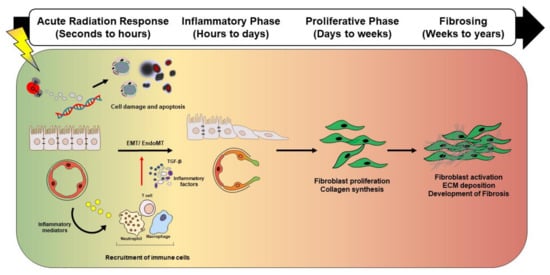
Figure 1.
Schematic representation showing distinct and overlapping stages of radiation-induced lung injury. Radiation-induced lung injury consists of overlapping and highly coordinated stages of acute radiation response, inflammation, proliferation, and fibrosis. After lung injury, ionizing radiation induces reactive oxygen species (ROS) induction, DNA damage, and vascular damage. Damaged epithelial cells and/or endothelial cells release inflammatory mediators that recruit immune cells. The recruited immune cells secrete profibrotic cytokines such as IL-1β, TNF, IL-13, and TGF-β. Secreted cytokines amplify the inflammatory response and trigger fibroblast proliferation and recruitment, which eventually culminates in fibrotic changes. Abbreviation: ROS: Reactive oxygen species, IL-1β: Interleukin 1 beta, TNF: Tumor necrosis factor, IL-13: Interleukin 13 beta, TGF-β: Transforming growth factor-β, EMT: Epithelial to mesenchymal transition, EndMT: Endothelial to mesenchymal transition, ECM: Extracellular matrix
2.1. Radiation Pneumonitis
Radiation pneumonitis as an acute reaction occurs within 4–12 weeks after RT. The acute pneumonitis stage is characterized by recruiting various immune cells into the alveolar space, thickening the alveolar septum, and destroying the integrity of the alveoli. At this time, infiltration of myeloid and lymphoid cells occurs, which results in lung inflammation and edema of alveolar interstitium and the air space [9]. In the lung alveoli, which is the end of the respiratory tree, there are two types of alveolar epithelial cells (AECs or pneumocytes) known as AECI and AECII. In addition to epithelial cells, alveolar macrophages are present in the alveolar space, which are essential for tissue homeostasis, early pathogen recognition, and the initiation of local immune responses and resolution of inflammation. Resident fibroblasts are the most prominent cell type in the alveolar interstitium. Under certain pathological situations, resident fibroblasts can be activated and transformed into myofibroblasts. Myofibroblasts are the main effector cells of tissue fibrosis [10,11,12]. When ACEI undergoes apoptosis or necrosis, depletion of the basement membrane occurs, which is followed by hyperplasia of ACEII. However, the accelerated proliferation of ACEII inhibits the ability of lung surfactants production, which causes surface tension to be lost. This is followed by edema and lethargy of lung tissue [13]. Edema increases vascular permeability, which causes plasma proteins and fibrin-rich exudate to leak into the alveolar space. Radiation pneumonitis without specific treatment remains a major dose-limiting complication in patients. The current treatments including steroids, diuretics, hormones, enzymes, and antioxidants are still non-specific and symptomatic. If pulmonary damage is so extensive that homeostasis cannot be restored by any repair mechanisms, the integrity of the alveolar-capillary barrier begins to decompose at certain points where it does not recover. This makes RIPF worse due to inadequate regeneration and subsequent processing of lung tissue (Figure 2).
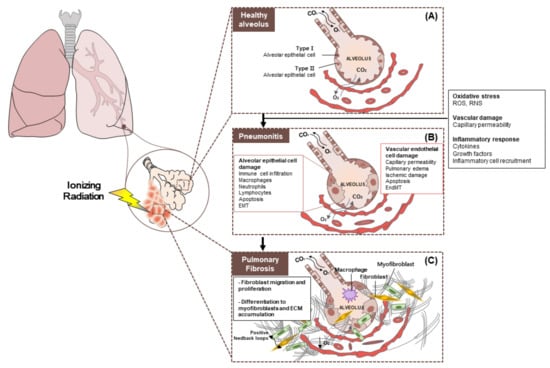
Figure 2.
Pathobiology of radiation pneumonitis and radiation-induced lung injury. (A) Healthy alveolus. The epithelium, which is the outer layer of the alveoli, consists of two types of cells (type 1 and type 2 cells). Type 1 alveolar cells cover 95% of the alveolar surface and constitute an air-blood barrier. Type 2 alveolar cells are smaller and are responsible for the production of surfactants that coat the inside of each alveolus when inhaling and breathing. (B) Radiation pneumonitis is inflammation of the lungs caused by radiotherapy. Chronic pneumonitis can cause scarring of the lungs called pulmonary fibrosis. (C) Pulmonary fibrosis. General changes seen in interstitial lung disease. There is proliferation of interstitial fibroblasts and recruitment of fibrocytes into the interstitium (extracellular matrix (ECM)). These cells begin secreting components of the ECM such as collagen and other fibers. Cells also convert to myofibroblasts, which produce more ECM. Prolonged fibroblast activity leads to fibrosis, which is a hallmark of interstitial lung disease and restrictive lung patterns. Abbreviation: EMT: Epithelial to mesenchymal transition; EndMT: Endothelial to mesenchymal transition; ECM: Extracellular matrix
2.2. Radiation-Induced Pulmonary Fibrosis
RIPF occurs at an irreversible stage more than six months after irradiation. The characteristic RIPF is the accumulation of fibroblasts and myofibroblasts, causing extensive production of collagen, infiltration of inflammatory cells, and remodeling of ECM. This is followed by fibrosis of the alveolar septum, which, in turn, leads to extensive occlusion of the alveoli. RIPF is characterized by the accumulation of the ECM protein, which lowers the ability of the lungs to exchange oxygen. In response to IR, resident lung fibroblasts can differentiate into myofibroblasts, which can secrete ECM proteins. Activated myofibroblasts contribute to fibrogenesis. Therefore, inhibiting IR-induced myofibroblast differentiation is an important therapeutic strategy for preventing fibrosis [14,15]. IR activates fibroblasts/myofibroblasts, which leads to incorrect regulation and exaggerated repair processes, and eventually fibrosis [16]. Epithelial-mesenchymal transition (EMT) is one of the processes that causes lung fibrosis, which is activated by IR, but may also be involved in other pulmonary processes such as embryonic development [17] and tumorigenesis [18]. The major cytokine involving the EMT process is transforming growth factor-β (TGF-β). TGF-β signaling initiates the trans-differentiation of epithelial cells into activated myofibroblasts [17]. The main role of cells undergoing EMT is the synthesis and deposition of ECM proteins.
3. Animal Models of RIPF
3.1. Animal Models That Mimic Conventional RT
Classical studies using whole thorax irradiation have provided important information about the pulmonary IR responses of different rodent strains and defined dose thresholds for lung toxicities. Susceptibility to fibrosis to IR is genetically based in mice, and, most notably, C3H/HeJ and CBA/J mice are resistant to IR-induced fibrosis in comparison with the more susceptible C57BL/6 strain [19]. C3H/He mice are used as a classical model to develop early-stage pneumonitis, but do not develop fibrosis below a single dose of 20 Gy by 12–20 weeks. In contrast, 20 Gy in C57BL/6 mice are sufficient to induce fibrosis, while other strains are intermediate between these extremes [20]. To avoid a systemic complication, RT that usually delivers thorax-limited IR is applied and, in this case, fibrosis takes up to 24 weeks [21,22]. During the pulmonary fibrosis, inflammatory responses affect the severity of lung remodeling but is rarely associated with mortality [19]. The pathology of RIPF is believed to involve ROS-mediated DNA damage and TGF-β production [23]. The introduction of the small animal irradiator significantly improves the precision and accuracy of the dose delivery to the mice, so that more clinically relevant dose and fractionation schedules could be experimentally achieved. When using a small animal irradiator targeting a 10% to 30% sub-volume of the lungs or the near whole lung, representative examples of the dose distribution is shown in Figure 3. Recently, only a small number of published studies focused on RIPF have aimed to improve understanding of the major biological and physical factors supporting the lung’s IR response as preclinical technology advances using a small animal irradiator. This suggests that, as the preclinical techniques advance, there are many challenges and opportunities to understand the biological and physical factors of the lung’s IR response. Fibrosis developed in response to application of 20 Gy to the entire left lung of C57BL/6 mice, and a significant amount of lung fibrosis was observed at 12 months after IR. Collagen deposition was observed at three months after IR with peak induction at 12 months. Fibrosis-related genes identified revealed several differences between 20 Gy IR and 75–90 Gy SBRT (stereotactic body radiation therapy)-mimicking irradiation, which suggested different molecular mechanisms of a low dose whole lung irradiation and a high dose focal irradiation in developing lung fibrosis [24].
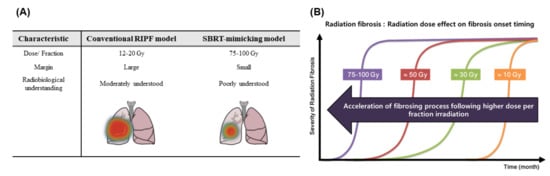
Figure 3.
General comparison of conventional and stereotactic SBRT-mimicking RIPF models. (A) Summary of conventional and SBRT-mimicking RIPF models. In the conventional RIPF model, a large volume of the lung is irradiated with a relatively low dose of radiation, and an abundance of radiobiological research data has been generated. In contrast, the SBRT-mimicking RIPF model mimics modern radiotherapy whereby there is local irradiation with high radiation doses. (B) Both the dose and fractionation of radiation contribute to the severity of radiation fibrosis. RIPF is known to be accelerated with a higher radiation dose per fraction. Therefore, the radiation dose is very important for the development of RIPF. Abbreviations: SBRT: Stereotactic body radiation therapy; RIPF: Radiation-induced pulmonary fibrosis
3.2. Animal Models to Mimic Stereotactic Body Radiation Therapy (SBRT)
More sophisticated conformal RT techniques such as intensity-modulated RT (IMRT), volumetric arc RT (VMAT), stereotactic body radiation therapy (SBRT), and stereotactic radiosurgery (SRS) have a lower RILI incidence than standard 3D conformal RT. This can be due to the increased precision of IR delivery to the tumor, which, thereby, protects the surrounding normal tissue. Moreover, due to the diversity of the technology, it is possible to guide the intensity and direction of the radiation beam by taking into account the anatomy, breathing pattern, and organ motion of the patient. All of these modalities are expected to lower the volume of lung irradiation and the irradiation dose while maintaining millimeter-range accuracy. Moreover, SBRT is the standard of care for patients with medically inoperable stage I NSCLC (Non-small-cell lung carcinoma) [25]. The steep dose gradient and sharp dose fall-off achieved with SBRT substantially reduces treatment volume, and, thereby, reduces the dose to surrounding normal tissues. However, based on published data, the rate of symptomatic IR pneumonitis ranges from 0–49%. The rate of RILI, which can continue to evolve one year after SBRT, is still 70% to 80% in high-dose regions [26]. A mouse model simulating clinical SBRT in terms of ablative dose delivery in a few fractions has been used to show the induction of pulmonary fibrosis using image-guided high-dose-per-fraction irradiation system [27]. Fibrosis-related gene expression in the 90 Gy SBRT-mimicking model was different from that of the 20 Gy conventional RT model [24]. In the SBRT mimicking model, no difference in fibrosis progression between radiosensitive C57BL/6 and radioresistant C3H mice was detected [28]. Because fibrosis development was rapidly induced in this model (4–6 weeks) compared to the conventional RT animal model (6–12 months), it may be a good preclinical model to evaluate anti-fibrotic drugs.
4. Characteristics of RIPF
The pathological mechanism of RIPF is complex and involves numerous cell types. Thorax IR induces delayed damage to resident lung cells, which, primarily, leads to apoptosis of bronchial epithelial cells and loss of barrier function [29]. Recent studies have shown that damaged epithelial cells can become a source of myofibroblasts called EMT/EndMT (Epithelial and endothelial-to-mesenchymal transition) [30,31,32]. Myofibroblasts are known to play a central role in the pathogenesis of RIPF because these cells produce collagens, fibronectins, and other matrix molecules [33,34,35]. Moreover, thorax IR has been shown to induce recruitment of various immune cells into the lungs. After IR exposure, the expression of Th2-related cytokines increased, and the expression of Th1-related cytokines decreased. Thereafter, alveolar macrophage accumulation in the irradiated tissue increased, and TGF-β was dramatically expressed [36]. Macrophages can be divided into two subsets according to their distinct functions [37,38,39]. The two subsets are known as classically activated macrophages (M1 macrophages) and alternatively activated macrophages (M2 macrophages). M1 macrophages are known to play a primary role in the onset of injury [40,41,42]. They secrete pro-inflammatory cytokines that exacerbate damage, amplify the inflammatory response, and contribute to myofibroblast proliferation and recruitment of fibroblasts [43,44]. M1 macrophages are responsible for the release of matrix metalloproteinases (MMP) that break down ECM and promote EMT/EndMT [45], and M2 macrophages contribute to the control of inflammatory processes [46]. When the acute phase of inflammation is complete, Th2 cytokines are produced, which promote the polarization and recruitment of M2 macrophages [47]. In addition, M2 macrophages create an anti-inflammatory environment and promote wound healing [48] and regeneration [49]. However, in the lesion, M2 macrophages may play an important role in the pro-fibrotic process by secreting large amounts of pro-fibrotic factors [50] (Table 1).

Table 1.
Related cells in the development of radiation-induced pulmonary fibrosis (RIPF).
4.1. Cytokine Responses in RIPF
Since various cytokines affect the particular processes of RIPF [51], cytokines have been intensively studied as signaling molecules related to the RILI process and as candidate biomarkers that can identify the risk of RIPF development. Although each cytokine has a unique expression profile, within 4–24 h after irradiation, there is often a nonspecific acute reaction (so-called ‘cytokine storm’) characterized by elevated expression of numerous cytokines. This is followed by a decrease in cytokine levels to baseline within a period of 24 h to a few days [52]. Hundreds of studies have examined the expression of cytokines after irradiation, and a wide spectrum of cytokine profiles are available for both patients and animal models. Damaged pneumocytes, resident macrophages, and endothelial cells release inflammatory cytokines such as TGF-β (Transforming growth factor-β), TNFα (Tumor necrosis factor-α), PDGF (platelet-derived growth factor), IL-1 (Interleukin-1) and IL-6 (Interleukin-6) as well as FGF (Fibroblast growth factor) [31,53,54,55,56]. The characteristic changes in the expression of local and systemic cytokines as well as chemokines are associated with the recruitment of immune cells [57]. Additional studies are necessary to define how the various cells interact and are coordinated during irradiation-induced pulmonary injury (Table 2).

Table 2.
Cytokines in the development of RIPF.
4.2. EMT (Epithelial-Mesenchymal Transition) in RIPF
IR induced a series of complex injuries mediated by oxidative stress, cell death, senescence, and loss of normal lung barrier functions [75]. Some alveolar epithelial cells may undergo EMT and EMT is controlled by transcription factors such as Snail and Twist. Activation of these transcription factors inhibits E-cadherin and increases the expression of contractile protein α-SMA (Smooth muscle actin) [76,77,78,79]. The EMT associated with wound healing, tissue regeneration, and organ fibrosis is considered a second type of EMT. Type 2 EMT generally initiates the production of fibroblasts and other related cells to reconstruct tissues after trauma and inflammatory injury [80]. The production of numerous molecules by inflammatory cells and resident myofibroblasts is associated with Type 2 EMT. These molecules destroy the epithelial layer through the breakdown of the basement membrane. The loss of epithelial cells polarity induces apoptosis or EMT [17]. However, unlike to type 1 EMT, type 2 EMT is associated with inflammation and stops when inflammation is weakened during processes of wound healing and tissue regeneration. The combination of sustained myofibroblast activation, collagen deposition, EMT, and sustained activation of inflammatory cytokine signaling is likely to lead to fibrosis and collagen produced primarily by fibroblasts. This collagen is the most abundant matrix in fibrotic lesions.
5. Therapeutic Targets and Their Inhibitors in RIPF
5.1. Inflammatory Cells
The therapeutic strategy for treating lung injury after IR is restoring the immunological balance. With this treatment strategy, corticosteroids play a major role in managing acute IR pneumonitis, but its role in advanced lung fibrosis remains unclear. Inhibition of neutrophil elastase (NE) prevents the development of lung fibrosis after acute lung injury [81]. Irradiated mice that received sivelestat survived longer than mice that received IR alone. Sivelestat reduced RIPF in mice by suppressing NE activity and an excessive inflammatory reaction [82]. CSF1/CSF1R (colony-stimulating factor receptor-1) pathway is a novel therapeutic candidate for RIPF inhibition based on the results from a preclinical mouse model following thorax IR and also from using human lung biopsies from patients who underwent thorax irradiation. The murine counterpart of the human CS4 antibody known as the anti-CSF1R antibody (CS7) is currently in clinical trials for treating prostate and breast cancer [83]. Specific depletion of infiltrating macrophages in RIPF with CSF1R mAb had an anti-fibrotic effect, and CSF1R was identified as a potent antifibrotic target [84]. Navitoclax (ABT-263), a B cell lymphoma 2/extra-large (Bcl-2/xl) inhibitor, and a newly identified senolytic drug can reverse RIPF even at the late stages of fibrosis [85]. This finding suggests that ABT-263 is a potentially effective treatment for RIPF.
5.2. ROS (Reactive Oxygen Species)
The thiophosphate, amifostine, is the only agent approved by the FDA as a clinical IR protector and its active metabolite acts as a radical scavenger [52] to protect against lung and soft tissue fibrosis. Mammary adenocarcinoma bearing rats that received fractionated RT and were treated with amifostine showed reduced lung damage with low plasma TGF-β levels. In addition, administration of amifostine before IR also reduced macrophage accumulation and TGF-β1 expression in lung tissue [86]. Superoxide dismutase (SOD) is a metalloprotease that protects against oxidative damage [36]. Recombinant SOD-TAT (Superoxide dismutase fusion of TAT) protein protected against RILI in mice. When irradiated mice were examined after pretreatment with SOD-TAT, improved growth rate and reduced lung hydroxyproline content as well as sustained SOD activity, glutathione peroxide (GSH-Px) activity, and total antioxidant capacity were detected [87].
5.3. Cytokines/Chemokines
Toll-like receptor (TLR) activation requires a number of intracellular adaptor proteins proximal to TLR and the most prominent one is a myeloid differentiation primary response factor 88 (MyD88). MyD88 contains two prominent domains, a TLR/IL1 receptor domain, and a death domain. MyD88 is a key control factor for innate immune signaling by pathogenic and environmental stresses. MyD88 regulates innate immunity, reduces long-term RILI, and alleviates fibrosis development by preventing chronic lung damage [29,88]. Activation of the chemokine receptor CXCR4 by its ligand stromal cell-derived factor 1 (SDF-1/CXCL12) may also involve the development of RIPF [89]. MSX-122 is a novel small molecule and partial inhibitor of CXCR4, and the CXCR4/CXCL12 axis has been shown to be critical for the development of RIPF in a mouse model. CXCR4 inhibition by MSX-122 may alleviate potential RILI, which represents a therapeutic opportunity for patients requiring chest irradiation [89].
5.4. TGF-β (Transforming Growth Factor-β)
Activation of the TGF-β/Smad signaling pathway, which is an important step for fibrosis development, has been explored as a potential intervention strategy. SM16, a small molecule inhibitor of the TGF-β receptor I, reduced the degree of RILI in a rat model [29]. Rats that received SM16 experienced a significant decrease in PF, inflammatory response, and TGF-β1 activity [90]. LY2109761 is a novel small-molecule TGF-β receptor I serine/threonine kinase inhibitor that markedly reduces inflammation and PF by IR, which results in prolonged survival. This inhibitor directly attenuates profibrotic signaling pathways as well as mediates TGF-β/bone morphogenetic protein (BMP) signaling [91]. Galunisertib is a highly selective inhibitor of TGF-β receptor I by complete inhibition of its phosphorylation and activation [92]. Treatment with galunisertib attenuated IR-induced pulmonary inflammation and fibrosis. SB203580 and WP631 inhibited the Smad signal transduction pathway, abrogated excessive proliferation, and reduced the expression of p21 (WAF1/CIP1) and PAI-1 (Plasminogen Activator inhibitor-1) induced by IR and TGF-β1 [29]. SB203580, which is a pyridinylimidazole, can highly and specifically inhibit TGF-β receptor I. WP631, which is a serine proteinase inhibitor, inhibited the expression of PAI-1. An increase in expression and activation of PAI-1 could effectively prevent hydrolysis of fibrous proteins and degradation of the ECM, which causes the precipitation of fibrous proteins within tissues and the accumulation of ECM [93]. Thalidomide was shown to inhibit TGF-β1-induced α-SMA and vimentin production as well as pulmonary epithelial cell morphological changes by suppressing both Smad-dependent and non-Smad-dependent pathways. Thus, thalidomide may have potential as a therapeutic agent to inhibit pulmonary fibrosis [89].
5.5. PDGF (Platelet-Derived Growth Factor)
The platelet-derived growth factor (PDGF) receptor tyrosine kinase inhibitors significantly attenuate the development of fibroblast foci, characteristics of PF, and subsequent remodeling of the pulmonary structure. SU9518 is a highly selective inhibitor of the PDGF receptors α and β, which blocks PDGF receptor kinase activity and PDGF receptor-induced cell proliferation [36]. SU9518, SU1167, and imatinib mesylate with all activity against PDGF receptor α and PDGF receptor β have been shown to attenuate PF in an animal model [94]. SU9518 treatment significantly reduced collagen deposition, alveolar wall thickness, and other histological signs of fibrosis [95,96].
5.6. CTGF (Connective Tissue Growth Factor)
Connective tissue growth factor (CTGF) is known as a molecule for TGF-β-mediated fibrosis. However, CTGF, once activated, has been reported to promote and maintain PF by exerting a direct fibrosis effect out of control by TGF-β. This hypothesis is supported by data demonstrating that CTGF activates collagen type 1 and promotes ECM synthesis. CTGF depletion in fibroblasts and smooth muscle cells significantly reduced fibrosis [97]. Pamrevlumab (FG-3019), which is a human monoclonal antibody against CTGF, exhibits reprogrammed fibrogenesis through normalization of IR-induced genes. These genes regulate inflammation associated with M2 macrophage influx, EMT, myofibroblast activation, tissue remodeling, and ECM deposition. Treatment of FG-3019 to irradiated mice reversed lung regeneration, preserved lung function, and increased survival [36,98,99].
5.7. Miscellaneous Targets
The renin-angiotensin system is known to regulate blood pressure and fluid balance and has also been reported to be involved in the development of RILI. Some angiotensin-converting enzyme (ACE) inhibitors have proven to protect the lungs from fibrosis after IR exposure [97]. Treatment of three structurally different ACE inhibitors (fosinopril, captopril, and enalapril) in drinking water after whole thorax IR reduced collagen synthesis [100]. Methoxystradiol (2-ME), which is a metabolite of 17-beta-estradiol, has been proposed to effectively inhibit HIF-1α. 2-ME suppressed the IR-induced increase of HIF-1α (Hypoxia-inducible factor 1 alpha), which led to a decreased EndMT. The deposition of vascular collagen during RIPF development was also inhibited [33,36]. Myriocin is a specific inhibitor of serine palmitoyltransferase (SPT) and can block the de novo biosynthesis of sphingolipids. Targeting SPT with myriocin can counteract TGF-β–mediated signaling and RIPF progression, suggesting that SPT may be a novel therapeutic target for managing RIPF [36,101]. IR of the lung is associated with increased IL-4 expression and that of its receptor, IL4 receptor α1, as well as the dual oxidase 2 (DUOX2) gene. Treatment with metformin suppressed upregulation of IR-induced genes and IL-4, which was associated with amelioration of pathological changes. Treatment with metformin attenuated the upregulation of the IL-4–DUOX2 pathway and other pathological damages to the lung after exposure to a high dose of IR [102]. The heat shock protein 27 (HSP27) was also identified as a candidate target for treating PF in a mouse model of clinical SBRT. Inhibition of HSP27 using a recently identified small-molecule inhibitor, J2, induces crosslinking of HSP27 attenuated RIPF development. Mechanism underlying HSP27-mediated fibrosis development is IkBa-NFkB signaling activation, which is involved in the EMT process (Table 3) [103].

Table 3.
Therapeutic targets for radiation-induced pulmonary fibrosis (RIPF).
6. Conclusions
Tolerance of lung tissue to IR and subsequent development of IR pneumonitis and fibrosis limit the effectiveness of RT. However, despite the urgent need for improved treatment of RIPF, there are no clinically used methods for RIPF treatment. In addition to drug development, it is important to identify the predictive biomarker that can help identify patients susceptible to RIPF, so that a lower IR dose can be administered to these patients. Future research efforts should focus on identifying novel RIPF inhibitors to improve the treatment of patients with RIPF. Combining small animal irradiators with appropriate animal models and preclinical molecular and functional imaging studies has great potential to further enhance our understanding of the IR response of lung tissue. This approach may help address long-standing questions about the inter-relationships of acute and late effects of IR. This will further promote the development of effective novel treatments that can preserve lung function and quality of life after RT.
In this review, we emphasized the importance of small animal models that can mimic SBRT in terms of delivering high-dose focal irradiation. Regarding the level of vascular dysfunction in the lung tissue, it may occur after high-dose IR. Additional mechanisms are significantly affected in normal tissues after SBRT when compared to conventional RT. However, due to the limited understanding of basic mechanisms and the quantitative impact of clinical outcomes, many questions and investigations are needed to alleviate the effects of high-dose radiation on lung injury. Therefore, more examination using additional animal models are needed.
In conclusion, recent advances have led to the development of more persistent experimental RIPF models and have allowed investigation of targeted epithelial damage, fibroblast-specific alterations, regulation of inflammatory cells during fibrosis, and epithelial-mesenchymal crosstalk. Although these models are still unable to replicate all the features of human RIPF pathology, specific analysis of signal pathways and interaction analysis among various cell types may be possible. More persistent models can enhance our ability to study mechanisms that are operative during the fibrogenic stage, which will increase the likelihood of translating the animal model findings to human disease and facilitate the assessment of therapeutic efficacy on fibrotic remodeling. This may enable a more precise prediction of which compounds have the ability to improve results after fibrosis is established.
Author Contributions
Conceptualization: Y.-S.L. and J.C. Writing–original draft preparation: Y.-S.L., Y.K. (Younghwa Kim), and Y.K. (Yeijin Kim) Investigation: Y.K. (Younghwa Kim) and Y.K. (Yeijin Kim). Visualization: H.J. and Y.Y. Writing–review and editing: Y.-S.L., J.C., and H.J. Supervision: Y.-S.L. The author read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the National Research Foundation of Korea (NRF) grants (NRF-2018R1A5A2025286, 2019R1A2C2086448 and 2020R1A2C3013255), funded by the Korean government (Ministry of Science and ICT).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kong, F.M.; Hayman, J.A.; Griffith, K.A.; Kalemkerian, G.P.; Arenberg, D.; Lyons, S.; Turrisi, A.; Lichter, A.; Fraass, B.; Eisbruch, A.; et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): Predictors for radiation pneumonitis and fibrosis. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1075–1086. [Google Scholar] [CrossRef]
- Ueki, N.; Matsuo, Y.; Togashi, Y.; Kubo, T.; Shibuya, K.; Iizuka, Y.; Mizowaki, T.; Togashi, K.; Mishima, M.; Hiraoka, M. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J. Thorac. Oncol. 2015, 10, 116–125. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- McElroy, M.C.; Kasper, M. The use of alveolar epithelial type I cell-selective markers to investigate lung injury and repair. Eur. Respir. J. 2004, 24, 664–673. [Google Scholar] [CrossRef]
- Bledsoe, T.J.; Nath, S.K.; Decker, R.H. Radiation Pneumonitis. Clin. Chest. Med. 2017, 38, 201–208. [Google Scholar] [CrossRef]
- Johnston, C.J.; Hernady, E.; Reed, C.; Thurston, S.W.; Finkelstein, J.N.; Williams, J.P. Early alterations in cytokine expression in adult compared to developing lung in mice after radiation exposure. Radiat. Res. 2010, 173, 522–535. [Google Scholar] [CrossRef][Green Version]
- Siva, S.; MacManus, M.; Kron, T.; Best, N.; Smith, J.; Lobachevsky, P.; Ball, D.; Martin, O. A pattern of early radiation-induced inflammatory cytokine expression is associated with lung toxicity in patients with non-small cell lung cancer. PLoS ONE 2014, 9, e109560. [Google Scholar] [CrossRef] [PubMed]
- Hillman, G.G.; Singh-Gupta, V.; Hoogstra, D.J.; Abernathy, L.; Rakowski, J.; Yunker, C.K.; Rothstein, S.E.; Sarkar, F.H.; Gadgeel, S.; Konski, A.A.; et al. Differential effect of soy isoflavones in enhancing high intensity radiotherapy and protecting lung tissue in a pre-clinical model of lung carcinoma. Radiother. Oncol. 2013, 109, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Barkauskas, C.E.; Cronce, M.J.; Xue, Y.; Harris, J.R.; Liang, J.; Noble, P.W.; Hogan, B.L. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl. Acad. Sci. USA 2011, 108, E1475–E1483. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, I.E.; Eickelberg, O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet 2012, 380, 680–688. [Google Scholar] [CrossRef]
- Barron, L.; Gharib, S.A.; Duffield, J.S. Lung Pericytes and Resident Fibroblasts: Busy Multitaskers. Am. J. Pathol. 2016, 186, 2519–2531. [Google Scholar] [CrossRef]
- Kasper, M.; Haroske, G. Alterations in the alveolar epithelium after injury leading to pulmonary fibrosis. Histol. Histopathol. 1996, 11, 463–483. [Google Scholar] [PubMed]
- Judge, J.L.; Lacy, S.H.; Ku, W.Y.; Owens, K.M.; Hernady, E.; Thatcher, T.H.; Williams, J.P.; Phipps, R.P.; Sime, P.J.; Kottmann, R.M. The Lactate Dehydrogenase Inhibitor Gossypol Inhibits Radiation-Induced Pulmonary Fibrosis. Radiat. Res. 2017, 188, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ahn, J.Y.; Lim, M.J.; Kim, M.H.; Yun, Y.S.; Jeong, G.; Song, J.Y. Sustained expression of NADPH oxidase 4 by p38 MAPK-Akt signaling potentiates radiation-induced differentiation of lung fibroblasts. J. Mol. Med. Berl. 2010, 88, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.M.; Waters, C.M. Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung. Cell Mol. Physiol. 2010, 298, L715–L731. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Morrison, C.D.; Parvani, J.G.; Schiemann, W.P. The relevance of the TGF-beta Paradox to EMT-MET programs. Cancer Lett. 2013, 341, 30–40. [Google Scholar] [CrossRef]
- Karvonen, R.L.; Fernandez-Madrid, F.; Maughan, R.L.; Palmer, K.C.; Fernandez-Madrid, I. An animal model of pulmonary radiation fibrosis with biochemical, physiologic, immunologic, and morphologic observations. Radiat. Res. 1987, 111, 68–80. [Google Scholar] [CrossRef]
- Franko, A.J.; Sharplin, J.; Ward, W.F.; Hinz, J.M. The genetic basis of strain-dependent differences in the early phase of radiation injury in mouse lung. Radiat. Res. 1991, 126, 349–356. [Google Scholar] [CrossRef]
- Haston, C.K.; Travis, E.L. Murine susceptibility to radiation-induced pulmonary fibrosis is influenced by a genetic factor implicated in susceptibility to bleomycin-induced pulmonary fibrosis. Cancer Res. 1997, 57, 5286–5291. [Google Scholar] [PubMed]
- Johnston, C.J.; Wright, T.W.; Rubin, P.; Finkelstein, J.N. Alterations in the expression of chemokine mRNA levels in fibrosis-resistant and -sensitive mice after thoracic irradiation. Exp. Lung Res. 1998, 24, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Epperly, M.W.; Guo, H.; Gretton, J.E.; Greenberger, J.S. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2003, 29, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kang, G.Y.; Jeon, S.; Kim, J.M.; Park, Y.N.; Cho, J.; Lee, Y.S. Identification of molecular signatures involved in radiation-induced lung fibrosis. J. Mol. Med. Berl. 2019, 97, 37–47. [Google Scholar] [CrossRef]
- Baumann, P.; Nyman, J.; Hoyer, M.; Wennberg, B.; Gagliardi, G.; Lax, I.; Drugge, N.; Ekberg, L.; Friesland, S.; Johansson, K.A.; et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J. Clin. Oncol. 2009, 27, 3290–3296. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Kunieda, E.; Takeda, T.; Tanaka, M.; Sanuki, N.; Fujii, H.; Shigematsu, N.; Kubo, A. Possible misinterpretation of demarcated solid patterns of radiation fibrosis on CT scans as tumor recurrence in patients receiving hypofractionated stereotactic radiotherapy for lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1057–1065. [Google Scholar] [CrossRef]
- Cho, J.; Kodym, R.; Seliounine, S.; Richardson, J.A.; Solberg, T.D.; Story, M.D. High dose-per-fraction irradiation of limited lung volumes using an image-guided, highly focused irradiator: Simulating stereotactic body radiotherapy regimens in a small-animal model. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 895–902. [Google Scholar] [CrossRef]
- Hong, Z.Y.; Lee, C.G.; Shim, H.S.; Lee, E.J.; Song, K.H.; Choi, B.W.; Cho, J.; Story, M.D. Time, Dose, and Volume Responses in a Mouse Pulmonary Injury Model Following Ablative Irradiation. Lung 2016, 194, 81–90. [Google Scholar] [CrossRef]
- Ding, N.H.; Li, J.J.; Sun, L.Q. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr. Drug. Targets 2013, 14, 1347–1356. [Google Scholar] [CrossRef]
- Grande, M.T.; Sánchez-Laorden, B.; López-Blau, C.; De Frutos, C.A.; Boutet, A.; Arévalo, M.; Rowe, R.G.; Weiss, S.J.; López-Novoa, J.M.; Nieto, M.A. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med. 2015, 21, 989–997. [Google Scholar] [CrossRef]
- Choi, S.H.; Hong, Z.Y.; Nam, J.K.; Lee, H.J.; Jang, J.; Yoo, R.J.; Lee, Y.J.; Lee, C.Y.; Kim, K.H.; Park, S.; et al. A Hypoxia-Induced Vascular Endothelial-to-Mesenchymal Transition in Development of Radiation-Induced Pulmonary Fibrosis. Clin. Cancer. Res. 2015, 21, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Péchoux, C.; Bogaard, H.J.; Dorfmüller, P.; Remy, S.; Lecerf, F.; Planté, S.; et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Yarnold, J.; Brotons, M.C. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Grgic, I.; Duffield, J.S.; Humphreys, B.D. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr. Nephrol. 2012, 27, 183–193. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Z.; Ning, W. Advances in Molecular Mechanisms and Treatment of Radiation-Induced Pulmonary Fibrosis. Transl. Oncol. 2019, 12, 162–169. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Varin, A.; Gordon, S. Alternative activation of macrophages: Immune function and cellular biology. Immunobiology 2009, 214, 630–641. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Kis, K.; Liu, X.; Hagood, J.S. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev. Mol. Med. 2011, 13, e27. [Google Scholar] [CrossRef]
- Mahdavian Delavary, B.; van der Veer, W.M.; van Egmond, M.; Niessen, F.B.; Beelen, R.H. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G. Tissue mechanics and fibrosis. Biochim. Biophys. Acta 2013, 1832, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver. Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Zimmermann, H.W. Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 2014, 60, 1090–1096. [Google Scholar] [CrossRef]
- Cheng, S.; Lovett, D.H. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am. J. Pathol. 2003, 162, 1937–1949. [Google Scholar] [CrossRef]
- Eddy, A.A. Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int. Suppl. 2014, 4, 2–8. [Google Scholar] [CrossRef]
- Ricardo, S.D.; van Goor, H.; Eddy, A.A. Macrophage diversity in renal injury and repair. J. Clin. Investig. 2008, 118, 3522–3530. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Braga, T.T.; Moura, I.C.; Lepique, A.P.; Camara, N.O.S. Editorial: Macrophages Role in Integrating Tissue Signals and Biological Processes in Chronic Inflammation and Fibrosis. Front. Immunol. 2017, 8, 845. [Google Scholar] [CrossRef]
- Vernon, M.A.; Mylonas, K.J.; Hughes, J. Macrophages and renal fibrosis. Semin. Nephrol. 2010, 30, 302–317. [Google Scholar] [CrossRef]
- Todd, N.W.; Luzina, I.G.; Atamas, S.P. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair 2012, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Talas, M.; Szolgay, E.; Varteresz, V.; Koczkas, G. Influence of acute and fractional X-irradiation on induction of interferon in vivo. Arch. Gesamte Virusforsch 1972, 38, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Johnston, C.J.; Finkelstein, J.N. Treatment for radiation-induced pulmonary late effects: Spoiled for choice or looking in the wrong direction? Curr. Drug Targets 2010, 11, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef]
- Vallee, A.; Lecarpentier, Y.; Guillevin, R.; Vallee, J.N. Interactions between TGF-beta1, canonical WNT/beta-catenin pathway and PPAR gamma in radiation-induced fibrosis. Oncotarget 2017, 8, 90579–90604. [Google Scholar] [CrossRef]
- Rube, C.E.; Uthe, D.; Schmid, K.W.; Richter, K.D.; Wessel, J.; Schuck, A.; Willich, N.; Rube, C. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1033–1042. [Google Scholar] [CrossRef]
- Lierova, A.; Jelicova, M.; Nemcova, M.; Proksova, M.; Pejchal, J.; Zarybnicka, L.; Sinkorova, Z. Cytokines and radiation-induced pulmonary injuries. J. Radiat. Res. 2018, 59, 709–753. [Google Scholar] [CrossRef]
- Du Bois, R.M. Strategies for treating idiopathic pulmonary fibrosis. Nat. Rev. Drug Discov. 2010, 9, 129–140. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, W.; Yu, F.; Gao, F. The Cellular and Molecular Mechanism of Radiation-Induced Lung Injury. Med. Sci. Monit. 2017, 23, 3446–3450. [Google Scholar] [CrossRef]
- Abraham, D.J.; Eckes, B.; Rajkumar, V.; Krieg, T. New developments in fibroblast and myofibroblast biology: Implications for fibrosis and scleroderma. Curr. Rheumatol. Rep. 2007, 9, 136–143. [Google Scholar] [CrossRef]
- Holmes, A.; Abraham, D.J.; Sa, S.; Shiwen, X.; Black, C.M.; Leask, A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J. Biol. Chem. 2001, 276, 10594–10601. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ivashkiv, L.B. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity 2009, 31, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Geiser, T.; Atabai, K.; Jarreau, P.H.; Ware, L.B.; Pugin, J.; Matthay, M.A. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1beta-dependent mechanism. Am. J. Respir. Crit. Care Med. 2001, 163, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Broekman, W.; Amatngalim, G.D.; de Mooij-Eijk, Y.; Oostendorp, J.; Roelofs, H.; Taube, C.; Stolk, J.; Hiemstra, P.S. TNF-alpha and IL-1beta-activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor. Respir. Res. 2016, 17, 3. [Google Scholar] [CrossRef]
- Wynn, T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef]
- Zurawski, S.M.; Vega, F., Jr.; Huyghe, B.; Zurawski, G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J. 1993, 12, 2663–2670. [Google Scholar] [CrossRef]
- Pesce, J.; Kaviratne, M.; Ramalingam, T.R.; Thompson, R.W.; Urban, J.F., Jr.; Cheever, A.W.; Young, D.A.; Collins, M.; Grusby, M.J.; Wynn, T.A. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J. Clin. Investig. 2006, 116, 2044–2055. [Google Scholar] [CrossRef]
- Doucet, C.; Brouty-Boye, D.; Pottin-Clemenceau, C.; Canonica, G.W.; Jasmin, C.; Azzarone, B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J. Clin. Investig. 1998, 101, 2129–2139. [Google Scholar] [CrossRef]
- Fertin, C.; Nicolas, J.F.; Gillery, P.; Kalis, B.; Banchereau, J.; Maquart, F.X. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol. Biol. 1991, 37, 823–829. [Google Scholar]
- Tiggelman, A.M.; Boers, W.; Linthorst, C.; Sala, M.; Chamuleau, R.A. Collagen synthesis by human liver (myo)fibroblasts in culture: Evidence for a regulatory role of IL-1 beta, IL-4, TGF beta and IFN gamma. J. Hepatol. 1995, 23, 307–317. [Google Scholar] [CrossRef]
- Pradere, J.P.; Gonzalez, J.; Klein, J.; Valet, P.; Gres, S.; Salant, D.; Bascands, J.L.; Saulnier-Blache, J.S.; Schanstra, J.P. Lysophosphatidic acid and renal fibrosis. Biochim. Biophys. Acta 2008, 1781, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Blonska, M.; You, Y.; Geleziunas, R.; Lin, X. Restoration of NF-kappaB activation by tumor necrosis factor alpha receptor complex-targeted MEKK3 in receptor-interacting protein-deficient cells. Mol. Cell Biol. 2004, 24, 10757–10765. [Google Scholar] [CrossRef] [PubMed]
- Osborn, L.; Kunkel, S.; Nabel, G.J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc. Natl. Acad. Sci. USA 1989, 86, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Baltimore, D. NF-kappa B: Ten years after. Cell 1996, 87, 13–20. [Google Scholar] [CrossRef]
- Citrin, D.E.; Prasanna, P.G.S.; Walker, A.J.; Freeman, M.L.; Eke, I.; Barcellos-Hoff, M.H.; Arankalayil, M.J.; Cohen, E.P.; Wilkins, R.C.; Ahmed, M.M.; et al. Radiation-Induced Fibrosis: Mechanisms and Opportunities to Mitigate. Report of an NCI Workshop, September 19, 2016. Radiat. Res. 2017, 188, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Nagarajan, D.; Tian, J.; Leal, S.W.; Wheeler, K.; Munley, M.; Blackstock, W.; Zhao, W. The role of alveolar epithelium in radiation-induced lung injury. PLoS ONE 2013, 8, e53628. [Google Scholar] [CrossRef] [PubMed]
- Rubin, P.; Johnston, C.J.; Williams, J.P.; McDonald, S.; Finkelstein, J.N. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 99–109. [Google Scholar] [CrossRef]
- Kim, K.K.; Kugler, M.C.; Wolters, P.J.; Robillard, L.; Galvez, M.G.; Brumwell, A.N.; Sheppard, D.; Chapman, H.A. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. USA 2006, 103, 13180–13185. [Google Scholar] [CrossRef]
- Sahlgren, C.; Gustafsson, M.V.; Jin, S.; Poellinger, L.; Lendahl, U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl. Acad. Sci. USA 2008, 105, 6392–6397. [Google Scholar] [CrossRef]
- Lee, K.; Nelson, C.M. New insights into the regulation of epithelial-mesenchymal transition and tissue fibrosis. Int. Rev. Cell Mol. Biol. 2012, 294, 171–221. [Google Scholar] [CrossRef]
- Fujino, N.; Kubo, H.; Suzuki, T.; He, M.; Suzuki, T.; Yamada, M.; Takahashi, T.; Ota, C.; Yamaya, M. Administration of a specific inhibitor of neutrophil elastase attenuates pulmonary fibrosis after acute lung injury in mice. Exp. Lung Res. 2012, 38, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Inomata, T.; Okada, Y.; Shimbo, T.; Takahashi, M.; Akita, K.; Uesugi, Y.; Narumi, Y. Sivelestat sodium hydrate reduces radiation-induced lung injury in mice by inhibiting neutrophil elastase. Mol. Med. Rep. 2013, 7, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Meziani, L.; Mondini, M.; Petit, B.; Boissonnas, A.; Thomas de Montpreville, V.; Mercier, O.; Vozenin, M.C.; Deutsch, E. CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef]
- Contreras, Z.A.; Chen, Z.; Roumeliotaki, T.; Annesi-Maesano, I.; Baiz, N.; von Berg, A.; Bergstrom, A.; Crozier, S.; Duijts, L.; Ekstrom, S.; et al. Does early onset asthma increase childhood obesity risk? A pooled analysis of 16 European cohorts. Eur. Respir. J. 2018, 52. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sun, C.; Su, Q.; Wang, Y.; Li, J.; Guo, Z.; Chen, L.; Zhang, H. Radiation-induced lung injury: Latest molecular developments, therapeutic approaches, and clinical guidance. Clin. Exp. Med. 2019, 19, 417–426. [Google Scholar] [CrossRef]
- Tsoutsou, P.G.; Koukourakis, M.I. Radiation pneumonitis and fibrosis: Mechanisms underlying its pathogenesis and implications for future research. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1281–1293. [Google Scholar] [CrossRef]
- Pan, J.; Su, Y.; Hou, X.; He, H.; Liu, S.; Wu, J.; Rao, P. Protective effect of recombinant protein SOD-TAT on radiation-induced lung injury in mice. Life Sci. 2012, 91, 89–93. [Google Scholar] [CrossRef]
- Brickey, W.J.; Neuringer, I.P.; Walton, W.; Hua, X.; Wang, E.Y.; Jha, S.; Sempowski, G.D.; Yang, X.; Kirby, S.L.; Tilley, S.L.; et al. MyD88 provides a protective role in long-term radiation-induced lung injury. Int. J. Radiat. Biol. 2012, 88, 335–347. [Google Scholar] [CrossRef][Green Version]
- Shu, H.K.; Yoon, Y.; Hong, S.; Xu, K.; Gao, H.; Hao, C.; Torres-Gonzalez, E.; Nayra, C.; Rojas, M.; Shim, H. Inhibition of the CXCL12/CXCR4-axis as preventive therapy for radiation-induced pulmonary fibrosis. PLoS ONE 2013, 8, e79768. [Google Scholar] [CrossRef]
- Anscher, M.S.; Thrasher, B.; Zgonjanin, L.; Rabbani, Z.N.; Corbley, M.J.; Fu, K.; Sun, L.; Lee, W.C.; Ling, L.E.; Vujaskovic, Z. Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 829–837. [Google Scholar] [CrossRef]
- Flechsig, P.; Dadrich, M.; Bickelhaupt, S.; Jenne, J.; Hauser, K.; Timke, C.; Peschke, P.; Hahn, E.W.; Grone, H.J.; Yingling, J.; et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin. Cancer Res. 2012, 18, 3616–3627. [Google Scholar] [CrossRef] [PubMed]
- Bueno, L.; de Alwis, D.P.; Pitou, C.; Yingling, J.; Lahn, M.; Glatt, S.; Troconiz, I.F. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur. J. Cancer 2008, 44, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, L.W.; Peng, R.Y.; Wang, D.W.; Jin, M.H.; Gao, Y.B.; Ma, J.J. Effects of SB203580 and WP631 on Smad signal transduction pathway in lung fibroblasts after irradiation. Ai Zheng 2008, 27, 698–702. [Google Scholar] [PubMed]
- Westbury, C.B.; Yarnold, J.R. Radiation fibrosis—current clinical and therapeutic perspectives. Clin. Oncol. R Coll. Radiol. 2012, 24, 657–672. [Google Scholar] [CrossRef]
- Abdollahi, A.; Li, M.; Ping, G.; Plathow, C.; Domhan, S.; Kiessling, F.; Lee, L.B.; McMahon, G.; Grone, H.J.; Lipson, K.E.; et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J. Exp. Med. 2005, 201, 925–935. [Google Scholar] [CrossRef]
- Li, M.; Abdollahi, A.; Grone, H.J.; Lipson, K.E.; Belka, C.; Huber, P.E. Late treatment with imatinib mesylate ameliorates radiation-induced lung fibrosis in a mouse model. Radiat. Oncol. 2009, 4, 66. [Google Scholar] [CrossRef]
- Sottili Mariangela, M.M.; Terziani, F.; Trombetta, L.; Loi, M.; Cappelli, S.; Di Brina, L.; Livi, L. Biological basis of radiation-induced pulmonary fibrosis. J. Tumor 2015, 3, 325–331. [Google Scholar]
- Bickelhaupt, S.; Erbel, C.; Timke, C.; Wirkner, U.; Dadrich, M.; Flechsig, P.; Tietz, A.; Pfohler, J.; Gross, W.; Peschke, P.; et al. Effects of CTGF Blockade on Attenuation and Reversal of Radiation-Induced Pulmonary Fibrosis. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012, 5, S24. [Google Scholar] [CrossRef]
- Kma, L.; Gao, F.; Fish, B.L.; Moulder, J.E.; Jacobs, E.R.; Medhora, M. Angiotensin converting enzyme inhibitors mitigate collagen synthesis induced by a single dose of radiation to the whole thorax. J. Radiat. Res. 2012, 53, 10–17. [Google Scholar] [CrossRef]
- Gorshkova, I.; Zhou, T.; Mathew, B.; Jacobson, J.R.; Takekoshi, D.; Bhattacharya, P.; Smith, B.; Aydogan, B.; Weichselbaum, R.R.; Natarajan, V.; et al. Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase-1 expression. J. Lipid. Res. 2012, 53, 1553–1568. [Google Scholar] [CrossRef] [PubMed]
- Azmoonfar, R.; Amini, P.; Saffar, H.; Rezapoor, S.; Motevaseli, E.; Cheki, M.; Yahyapour, R.; Farhood, B.; Nouruzi, F.; Khodamoradi, E.; et al. Metformin Protects Against Radiation-Induced Pneumonitis and Fibrosis and Attenuates Upregulation of Dual Oxidase Genes Expression. Adv. Pharm. Bull. 2018, 8, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jeon, S.; Yoo, Y.J.; Jin, H.; Won, H.Y.; Yoon, K.; Hwang, E.S.; Lee, Y.J.; Na, Y.; Cho, J.; et al. The Hsp27-Mediated IkBalpha-NFkappaB Signaling Axis Promotes Radiation-Induced Lung Fibrosis. Clin. Cancer Res. 2019, 25, 5364–5375. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).